|
|
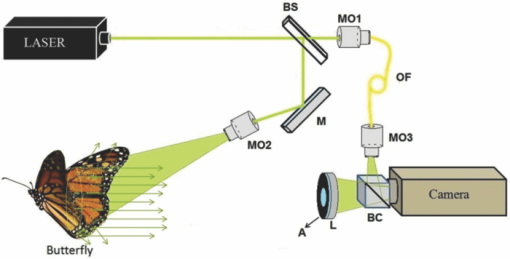
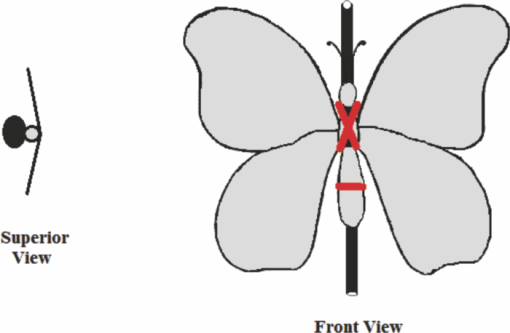
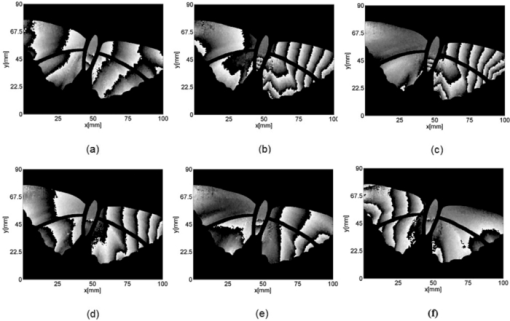
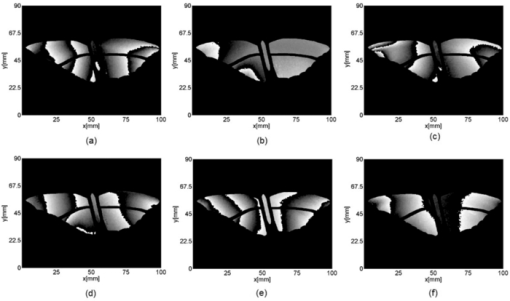
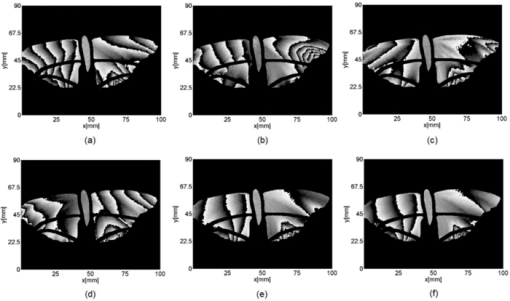
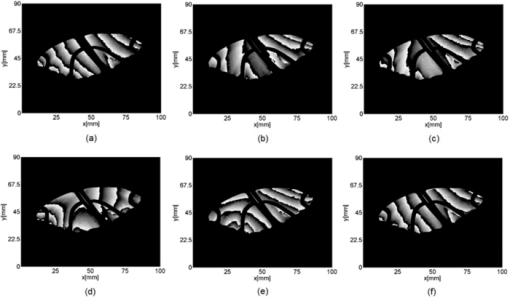
1.IntroductionBirds and insects have been taken as reference models to reproduce the movements performed by their wings during flight, giving way to the design of many experimental, simulated, and computing methods aimed at obtaining much better and more accurate results.1, 2 Knowing that one of the main factors of insects to survive on earth is their flying capability;3 winged insects are considered a perfect mechanical machine with different functions and mechanisms.4 The insect at an adult age presents wings with a flat appearance and well structured vein system. Butterflies and moths have a pair of forewings and hindwings that sometimes may move as one, with a type of sensor that controls their wing movement.5 Insect wings structure is semitransparent, and sometimes it can be colored by epidermis pigments. The Lepidoptera family presents scales with a form similar to human hair, giving the wing a soft appearance to the touch. Scales on wings are overlapped one over the other creating grooves and seem to act as a whole unit. Scales have a concavity where the pigments that provide color to wings are found. The wing color sometimes depends on the scales direction. The latter plays an important role as it smoothes the air flux over the body and wing surface.5 Wings present a resistance to water due to a spindlelike microform on the scales.5, 6 It is also worth mentioning that wings have a very important function as a body temperature regulator according to their position (see Ref. 3). The wings are capable to displace an air amount that generates the required lift to support the insects’ body and allow it to move in several positions/directions. Furthermore, only 0.5% to 5% of the insects’ body weight is used to beat the force generated by the air surrounding its wings. This also helps to compensate the acceleration and deceleration caused by its own mass doing this action several times per second.7 The wings have a passive response due to the fact that their structure has no complete muscularly control: it is supported by a vein system that creates the wing structure. They suffer large amplitude deformations particularly when flapping is slow.8 The structure varies from male to female, with that of the female body having a more resistant central structure prepared to egg carrying (see Ref. 7). It is the purpose of this research to present the latest results that will no doubt help to better understand these wings’ mechanical characteristics, for instance, knowing the speed and the magnitude of a wing's deformation it is possible to determine some mechanical properties of the wing's tissue, such as its stiffness and elasticity. It is worth pointing out here that there is a vast number of winged insect species (particularly butterflies and moths) in the Mexican environment to provide enough subjects to perform an insect wing study without affecting or endangering the species used.9, 10, 11, 12 The four chosen species were selected because they are abundantly present in the central area of Mexico: a. Nymphalis antiopa (Lepidoptera: Nymphalidae) commonly known as Mourning Cloak, b. Agraulis vanillae Incarnata (Lepidoptera: Heliconiidae) known as Gulf fritillary, c. Danaus gilippus Cramer (Lepidoptera: Danaidae) also called Queen Buttefly, and d. Precis evarete Felder (Lepidoptera: Nymphalidae) known as Buckeye butterfly. Figure 1 shows an image of the butterflies and moth mentioned above. They can also be found in tropical rainforests and perennial forests through several Mexican regions. Fig. 1Four Mexican butterflies used for wing flapping comparison: (a) Western Viceroy, (b) Gulf fritillary, (c) Mourning cloak, and (d) Buckeye butterfly. The black bar indicates a scale of 10 mm. 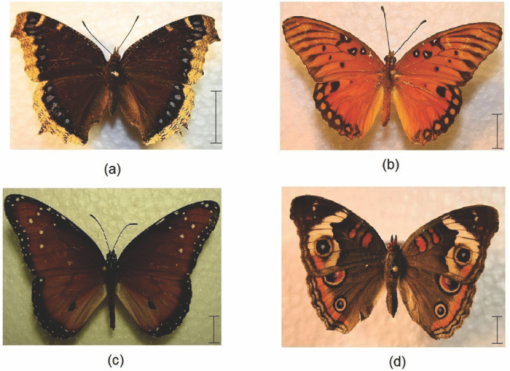 Noninvasive optical methods are used as an alternative to classic measurement techniques such as microrobotics models, computer simulation, pressure sensors, flow visualization, and photogrametry; 13, 14, 15, 16, 17, 18, 19, 20 allowing the gathering of new data about transient and nonrepeatable events.20, 21 These optical methods have been applied in a wide variety of samples such as metallic, polymeric, and biological tissues to obtain new information about particular parameters in them. From the available optical noninvasive methods digital holographic interferometry (DHI) is one that has a rather high accuracy measurement precision and is readily available in our laboratories.22, 23, 24, 25 Our first attempt to demonstrate the utility and performance of DHI to study fast and non-repeatable events, such as wings flapping, was performed in a small area of the wing surface of a butterfly and a mosquito.26 Once DHI proved successful, a study followed on a butterfly known as Eastern Swallowtail (Pterourus multicaudata) with data obtained on a micrometric scale.27 This research manuscript presents the flapping behavior over the wings surface of four different butterflies’ species having different characteristics such as size, wing form, scales, and flapping speed, a feature that affects the performance during flight. High speed DHI is used with a high power cw laser to record the raid deformation changes suffered on the wings. The results show uniqueness in the in flight wing deformations when the four insects are compared demonstrating that there is not a single flapping pattern for the wings, and that rather the combination of wing flapping patterns have to be given a serious consideration in aerodynamical design. 2.ModelCare was taken to perform a repeatable and controlled testing to avoid damaging the insects. It is well-known that the butterfly thorax is an important and fragile part that contains the equivalent to the circulatory system of a human body, also containing the structure that allows the insect to breath.28, 29 To have an almost free insect flapping, the insect cannot be pinned or glued in the thorax because this not only will kill it, but will modify its flapping movement, so it was necessary to look for another method to fix the insect and avoid damages to wing structures (this method is described in Sec. 3). DHI is used to perform the measurement in a noninvasive way, and to determine the object displacement a subtraction between two consecutive holograms is performed and to recover deformations presented in the wing surface with an outplaned sensitivity.30 DHI is based on an interferometric principle that involves the overlapping of a reference and an object beam (see Ref. 21). The resulting interference pattern is detected and recorded as intensity in the camera sensor (in this case a CMOS sensor). This intensity image is widely known as a digital hologram and contains the deformation recorded: the first one can be considered a base state, with no deformation, and the second one is a deformed state of the object. Comparison between these two digital holograms yields a wrapped phase map that contains the encoded information produced by changes undergone by the object under testing. Defining I(x, y) as the intensity that contains reference and object beam waves, expressed by I A(x, y) and I B(x, y) respectively, an equation for the intensity can be written as Eq. 1[TeX:] \documentclass[12pt]{minimal}\begin{document}\begin{eqnarray} I(x,y) &=& I_A (x,y) + I_B (x,y) + 2\left[ {I_A (x,y)I_B (x,y)} \right]^{{1 / 2}}\nonumber\\ && \times\,{\rm cos}\left({\phi + \psi } \right),\end{eqnarray}\end{document}3.Experimental Method3.1.Experimental SetupThe out-of-plane optical setup configured is schematically shown in Fig. 2. The light source used to illuminate the object under test is a high output power Verdi laser (Coherent V6) that is able to deliver a maximum power of 6 W at 532 nm. As is commonly done, the laser beam is divided at beam splitter into an object and reference beams with a relation 70:30, respectively. The object beam completely illuminates the insect wings surface using a 10× microscope objective (MO2). The backscattered light from the object passes through an aperture (A), and then is collected by a 125 mm focal length lens (L) located behind the aperture. The reference beam is launched into a single mode optical fiber using two 20× microscope objectives (M01 and M03), fixed in each extreme point of the fiber. The object and the reference beams are combined at the CMOS sensor using a 50:50 beam combiner. The field of view that allows observing the entire insect has an image area of 90×100 mm. The high speed camera (NAC GX-1) used has an image resolution of 800×800 pixels and 10 bits dynamic range. As mentioned before, the butterflies and moths chosen for the purpose of this research are abundant in the local ecosystem so they are captured in their usual habitat. 3.2.Insect Holding MethodIn vivo experimental measurements were performed by carefully fixing the butterflies and the moth onto a rigid surface, avoiding any wing damage and hence being able to perform the tests under the best experimental circumstances. The fixing method was suggested by an entomologist. The procedure followed was to first glue the insect legs to a metal post and with the help of a soft thread the main body was held by forming an “X” around it, as it is shown in Fig. 3. Care was taken to allow the insect to move the wings freely and at the same time holding it strongly enough to perform the test. Each test lasted only a few seconds and then each insect was released from its fixings and set free. This procedure was the best option found to avoid the use of entomologic needles to pin the insect, a situation that no doubt would have changed the wings natural movements. So this method assured the minimum restriction to insects’ natural movements and therefore it can be safely said that their wings were free to flap. 3.3.Experimental ResultsThe butterflies shown in Figs. 1a, 1b, 1c, 1d were placed in front of the camera and several images were recorded at different frames per second. One feature that is readily noticed is that each species has completely different wing displacement behavior, even between specimens from the same species. Several adjustments were done to the speed camera recording to find the ideal repetition rate to perform the experiments, and the best frame rate for all is 4000 fps. During all the recording processes the electronic shutter of the CMOS camera was significantly smaller than the exposure time of 250 μs, a feature that avoids data averaging. Wrapped phase maps obtained from experimental recordings for Nymphalis antiopa are shown in Fig. 4, while Figs. 5, 6, 7 show wrapped phase maps for Agraulis vanillae Incarnata, Danaus gilippus Cramer, Precis evarete Felder butterflies, respectively. All wrapped phase maps are for different and not controlled instants of the wing movement, and represent the insects’ wing deformations with variations from −π to π. All wrapped phase maps shown in Figs. 4, 5, 6, 7 represent particular states of each species during flapping at different moments. Comparison with other wrapped maps taken at random (the ones shown here were taken at random from a series of images), proved that there is not equal motion patterns from one to another insects’ wings. Images in Fig. 4 present an interval of 1.5 ms between them, while images in Fig. 5 show a time separation of 1.25 ms between each image; also Figs. 6, 7 have a time separation between images of 1.75 and 1.25 ms, respectively. Tthis shows how fast the insect wing can change during the flight movement. Figures 8a, 8b, 8c, 8d show the unwrapped phase maps from the wrapped phase maps in Figs. 4a, 5b, 6c, 7d and they depict the out-of-plane displacement of the insects’ wings, and all units are in micrometers. The media file corresponds to wings’ surface displacement images spaced in time intervals of 250 μs. Fig. 8(a)–(d) represent butterfly wings’ surface deformation from DHI measuring experiments, showing different moments of the flapping for the butterflies shown in Fig. 1. Video 1: Mesh grid view of the superficial deformation observed in the Agraulis vanillae Incarnata shown in (b). (QuickTime, 50 KB) . Video 2: Comparison of wrapped phase maps for insects shown in Fig. 1. (QuickTime, 152 KB) 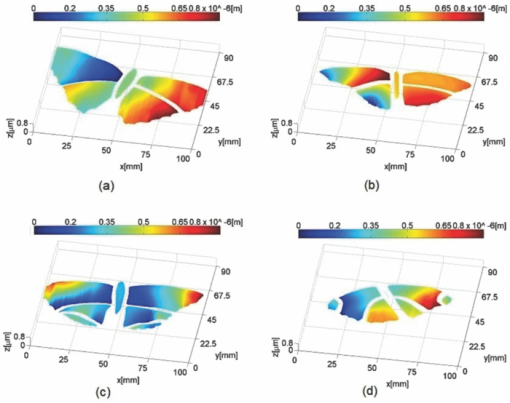 Figures 9, 10 present the results of tracking changes for each wing section among all the butterflies in a 50 frames series with a time interval of 250 μs between consecutive frames, for two different set of tests. Figure 9a represents displacement differences among the butterflies’ left forewing, while Fig. 9b corresponds to the right forewing variations. Changes measured on left and right hindwings are plotted on Figs. 9c, 9d, respectively. Also Figs. 10a, 10b, 10c, 10d plotted the tracking changes with the same insect’ wing distribution as Fig. 9. For both figures, curves are labeled representing wing changes for Mourning Cloak, Gulf fritillary, Queen Butterfly, and Buckeye respectively. Fig. 9(a)–(d) Butterfly wing displacement comparison between each wing section for each corresponding to insect shown in Fig. 1 (first data set). 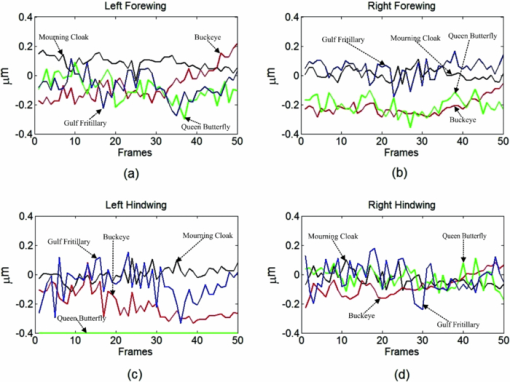 Fig. 10(a)–(d) Butterfly wing displacement comparison between each wing section for each corresponding to insect shown in (second data set). 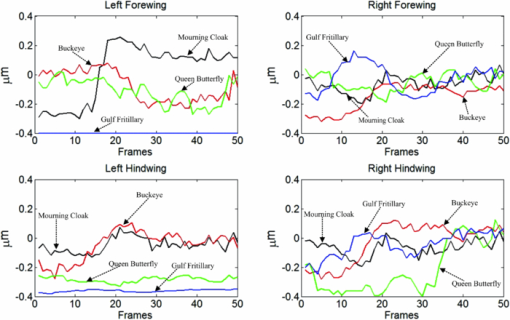 It can be seen that for each specimen, each wing has an independent movement as Fig. 9c shows any movement in this wing section for the Queen Butterfly instead of its three other sections, while the Gulf Fritillary left forewing does not present any type of movement as is shown in Fig. 10. These figures also show how fast a wing can compensate different deformations suffered in a relatively short period of time. Changes on butterflies wings’ surface can be interpreted as smooth if we take a time lapse of seconds, but when it is recorded in short time lapses, microseconds as in these experiments, it can be seen that wings’ structure adapt to fast changes and compensate these movements to keep a relative steady flight. 4.Conclusions and DiscussionWe believe that the results presented here using a noninvasive optical method to study winged insect species with different characteristics and wing shapes represent a step ahead in insect flight behavior understanding, having done the experiments relatively free to move wings. The results show a different response for each of the tested species under in-flight wing movement, a feature that can be seen from Figs. 4, 5, 6, 7. The unwrapped phase maps obtained for each specimen show neither symmetry nor similar movements, but show a rapid change from one instant to another. The data obtained show that each wing part has a particular contribution during flight, and from the experimental results it can be concluded that each wing does not have the same change. It was also noticed that wings can stay apparently in a static state while others keep on moving, a feature observed at least during the 50 images sampled (12.5-ms tracking lapse), see Figs. 9, 10. Studying four different Mexican butterflies with different sizes and characteristics can give an idea of how fast an insect is able to change its position and compensate pressure changes during flight in time intervals as short as several microseconds: this will never be detected with the naked eye. High speed digital holographic interferometry is providing an option to measure non repeatable events and whole field measurements with high precision, describing deformation on biological tissues with a micrometer resolution and accuracy. AcknowledgmentsThe all authors wish to thank Consejo Nacional de Ciencia y Tecnología (CONACyT, MEXICO) for partially supporting this research through Grant No. 42971, and for the PhD scholarship given to D. A. Aguayo and C. Caloca-Mendez. ReferencesM. H. Dickinson, F. O. Lehmann, and
S. P. Sane,
“Wing rotation and the aerodynamic basis of insect flight,”
Science, 284 1954
–1960
(1999). https://doi.org/10.1126/science.284.5422.1954 Google Scholar
M. Dickinson,
“Solving the mystery of insect flight,”
Scientific American, 34
–41
(2001). Google Scholar
J. G. Kingsolverg and
M. A. R. Koehl,
“Selective factors in the evolution of insects kings,”
Ann. Rev. Entomol., 30 425
–451
(1994). https://doi.org/10.1146/annurev.en.39.010194.002233 Google Scholar
S. Sudo and
K. Tsuyuki,
“Wing morphology of some insects,”
JSME International Journal Series C, 43
(4), 895
–900
(2000). Google Scholar
R. F. Chapman, The Insects. Structure and Function, Hodder and Stoughton, London
(1982). Google Scholar
Y. Fang, S. Gang, Q. Cong, G. Chen, and
L. Ren,
“Effects of methanol on wettability of the nonsmooth surface on butterfly wing,”
J. Bionic Eng., 5
(2), 127
–133
(2008). https://doi.org/10.1016/S1672-6529(08)60016-5 Google Scholar
S. A. Combes and
T. L. Daniel,
“Flexural stiffness in insect wings II. Spatial distribution and dynamics wing bending,”
J. Exp. Biol., 206 2989
–2997
(2003). https://doi.org/10.1242/jeb.00524 Google Scholar
R. J. Wootton,
“Functional morphology of insect wings,”
Ann. Rev. Entomology, 37 113
–140
(1992). https://doi.org/10.1146/annurev.en.37.010192.000553 Google Scholar
J. E. De la Maza,
“Mariposas Mexicanas: Guía para su colecta y determinación,”
(1987) Google Scholar
C. R. Beultelspacher, Mariposas diurnas del Valle de México, Ediciones Científicas L.P.M.M.(1980). Google Scholar
D. J. Borror, M. De Long, A. Triplehorn, and
J Norman, Introduction to the Study of Insects, Thomson Brooks, Belmont, CA
(2005). Google Scholar
H. L. Lewis, Butterflies of the World, Harrison House, New York
(1987). Google Scholar
J. Yan, R. J. Wood, S. Avadhanula, M. Sitti, and
R. S. Fearing,
“Towards flapping wing control for a micromechanical flying insect,”
3901
–3908
(2001). Google Scholar
S. Avadhanula, R. J. Wood, E. Steltz, J. Yan, and
R. S. Fearing,
“Lift force improvements for the micromechanical flying insect,”
1350
–1356
(2003). Google Scholar
C. P. Ellington,
“The novel aerodynamics of insect flight: applications to micro-air vehicles,”
J. Exp. Biol., 202 3439
–3448
(1999). Google Scholar
J. R. Usherwood and
C. P. Ellington,
“I. Model hawkmoth wings,”
J. Exp. Biol., 205 1547
–1564
(2002). Google Scholar
T. L. Hedrick, J. R. Usherwood, and
A. A. Biewener,
“Wing inertia and whole-body acceleration: an analysis of instantaneous aerodynamic force production in cockatiels (Nymphicus hollandicus) flying across a range of speeds,”
J. Exp. Biol., 207 1689
–1702
(2004). https://doi.org/10.1242/jeb.00933 Google Scholar
R. B. Srygley and
A. L. R. Thomas,
“Unconventional lift-generating mechanisms in free-flying butterflies,”
Nature (London), 420 660
–664
(2002). https://doi.org/10.1038/nature01223 Google Scholar
A. L. R. Thomas, G. K. Taylor, R. B. Srygley, R. L. Nudds, and
R. J. Bomphrey,
“Dragonfly flight: free-flight tethered flow visualizations reveal a diverse array of unsteady lift-generating mechanisms, controlled primarily via angle of attack,”
J. Exp. Biol., 207 4299
–4323
(2004). https://doi.org/10.1242/jeb.01262 Google Scholar
C. Jones and
A. J. Moore,
“High Speed photogrametry for measuring the kinematics of insect wings,”
Appl. Opt., 45 4165
–4173
(2006). https://doi.org/10.1364/AO.45.004165 Google Scholar
C. Pérez-López, M. H. De la Torre-Ibarra, and
F. M. Santoyo,
“Very high speed cw digital holographic interferometry,”
Opt. Exp., 14 9709
–9715
(2006). https://doi.org/10.1364/OE.14.009709 Google Scholar
R. Jones and
C. Wykes, Holographic and Speckle Interferometry, Cambridge University Press, UK
(1989). Google Scholar
R. K. Erf, Holographic Nondestructive Testing, Academic Press, New York/London
(1974). Google Scholar
C. M. Vest, Holographic Interferometry, John Wiley & Sons, New York
(1979). Google Scholar
P. K. Rastogi,
“Digital Speckle Pattern Interferometry and Related Techniques,”
(2001) Google Scholar
D. D. Aguayo, F. M. Santoyo, M. H. De la Torre, and
C. Caloca-Mendez,
“Insect wing displacement measurement using digital holography,”
(2007). Google Scholar
D. D. Aguayo, F M. Santoyo, M. H. De la Torre, and
M. D. Salas-Araiza, C. Caloca-Mendez, and
D. Asael Gutierrez Hernandez,
“Insect wing deformation measurements using high speed digital holographic interferometry,”
Opt. Exp., 18 5661
–5667
(2010). https://doi.org/10.1364/OE.18.005661 Google Scholar
D. L. Grodnitsky, Form and Function of Insect Wings, John Hopkins University, London
(1999). Google Scholar
R. Dudley, The Biomedical of Insect Flight, Princeton University Press, Princeton, NJ
(2000). Google Scholar
N. K. Mohan, A. Andersson, M. Sjödahl, and
N.-E. Molin,
“Optical configuration for TV holography measurement of in-plane and out-of-plane deformations,”
Appl. Opt., 39 573
–577
(2000). https://doi.org/10.1364/AO.39.000573 Google Scholar
M. Takeda, H. Ina, and
S. Kobayashi,
“Fourier-transform method of fringe-pattern analysis for computer-based topography and interferometry,”
J. Opt. Soc. Am., 72 156
–160
(1982). https://doi.org/10.1364/JOSA.72.000156 Google Scholar
|