|
|
1.IntroductionAccording to the World Oral Health Report published by the World Health Organization, periodontal disease is one of the major dental diseases that affects human populations worldwide at high prevalence rates.1 A correct diagnosis on the etiology of periodontal disease is a chief responsibility of the clinician. Dental calculus is defined as mineralized bacterial plaque and contains irritating substances such as endotoxins and bacterial antibodies.2, 3 Although it is not the initial cause of destructive periodontal disease, removal of it is essential to maintain periodontal health. Complete removal of subgingival calculus for successful periodontal therapy is based on the successful detection of calculus under gingiva.3 In general, subgingival calculus detection is more difficult than supragingival calculus because it is firmly attached to root surfaces within a periodontal pocket. Moreover, clinical detection of residual calculus following subgingival scaling and root planning is questioned.4, 5 Tugnait 5 showed that traditional assessment methods using an explorer, a probe, or radiography technique are usually not accurate. Residual calculus is difficult to be distinguished by manual probing when hidden on subgingival-covered root surfaces. It was known that subgingival calculus is more prevalent on lingual and interproximal surfaces than on buccal tooth surfaces.6 To evaluate extracted teeth after scaling, 77% of the root surfaces which are clinically evaluated as those free from subgingival deposits, have microscopically revealed considerable amounts of residual calculus.4 Recently, laser fluorescence was considered a potential diagnostic tool.7 The laser optical procedure can be an objective measuring system for the assessment of subgingival calculus on the root surfaces. Besides, the intimate contact of subgingival calculus with periodontal structures brings a possible risk of collateral damage when a dental probe is used. Detection of residual calculus after deep scaling within the periodontal pocket remains complicated. The residual calculus is also hard to be identified by probing or dental radiograph. Furthermore, the ionizing radiation exposure of dental radiograph is not advocated for regular monitoring either. When irregularities of the subgingival structure and that of the root surface characteristics are considered, current diagnostic ways suffer from limited efficiency in clinical situations. Due to a different mechanism, laser radiation is less prone to those factors mentioned above. Laser fluorescence detection is a noncontact and minimally invasive technique on calculus examination. The fluorescence emission of human dental calculus was strongest for excitation wavelengths from 400 to 420 nm (Ref. 8) when one-photon excitation is considered. Detection of dental calculus by ultraviolet excitation has the reliability of distinguishing calculus from a healthy and unaffected tooth surface.9 Although autofluorescence detection has recently become an alternative method to detect subgingival calculus, the precision and accuracy are still questionable.10 The examination usually suffers from shallow penetration depth, especially when it is used in the detection of subgingival calculus. Since a diseased periodontal root surface is covered by gingiva, an excitation light in a near-infrared (NIR) range is desired to increase the penetration depth through soft tissue due to less light scattering and less water absorption. Based on literature reports, fluorescence intensity is masked when the calculus surface is covered by soft tissue and blood clots. It was suggested that the accuracy of the laser-based calculus detection can be impaired by gingiva.11, 12 The feasibility of using a multiple-photon fluorescence technique on the detection of subgingival calculus is then an interesting topic. The major impact of nonlinear optics in microscopy began with the invention of two-photon microscopy by Denk in 1990 (Ref. 13). Different from a one-photon excited fluorescence technique, excitation of two-photon fluorescence is a nonlinear process: one electron is excited by two photons simultaneously in a two-photon fluorescence process instead of being excited by one single photon in a one-photon fluorescence process. Based on this physics mechanism, excitation light beam with the wavelength double of the one for one-photon excitation can then be used. But, the wavelength does not have to be exactly doubling. In order to receive two photons simultaneously, only can the volume illuminated by very high photon density be excited and emit fluorescence. When damage threshold of the optical power received by biological tissues or cells is considered, such technology usually favors the usage of an objective with a high focusing power or a high numerical aperture. In general, the multiphoton fluorescence imaging technology has demonstrated its advantages of better penetration depth, excellent optical sectioning, and good separation between the excitation and emission wavelengths in several biological applications. Our previous pilot study has shown the feasibility of subgingival calculus detection using a two-photon fluorescence imaging technique when a 20× objective lens (NA = 0.4) was used.14 However, the working distance of such an objective is only 1.30 mm. In this study, a 10× objective (NA = 0.25) with longer working distance (WD = 10.6 mm), but less focusing power was used. The smaller focusing power requires almost four times the laser power to obtain the same fluorescence intensity since the two-photon fluorescence response is proportional to the square of the peak power of the laser pulses. The detection of subgingival calculus by the multiphoton fluorescence imaging technique was further characterized here. The longer working distance that allows better physical penetration depth is important for the feasibility of the two-photon fluorescence microscopy technique on the detection of subgingival calculus in the clinic. The hypothesis of the multiple-photon excitation is a better candidate for autofluorescence imaging of gingiva covered calculus than the one-photon fluorescence confocal imaging technology that was experimentally verified in this study. The aim of the experiment was to assess the feasibility of detecting gingiva-covered subgingival calculus with the proposed multiple-photon fluorescence technique by a femtosecond pulsed Ti-sapphire laser in vitro. 2.Material and MethodsThe study protocol has applied IRB from the ethics committee (the Review Committee for Human Subjects) of the National Yang Ming University. Patients were informed about the nature of the study, and a signed consent form was obtained from each individual. Subgingival calculus was prepared from extracted teeth and rinsed under running tap water. To maintain the environment of a periodontal condition, the teeth were stored in a minimal amount of neutral buffered saline. This storage medium, at neutral pH value, was selected to avoid the dissolution of the inorganic constituents of the calculus. No mounting or fixation was used in the experiment. Around 20 extracted teeth partially covered with calculus on the root surfaces were used in the study. The gingival tissue was obtained from patients undergoing crown-lengthening procedures and from patients undergoing the extraction of impacted third molars under aseptic condition. The tested tissue was from 15 different patients. The tissue was placed in a Dulbecco's modified Eagle medium supplemented with antibiotics and taken to the laboratory. The experimental setup is shown in Fig. 1, A train of 200 femtosecond pulses generated by a Ti-sapphire laser at a wavelength of 790 nm with an average power of 10 to 20 mW was used to study the excitation of two-photon autofluorescence from gingiva and from subgingival calculus. The laser beam was directed to the tooth samples through a pair of scanning mirrors, a dichroic mirror, and a 10× objective with numerical aperture NA = 0.25. The pair of scanning mirrors was used to direct the laser beam for the point scanning on the tested samples. The excited fluorescence was separated from the excitation laser beam by the dichroic mirror (675DCSP, Chroma). An additional notch filter (E650SP, Chroma) with transmission ranged within 402 to 650 nm, was used to further filter out the excitation beam and the second harmonic signal, if there is any. A photomultiplier tube (PMT) was used to detect the fluorescence through a photon counting mode or an analog mode, which depends on the amount of photons. A time-correlated mechanism is incorporated in this two-photon fluorescence microscopy system to reduce optical noise in the environment. Fig. 1Configuration of experimental setup. (1) Ti-Sapphire laser; (2) pair of scanning mirrors; (3) 10× objective; (4) dichroic mirror; (5) mirror; (6) iris; (7) notch filter: 402-650 nm; (8) focus lens; (9) photomultiplier tube (PMT). 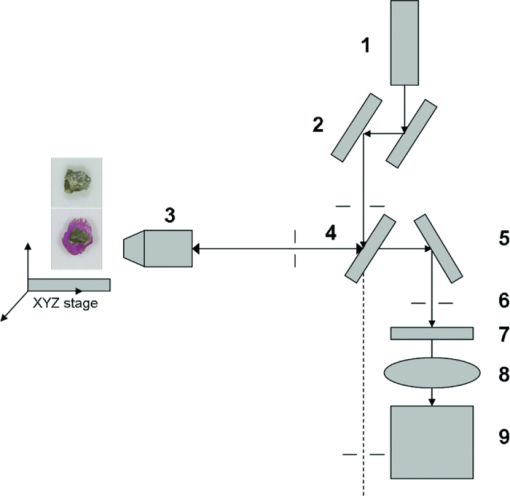 In this study, one-photon fluorescence images were obtained by a one-photon fluorescence confocal microscope (LSM 5 PASCAL, Zeiss). An argon laser with wavelength of 488 nm was used to excite autofluorescence of subgingival calculus based on one-photon fluorescence excitation. A filter LP505 was used to filter out the excitation laser beam. A fluorescence signal of subgingival calculus with and without gingiva covered from both fluorescence excitation modalities was processed by computer software, ImageJ 1.37c (National Institutes of Health), to carry out the analysis. The dental radiography pictures were obtained through a standard x-ray radiography instrument in a hospital. It is basically a 2D projection of a tooth. Calculus can only be shown on mesial and distal proximal sides of the tooth through a radiography instrument. The degree of calcification can be qualitatively characterized by the image contrast. 3.Experimental Result and DiscussionThe presence of subgingival calculus was a well known factor most strongly associated with periodontal diseases. A typical subgingival calculus covered on the root surfaces was shown in Fig. 2. In conventional methods, subgingival calculus is usually detected through dental probing and x-ray radiography. While a dental probe can bring a potential risk of collateral damage through close contact to subgingival calculus on teeth and periodontal structures, the x-ray radiograph usually has difficulty in precisely measuring the subgingival calculus. This was demonstrated in Figs. 2b and 2d that the x-rays image can only reveal the subgingival calculus appeared on the lateral sides of the tooth. The buccal- and lingual-side subgingival calculus attached on the molar tooth surface, shown in Figs. 2a and 2c, respectively, are hardly found by radiography. Fig. 2Photograph and x-ray radiograph of a tooth with subgingival calculus. The buccal- and lingual-side subgingival calculus was indicated by the arrows. The x-ray radiograph of one tested tooth revealed the disadvantage of radiography on the detection of subgingival calculus. 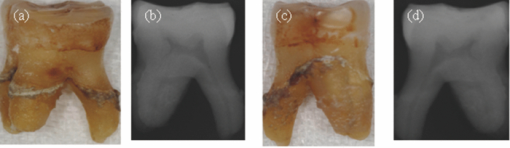 As was mentioned previously, fluorescence techniques can be classified as one-photon fluorescence techniques and two-photon fluorescence techniques. In the mechanism of one-photon fluorescence excitation, an electron absorbs one photon and jumps to a higher electric state. Fluorescence is emitted when the electron falls back to a lower electronic state. Therefore, a fluorescence image of a sample can be considered as the projection of the fluorescence from all the parts illuminated by the excitation light. In the two-photon fluorescence mechanism, however, an electron absorbs two photons simultaneously to obtain enough energy and jump to the higher electronic state. Therefore, high density of photons is required. This requirement provides excellent optical sectioning capability on a sample. While an electron absorbs two photons instead of one to emit fluorescence, from the energy point of view, the wavelength of the excitation light for two-photon fluorescence excitation is two times that for a corresponding one-photon fluorescence scheme. Of course, since it is not a parametric process, the excitation wavelength does not have to be exactly two times. This characteristic implies that NIR light is usually the candidate for two-photon fluorescence imaging. The longer wavelength implies less scattering and employment of NIR light indicates less absorption. Both effects result in a better penetration depth. Therefore, to study the feasibility of using the two-photon fluorescence mechanism for subgingival calculus detection, fluorescence response of healthy teeth, subgingival calculus, and gingiva, as well as the penetration depth of fluorescence imaging and capability of optical sectioning, were characterized in comparison with the one-photon fluorescence confocal scheme. The fluorescence response of healthy teeth has been studied previously using 20× objective and the result showed that a healthy tooth has no significant fluorescence response to two-photon excitation but has a significant response to one-photon excitation.14 This characteristic provides the foundation of background-free fluorescence imaging when two-photon absorption mechanism is employed. The advantage of this background-free imaging and capability of the optical sectioning can be further revealed in the fluorescence imaging of subgingival calculus shown in Fig. 3. The size of the subgingival calculus over the tooth was about 8 mm×4 mm. The one-photon fluorescence image of this sample is shown in Fig. 3a. The fluorescence image was optically sectioned through the confocal mechanism built in the confocal microscopy. When there is no confocal mechanism, the one-photon fluorescence image will be the projection of all the illuminated parts as a mixture of calculus, gingival cervicular fluid, and blood. The two-photon fluorescence image of the same sample is shown in Fig. 3b. As a comparison between these fluorescence images, two-photon fluorescence scheme also imaged a thin slice of the subgingival calculus. This is due to the automatic optical sectioning of the two-photon fluorescence mechanism. It does not need an extra spatial filter as in a confocal microscope to perform optical sectioning. When the fluorescence response of healthy teeth is concerned, it showed that the healthy teeth have a significant amount of fluorescence under one-photon excitation. The one- and two-photon excited fluorescence response of a representative healthy tooth is shown in Figs. 3c and 3d, respectively. The one-photon fluorescence image was shown on the photo of the tested sample to indicate the location of the fluorescence. Since the one-photon fluorescence was taken by a confocal microscope, only the part within the optically sectioned slide showed the fluorescence. Different from this, the healthy tooth has almost no response to the two-photon excitation. As is shown in Fig. 3d, only a few photons were detected in the two-photon fluorescence scheme. The computer obtained an almost black image. This characteristic of the background-free imaging revealed the advantage of the two-photon fluorescence imaging over the one-photon fluorescence imaging even when the calculus was not covered by gingiva: In the two-photon fluorescence imaging technique, only the calculus part has fluorescence; a better contrast was obtained. It is important to emphasize here that when an optical fiber bundle is used to direct the laser beam to patients, as in a clinical situation, it is difficult to employ a spatial filter as used in confocal microscopy. Fig. 3(a) Autofluorescence image of subgingival calculus obtained by one-photon excitation with the aid of a confocal mechanism. (b) Autofluorescence images of subgingival calculus obtained by time correlated two-photon excitation and fluorescence emission. (c) Autofluorescence image of tooth surface obtained by one-photon excitation The green band is the fluorescence. The gray part is a regular photo of the tested sample to show the location of the fluorescence. (d) Autofluorescence image of the tooth surface obtained by the two-photon excitation. The scale bar in is 100 μm. The sample has not been covered by gingiva yet. 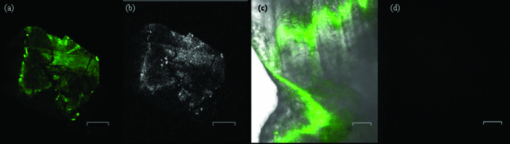 When detection of subgingival calculus is considered, fluorescence response of gingiva should be considered since subgingival calculus is hindered behind gingiva in a clinical situation. A significant amount of autofluorescence from gingiva can block or deteriorate that of calculus under it. The fluorescence response of gingiva subject to one- and two-photon excitation is shown in Fig. 4. The autofluorescence of a gingival tissue from one-photon excitation is shown in Fig. 4a. It is important to notice that the intensity of the fluorescence decreased from the rim of the optically sectioned gingiva toward the center of the gingiva within the excitation area. This is due to the optical sectioning of the confocal mechanism. When the optical sectioning mechanism cut the gingival, the center part is too thick for the laser beam to excite the fluorescence. Therefore, only the rim of the optically sectioned gingiva showed the fluorescence. Because of this reason, the illumination spot was chosen at the edge of the tested gingiva, located at the upper-right corner, to demonstrate the one-photon fluorescence response of the gingiva. This fact first revealed that the one-photon excitation can generate a very significant amount of fluorescence out of the gingiva. Second, the laser beam for the excitation of one-photon fluorescence has a very shallow penetration depth. This penetration issue will be further demonstrated in the next paragraphs. In comparison, the fluorescence image of the same gingiva from the two-photon excitation revealed almost no photon emission. The maximum number of the fluorescence photons counted by the PMT in the setup was less than 10 when the laser power was set at 13 mW. The little photon numbers cannot be observed from Fig. 4b. Therefore, an image of complete darkness was shown in Fig. 4b. This characteristic also showed its advantage of the two-photon fluorescence scheme over the one-photon scheme in the detection of subgingival calculus. Fig. 4(a) Autofluorescence image of gingiva obtained by one-photon excitation. (b) Autofluorescence images of gingiva obtained by time correlated two-photon fluorescence emission. The scale bar in (b) is 100 μm. 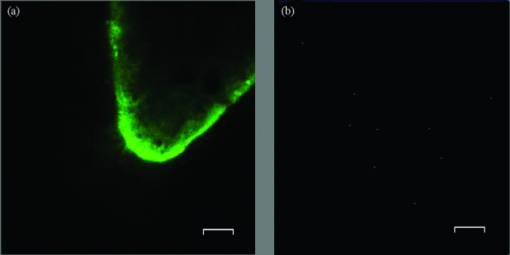 To verify if the detection of the subgingival calculus can benefit from the better penetration of the longer-wavelength excitation and the selected NIR radiation from the two-photon absorption mechanism, the subgingival calculus was covered by a slice of gingival tissue for the test. The gingival tissue is about 1 mm in thickness. It is important to examine this characteristic since subgingival calculus is hindered behind gingiva in a clinical situation. It is also the main purpose of this research. The experimental result is shown in Fig. 5. A sample under this test is shown in Fig. 5a. The one-photon fluorescence image of this sample is shown in Fig. 5b and the two-photon fluorescence image of the sample is shown in Fig. 5c. It is important to realize that the image scale in Fig. 5a is very different from the fluorescence images shown in Figs. 5b and 5c. The image scale in Figs. 5b and 5c is in hundreds of micrometers, but the scale in Fig. 5a is in centimeters. Therefore, the fluorescence images shown in Figs. 5b and 5c depicted only a very small portion of the subgingival calculus shown in Fig. 5a. The sample covered by a piece of gingiva is also shown in Fig. 5a. The corresponding one-photon and two-photon fluorescence images are shown in Figs. 5b and 5c, respectively. It revealed that the one-photon fluorescence image of the subgingival calculus was almost blocked by the gingival tissue. However, it can be observed that the two-photon fluorescence image of the subgingival calculus was not masked by the gingiva. This is due to relatively low light scattering in the gingiva and almost no fluorescence from the gingiva as the overall result of utilizing the longer wavelength, the optical sectioning of multiphoton mechanism, and the very small two-photon cross section of gingiva. Therefore, a good two-photon fluorescence image of calculus covered by subgingival was obtained. Fig. 5(a) Photograph of a subgingival calculus covered by gingiva. (b) One-photon autofluorescence confocal image of subgingival calculus covered by gingiva. (c) Two-photon autofluorescence image obtained by time correlated two-photon microscopy technique. The scale bar, 100 μm, in (c) indicates the scale of the two-photon image.  A series of images with different sectioning planes were obtained in gingiva covered subgingival calculus by time correlated two-photon microscopy technique. The images were taken using 790 nm excitation and the 10× objective. The thickness of the gingiva is about 1 mm. The fluorescence images, starting from the middle of the gingiva to different penetration depths, are shown in Fig. 6. As expected, no fluorescence of gingival section was shown at the reference plane, indicated by 0 μm, in Fig. 6a. In the penetration depth of 500 to 800 μm, shown from Figs. 6b, 6c, 6d, 6e, 6f, the two-photon excited autofluorescence images in different depth were obtained from subgingival calculus covered by tested gingiva. The scale bar, 100 μm, indicates the scale of the two-photon images. The result showed the penetration capability of the two-photon fluorescence method through the gingiva and the optical sectioning depth into the subgingival calculus. This result showed that the laser beam to excite two-photon fluorescence can penetrate the gingival with a thickness of 1 mm and further penetrate the calculus down to 800 μm. Fig. 6Two-photon autofluorescence images obtained by the time correlated two-photon microscopy technique. (a) Fluorescence image of subgingival calculus covered by gingiva at the reference depth, indicated as 0 μm. (b)–(f) The autofluorescence images of subgingival calculus covered by gingiva at 500, 550, 600, 650, and 800 μm, respectively. The scale bar, 100 μm, indicates the scale of the two-photon images. 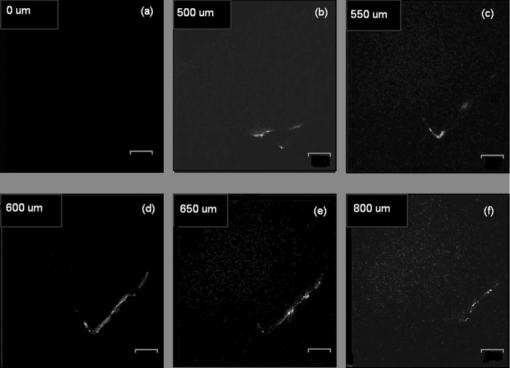 To statistically compare the laser-fluorescence intensity of the tested samples from the one-photon excitation and that from the two-photon laser excitation, sites of 25 from each sample were measured. This measurement included the healthy teeth, gingiva, uncovered subgingival calculus, and subgingival covered calculus. The fluorescence images from both modalities were analyzed by ImageJ 1.37c, National Institutes of Health. A box-and-whisker diagram was used to demonstrate the distribution of fluorescence intensity from the 25 sites on all the samples. The analysis result of one-photon fluorescence images is shown in Fig. 7a. The result shown in Fig. 7a depicts the contrast between healthy teeth, calculus, and gingiva was very low. The result was easily realized from the represented images shown in Figs. 3 and 4. Besides the issue of contrast, one-photon fluorescence of calculus was heavily blocked by the gingiva on top. Through the same analysis method, the distribution of the fluorescence intensity from two-photon autofluorescence images is shown in Fig. 7b. The difference from the one-photon excitation result shown in Fig. 7a and the two-photon fluorescence modality showed a good contrast between healthy teeth, calculus, and gingiva. As expected, the fluorescence of healthy teeth and gingiva was very low when compared to that of calculus. When the calculus was covered by gingiva, the fluorescence of calculus still remained from one-third to one-fourth of the intensity of uncovered calculus. It is important to point out that the fluorescence intensity obtained by the two different modalities used different detection systems. Therefore, the unit of intensity is different. However, the more important point is the contrast of the fluorescence intensity and the penetration of the excitation beam through the gingiva. The quantitative analysis of the fluorescence intensity further pronounced the superior of the two-photon excitation scheme to the one-photon excitation scheme when detection of subgingival calculus is concerned. Fig. 7Box-and-whisker diagrams for the fluorescence intensity of healthy tooth, gingiva, calculus, and gingival covered calculus. (a) Analysis of one-photon excitation fluorescence images. (b) Analysis of two-photon excitation fluorescence images. 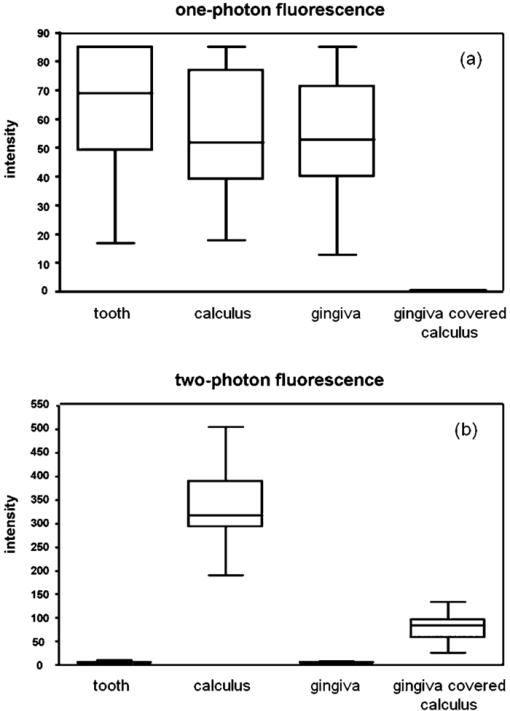 When the two-photon fluorescence imaging method was proposed for a new application, an issue of cellular damage should be verified,15 especially when the objective used is 10× instead of 20× which was used in our pilot study.14 The less focusing power usually requires higher illumination power to obtain the same fluorescence response as the 20× objective does. The issue of cell damage is related to the absorption of the cells to the excitation laser beam. The absorption coefficient strongly depends on the wavelength of the incoming laser irradiation. For the laser source used in this study, the influence of 790 nm near-infrared femtosecond laser pulses in two-photon microscope on human gingival fibroblast was examined. The result assured that the femtosecond laser beam of average power 13 mW may act as nondestructive excitation beams in the two-photon microscopy when the illumination time was less than 120 s on a single spot. A systematic study on this issue is currently being conducted. The result will be reported in the near future. Based on literature reports, fluorescence intensity is masked when the calculus surface is covered by soft tissue and blood clots. It was suggested that the accuracy of the laser-based calculus detection can be impaired by gingiva.11, 12 However, this situation was improved by the two-photon fluorescence imaging technology based on our study. While the detection of subgingival calculus by the conventional one-photon confocal microscopy technique suffered from the fluorescence of healthy cementum and the shallow penetration depth, the two-photon mechanism limited the fluorescence excitation within the focal plane and the gingiva-covered calculus. Only the focal plane part gained enough photon power to excite the fluorescence. Besides, the gingiva showed none or negligible fluorescence under the two-photon microscopy imaging technique with the selected excitation wavelength 790 nm. This characteristic gives the two-photon fluorescence microscopy technique the ability to discriminate against fluorescence originating from regions outside the focal plane, such as gingiva and healthy tooth cementum. On the other hand, the two-photon mechanism allows the use of near-infrared light, which provides a much better penetration performance in gingiva, and therefore is a potential candidate for the detection of subgingival calculus. Due to the influences of gingival thickness and tooth dimensions on the laser penetration, we should know that dental arch location, gender, age, and race are all factors which may possibly affect the accuracy and feasibility of such detection techniques.16, 17 We will consider those factors in our future studies. 4.ConclusionCharacteristics of two-photon fluorescence imaging technology employed on the detection of subgingival calculus were revealed in comparison with the one-photon confocal fluorescence imaging technique. The experimental result revealed that, different from the one-photon fluorescence imaging scheme, the two-photon fluorescence images of the subgingival calculus were not masked by the gingiva used in the experiment. Because the two-photon fluorescence was almost nondetectable on the gingiva and the healthy tooth, together with the optical sectioning capability of the nonlinear optical mechanism, fluorescence images of subgingival calculus with high contrast were obtained. Furthermore, the optical sectioning capability also provides the location of subgingival calculus in different depths without the aid of a confocal mechanism. The experimental result has revealed the potential of using a two-photon fluorescence imaging technique to detect subgingival calculus. AcknowledgmentsThe authors would like to thank Imaging Core at National Yang Ming University for the support of two-photon time-correlated fluorescence microscopy and the Department of Medical Research and Education, Taipei Veterans General Hospital for the technical support in one-photon laser scanning microscopy. This work was supported by research Grant Nos. NSC 97-2627-M-010-005 and NSC 99-2112-M-010-001-MY3 from National Science Council in Taiwan and by “A grant from Ministry of Education, Aim for the Top University Plan” from National Yang Ming University. ReferencesP. E. Petersen,
“The World Oral Health Report 2003: continuous improvement of oral health in the 21st century–the approach of the WHO Global Oral Health Programme,”
Community Dent. Oral Epidemiol., 31
(1), 3
–23
(2003). https://doi.org/10.1046/j..2003.com122.x Google Scholar
B. T. Tan, N. J. Mordan, J. Embleton, J. Pratten, and
P. N. Galgut,
“Study of bacterial viability within human supragingival dental calculus,”
J. Periodontol., 75
(1), 23
–29
(2004). https://doi.org/10.1902/jop.2004.75.1.23 Google Scholar
E. A. Roberts-Harry and
V. Clerehugh,
“Subgingival calculus: where are we now? A comparative review,”
J. Dent., 28
(2), 93
–102
(2000). https://doi.org/10.1016/S0300-5712(99)00056-1 Google Scholar
P. R. Sherman, L. G. Jewson, J. M. Moriarty, G. W. Greco, W. T. McFall , Jr.,
“The effectiveness of subgingival scaling and root planning. I. Clinical detection of residual calculus,”
J. Periodontol., 61
(1), 3
–8
(1990). Google Scholar
A. Tugnait, V. Clerehugh, and
P. N. Hirschmann,
“The usefulness of radiographs in diagnosis and management of periodontal diseases: a review,”
J. Dent., 28
(4), 219
–226
(2000). https://doi.org/10.1016/S0300-5712(99)00062-7 Google Scholar
J. Lindhe, Textbook of Clinical Periodontology, Munksgaard(1990). Google Scholar
F. Krause, A. Braun, and
M. Frentzen,
“The possibility of detecting subgingival calculus by laser-fluorescence in vitro,,”
Lasers Med. Sci., 18
(1), 32
–35
(2003). https://doi.org/10.1007/s10103-002-0241-7 Google Scholar
W. Buchalla, A. M. Lennon, and
T. Attin,
“Fluorescence spectroscopy of dental calculus,”
J. Periodontal Res., 39
(5), 327
–332
(2004). https://doi.org/10.1111/j.1600-0765.2004.00747.x Google Scholar
Y. L. Qin, X. L. Luan, L. J. Bi, Z. Lu, Y. Q. Sheng, G. Somesfalean, C. N. Zhou, and
Z. G. Zhang,
“Real-time detection of dental calculus by blue-LED-induced fluorescence spectroscopy,”
J. Photochem. Photobiol. B, 87
(2), 88
–94
(2007). https://doi.org/10.1016/j.jphotobiol.2007.03.002 Google Scholar
M. Folwaczny, R. Heym, A. Mehl, and
R. Hickel,
“Subgingival calculus detection with fluorescence induced by 655 nm InGaAsP diode laser radiation,”
J. Periodontol., 73
(6), 597
–601
(2002). https://doi.org/10.1902/jop.2002.73.6.597 Google Scholar
M. Folwaczny, R. Heym, A. Mehl, and
R. Hickel,
“The effectiveness of InGaAsP diode laser radiation to detect subgingival calculus as compared to an explorer,”
J. Periodontol., 75
(5), 744
–749
(2004). https://doi.org/10.1902/jop.2004.75.5.744 Google Scholar
E. Kurihara, T. Koseki, K. Gohara, T. Nishihara, T. Ansai, and
T. Takehara,
“Detection of subgingival calculus and dentine caries by laser fluorescence,”
J. Periodontal. Res., 39
(1), 59
–65
(2004). https://doi.org/10.1111/j.1600-0765.2004.00712.x Google Scholar
W. Denk, J. H. Strickler, and
W. W. Webb,
“Two-photon laser scanning fluorescence microscopy,”
Science, 248
(4951), 73
–76
(1990). https://doi.org/10.1126/science.2321027 Google Scholar
O. H. Tung, S. Y. Lee, Y. L. Lai, and
H. F. Chen,
“Detection of subgingival calculus through oral gum in vitro using two-photon fluorescence microscopy,”
Conf. Proc. IEEE Eng. Med. Biol. Soc., 2008 4051
–4054
(2008). Google Scholar
K. Konig, P. T. So, W. W. Mantulin, and
E. Gratton,
“Cellular response to near-infrared femtosecond laser pulses in two-photon microscopes,”
Opt. Lett., 22
(2), 135
–136
(1997). https://doi.org/10.1364/OL.22.000135 Google Scholar
K. L. Vandana and
B. Savitha,
“Thickness of gingiva in association with age, gender, and dental arch location,”
J. Clin. Periodontol., 32
(7), 828
–830
(2005). https://doi.org/10.1111/j.1600-051X.2005.00757.x Google Scholar
J. Y. Ling and
R. W. Wong,
“Tooth dimensions of Southern Chinese,”
Homo., 58
(1), 67
–73
(2007). https://doi.org/10.1016/j.jchb.2006.08.003 Google Scholar
|

