|
|
1.IntroductionThe outer layer of the human skin—the stratum corneum—consists of unnucleated corneocytes.1, 2 Representing the barrier of the human organism to the environment, the stratum corneum not only prevents the body from water loss3 and electrolytes (desiccation), but also environmental hazards4 and micro-organisms from penetrating.5 The skin is a sensitive organ which is susceptible to any kind of irritation.6 This is due to the high density of nerve cells located in the skin.7 However, the skin sensitivity of the population can vary significantly particularly in dry or inflamed skin that might respond intensively to external mechanical stress. Such irritations are generated, inter alia, by textile materials covering the major part of the skin. Today, an ample range of manufacturing and treatment processes aimed at producing clothing textiles with advantageous skin physiological properties are available.8 For atopic dermatitis, e.g., specific clothing has been developed.9, 10, 11 So far, textile fabrics have been characterized for their material properties only, such as type of material (wool, polyester, etc.), abrasion resistance, number of filaments, type of weaving, tensile strength, bending strength, elongation, and abrasion.12 Besides various mechanical measuring methods, optical analysis techniques, such as light microscopy and electron scanning microscopy have been applied.13 No use has been made of techniques, which identify the skin physiological properties of textile materials. In the present study, textile materials were characterized for their skin compatibility and surface structure, noninvasive, state-of-the-art optical methods, i.e., optical coherence tomography,14, 15 3D-surface profilometry16 and laser scanning microscopy (LSM).17, 18 These methods are widely used in in vivo dermatological and cosmetological research.19 For this purpose, two different fabrics were compared with different haptic properties according to the treatment exhibited. These materials were objectively evaluated by optical methods and, moreover, tested on volunteers to obtain a subjective haptic assessment on the skin friendliness of the samples. In a standardized rubbing experiment, the samples were then tested on the skin in vivo, and by subsequently analyzing changes in barrier properties with in vivo LSM.20 2.Materials and Methods2.1.Textile MaterialsThe investigation was carried out on two different textile materials [polyamide (PA 6.6) and polyester]. The mass per unit area of the desized and thermofixed tabby weave polyester material amounted to 207.5 g/m2. The desized tabby weave polyamide material exhibited a mass per unit area of 167.4 g/cm2. 2.2.VolunteersThe experiments were carried out on the volar forearm of 26 healthy volunteers between 20 and 50 yrs of age (mean age 35.7 yr) of both genders (8 men; 18 women). A mechanical stress test with the different textile samples and subsequent barrier assessment were performed on the forearm of six healthy volunteers. Twenty volunteers were questioned about their haptic sensations of the samples when the textiles were in contact with their skin. Approval for this study had been obtained from the Ethics Committee of the Charité–Universitätsmedizin Berlin. 2.3.Stress TestThe selected textile materials were tensioned on an applicator of 3×5 cm in size and 1000 g in weight. This applicator was moved 40times over a marked skin area of 4 cm ×10 cm on the volar forearm, the constant weight generating a constant pressure of the fabric onto the skin during the movement. 2.4.Optical Coherence TomographyThe bulk structure of the textile materials was analyzed by means of optical tomography using a “SkinDex 300” system (ISIS Optronics, Mannheim, Germany). This optical coherence tomomgraphy (OCT) system has been described in detail by Lademann 14 2.5.Surface ProfilometryThe surface structure of the employed textile was measured with a “PRIMOS” noncontact surface 3D-profilometer (GFMesstechnik GmbH, Teltow, Germany).16, 21 This system is generally used for analyzing the skin surface structure in vivo. In the present study, the 3D-profilometer delivered camera images, color-coded roughness images, and roughness profiles. In addition, it determined the surface roughness arithmetically. The system has been previously described.21 2.6.Laser Scanning MicroscopyThe in vivo LSM “Stratum” (Optilas Ltd., Melbourne, Australia) was used for analyzing the surface structure of the stratum corneum. In order to visualize the corneocyte architecture as a marker of epidermal barrier status, the fluorescent mode of the LSM was used. 0.1 mL/cm2 of an aqueous solution containing 0.1% of the fluorescent dye, fluorescein, was applied to the skin areas of the volunteers after the standardized contact with the textile material. After an exposure time of 1 min, the excess solution was carefully removed with filter paper. The measuring area of the LSM was 260×250 μm. Each treated skin area was measured at least 4times. The system was previously described in detail by Jung 22 3.Results and DiscussionWhile the investigation of textile materials hitherto had exclusively been aimed at characterizing the mechanical properties of these materials, the purpose of the optical methods applied in the present study was to gain insight into characteristics of the fabric and the subsequent effect on human skin. The applied methods are standard procedures as used in dermatology and cosmetology for in vivo investigations on humans. Figures 1a and 1b represent OCT images of the polyamide and the polyester fabric cross sections. It is visible that the bulk structure of the polyamide fabric [Fig. 1a] is essentially rougher than that of the polyester fabric [Fig. 1b]. On the photos of the two textile materials [Figs. 1c and 1d] taken with the PRIMOS surface 3D-profilometer, differences in structures of the samples are clearly visible. This impression is confirmed by the surface topography, which permits the estimation of the roughness of the two materials (polyamide fabric [Fig. 2a] and polyester fabric [Fig. 2b]). The measured roughness value of the polyamide surface (29.8=B10.6 =B5m SD roughness depth) compared to that of the polyester surface (24.6=B11.0 =B5m SD roughness depth) is increased. Fig. 1(a) Characterization by optical coherence tomography OTC picture of polyamide fabric. (b) Characterization by optical coherence tomography OTC picture of polyester fabric. (c) Top view picture captured by Primos system of polyamide fabric. (d) Top view picture captured by Primos system of polyester fabric.  Fig. 2(a) Surface structure 3D-profilometry with the Primos system of polyamide fabric. (b) Surface structure 3D-profilometry with the Primos system of polyester fabric. 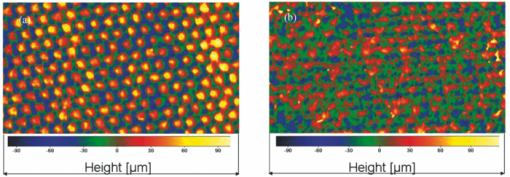 Twenty out of twenty volunteers assessing the haptic properties of the fabrics scored polyester favorable over polyamide regarding their sensation when the two textile materials were in contact with the skin. The volunteers considered the polyester material to be far more comfortable on their skin than the polyamide material. Thus, the feelings of the volunteers unambiguously reflected the objective results of the OCT measurements and the surface roughness profilometry. In the last part of the study, it was investigated whether the two textile materials affect the barrier functions when standardized rubbing on the skin with the fabric was performed. The results obtained by laser scanning microscopy showed that under standardized conditions the polyamide material disturbs the structure of the skin barrier [Fig. 3c] while polyester [Fig. 3d] induced almost no detectable changes in LSM. In Figs. 3a, 3b, 3c, 3d, the LSM images before [Figs. 3a and 3b] and after skin contact [Figs. 3c and 3d] with the textiles are compared. While the corneocytes have an intact homogeneous structure similar to a flat honeycomb before contact with the polyamide material [Figs. 3a and 3b], their arrangement after the textile-skin contact [Fig. 3c] resembles a somewhat mountainous structure, which corresponds to an extremely dry or damaged barrier.23 On the contrary, the polyester material had not caused damage to the barrier under the same conditions [Fig. 3d]. Thus, the objective results from the textile analysis obtained by OCT and skin surface profilometry were confirmed by both the subjective, haptic skin physiological sensation of the volunteers and the in vivo response of the skin barrier (stratum corneum).24, 25 Consequently, new perspectives are opening up for the optical examination methods used in this study when textile materials are evaluated during the development process and differentiation of multiple textile materials. Based on optical methods, time consuming, expensive in vivo studies can be reduced and new, more comfortable textile materials can be introduced to the market quicker, more cost-favorable, and more user-friendly. With the increasing number of people suffering from skin irritations and diseases like atopic dermatitis,11 the development of textiles with favorable properties is becoming increasingly important. In this context, objective optical procedures like optical coherence tomography, laser scanning microscopy, and surface profilometry are decisive instruments at early development stages. Fig. 3(a) Baseline picture (before standardized textile-skin rubbing) as barrier properties measured by in vivo LSM from the polyamide area. (b) Baseline picture (before standardized textile-skin rubbing) as barrier properties measured by in vivo LSM from the polyester area. (c) After polyamide rubbing: barrier properties measured by in vivo LSM from the polyamide area. Note the mountainous and inhomogeneous structure, which corresponds to a damaged barrier. (d) After polyester rubbing: barrier properties measured by in vivo LSM from the polyester area. Note the intact and homogeneous structure similar to a flat honeycomb before contact with the polyamide material. 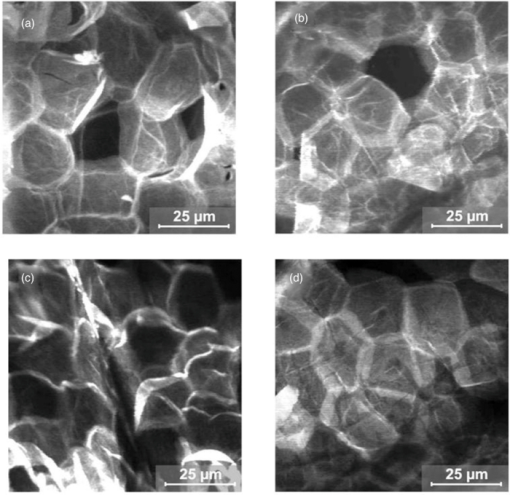 AcknowledgmentsWe would like to thank the Foundation of Skin Physiology incorporated in the Donor Association for the Promotion of Sciences and Humanities in Germany for its financial support. ReferencesD. Kessner, A. Ruettinger, M. A. Kiselev, S. Wartewig, and
R. H. H. Neubert,
“Properties of ceramides and their impact on the stratum corneum structure: a review—part 2: stratum corneum lipid model systems,”
Skin Pharmacol. Physiol., 21 58
–74
(2008). https://doi.org/10.1159/000112956 Google Scholar
M. Q. Man, S. J. Xin, S. P. Song, S. Y. Cho, X. J. Zhang, C. X. Tu, K. R. Feingold, and
P. M. Elias,
“Variation of skin surface pH, sebum content and stratum corneum hydration with age and gender in a large chinese population,”
Skin Pharmacol. Physiol., 22 190
–199
(2009). https://doi.org/10.1159/000231524 Google Scholar
J. Pinnagoda, R. A. Tupker, T. Agner, and
J. Serup,
“Guidelines for transepidermal water-loss (Tewl) measurement—a report from the standardization-group-of-the european-society-of-contact-dermatitis,”
Contact Dermatitis, 22 164
–178
(1990). https://doi.org/10.1111/j.1600-0536.1990.tb01553.x Google Scholar
V. Stone, H. Johnston, and
M. J. D. Clift,
“Air pollution, ultrafine and nanoparticle toxicology: cellular and molecular interactions,”
IEEE Trans. Nanobiosci., 6 331
–340
(2007). https://doi.org/10.1109/TNB.2007.909005 Google Scholar
J. Lai and
H. I. Maibach,
“Experimental models in predicting topical antifungal efficacy: practical aspects and challenges,”
Skin Pharmacol. Physiol., 22 231
–239
(2009). https://doi.org/10.1159/000235827 Google Scholar
D. Basketter and
I. Kimber,
“Re: updating the skin sensitization in vitro data assessment paradigm in 2009-a chemistry and QSAR perspective,”
J. Appl. Toxicol., 30 289
–289
(2010). Google Scholar
V. M. Riccardi,
“Skin, blood, nerve-cells, and heritability—new lessons from neurofibromatosis type-I,”
Arch. Dermatol., 131 944
–944
(1995). https://doi.org/10.1001/archderm.131.8.944 Google Scholar
W. Zhong, M. M. Q. Xing, N. Pan, and
H. I. Maibach,
“Textiles and human skin, microclimate, cutaneous reactions: an overview,”
Cutaneous and Ocular Toxicology., 25 23
–39
(2006). https://doi.org/10.1080/15569520500536600 Google Scholar
N. Bendsoe, A. Bjornberg, and
H. Asnes,
“Itching from wool fibers in atopic-dermatitis,”
Contact Dermatitis., 17 21
–22
(1987). https://doi.org/10.1111/j.1600-0536.1987.tb02638.x Google Scholar
S. Haug, A. Roll, P. Schmid-Grendelmeier, P. Johansen, B. Wuthrich, T. M. Kundig, and
G. Senti,
“Coated textiles in the treatment of atopic dermatitis,”
Curr. Probl Dermatol., 33 144
–151
(2006). https://doi.org/10.1159/000093941 Google Scholar
J. W. Fluhr, M. Breternitz, D. Kowatzki, A. Bauer, J. Bossert, P. Elsner, and
U. C. Hipler,
“Silver-loaded seaweed-based cellulosic fiber improves epidermal skin physiology in atopic dermatitis: safety assessment, mode of action and controlled, randomized single-blinded exploratory in vivo study,”
Exp. Dermatol., 19 E9
–E15
(2010). https://doi.org/10.1111/j.1600-0625.2009.00943.x Google Scholar
C. Dormor,
“Skin:textile:film,”
Textile-the Journal of Cloth & Culture., 6 238
–253
(2008). https://doi.org/10.2752/175183508X377591 Google Scholar
B. Gotter, W. Faubel, and
R. H. Neubert,
“Optical methods for measurements of skin penetration,”
Skin Pharmacol. Physiol., 21 156
–165
(2008). https://doi.org/10.1159/000131081 Google Scholar
J. Lademann, A. Knuttel, H. Richter, N. Otberg, R. V. Pelchrzim, H. Audring, H. Meffert, W. Sterry, and
K. Hoffmann,
“Application of optical coherent tomography for skin diagnostics,”
Laser Phys., 15 288
–294
(2005). Google Scholar
J. Enfield, M. L. O’Connell, K. Lawlor, E. Jonathan, C. O’Mahony, and
M. Leahy,
“In-vivo dynamic characterization of microneedle skin penetration using optical coherence tomography,”
J. Biomed. Opt., 15 046001
(2010). https://doi.org/10.1117/1.3463002 Google Scholar
U. Jacobi, M. Chen, G. Frankowski, R. Sinkgraven, M. Hund, B. Rzany, W. Sterry, and
J. Lademann,
“In vivo determination of skin surface topography using an optical 3D device,”
Skin Res. Technol., 10 207
–214
(2004). https://doi.org/10.1111/j.1600-0846.2004.00075.x Google Scholar
C. Antoniou, J. Lademann, H. Richter, S. Astner, A. Patzelt, L. Zastrow, W. Sterry, and
S. Koch,
“Analysis of the melanin distribution in different ethnic groups by in vivo laser scanning microscopy,”
Laser Phys. Lett., 6 393
–398
(2009). https://doi.org/10.1002/lapl.200910013 Google Scholar
J. Lademann, H. Richter, S. Astner, A. Patzelt, F. Knorr, W. Sterry, and
C. Antoniou,
“Determination of the thickness and structure of the skin barrier by in vivo laser scanning microscopy,”
Laser Phys. Lett., 5 311
–315
(2008). https://doi.org/10.1002/lapl.200710122 Google Scholar
J. Lademann, N. Otberg, H. Richter, L. Meyer, H. Audring, A. Teichmann, S. Thomas, A. Knuttel, and
W. Sterry,
“Application of optical non-invasive methods in skin physiology: a comparison of laser scanning microscopy and optical coherent tomography with histological analysis,”
Skin Res. Technol., 13 119
–132
(2007). https://doi.org/10.1111/j.1600-0846.2007.00208.x Google Scholar
B. Lange-Asschenfeldt, A. Alborova, D. Kruger-Corcoran, A. Patzelt, H. Richter, W. Sterry, A. Kramer, E. Stockfleth, and
J. Lademann,
“Effects of a topically applied wound ointment on epidermal wound healing studied by in vivo fluorescence laser scanning microscopy analysis,”
J. Biomed. Opt., 14 054001
(2009). https://doi.org/10.1117/1.3213603 Google Scholar
C. Roques, L. Teot, N. Frasson, and
S. Meaume,
“PRIMOS: an optical system that produces three-dimensional measurements of skin surfaces,”
J. Wound Care, 12 362
–364
(2003). Google Scholar
S. Jung, N. Otberg, G. Thiede, H. Richter, W. Sterry, S. Panzner, and
J. Lademann,
“Innovative liposomes as a transfollicular drug delivery system: penetration into porcine hair follicles,”
J. Invest. Dermatol., 126 1728
–1732
(2006). https://doi.org/10.1038/sj.jid.5700323 Google Scholar
V. Brazzelli, E. Berardesca, C. Rona, and
G. Borroni,
“The influence of a non-occlusive bi-layer composite membrane on skin barrier properties. A non-invasive evaluation with a right-left intra-individual pre/post comparison study,”
Skin Pharmacol. Physiol., 21 50
–55
(2008). https://doi.org/10.1159/000112519 Google Scholar
G. K. Menon and
A. M. Kligman,
“Barrier functions of human skin: a holistic View,”
Skin Pharmacol. Physiol., 22 178
–189
(2009). https://doi.org/10.1159/000231523 Google Scholar
B. N. Garcia, A. Mleczko, T. Schink, H. Proquitte, R. R. Wauer, and
U. Blume-Peytavi,
“Influence of bathing or washing on skin barrier function in newborns during the first four weeks of life,”
Skin Pharmacol. Physiol., 22 248
–257
(2009). https://doi.org/10.1159/000235552 Google Scholar
|

