|
|
1.IntroductionSince the introduction of photobiomodulation in medicine, the effectiveness of a variety of light sources has been evaluated. In 1983, an experiment carried out at the cellular level showed that coherent and noncoherent lights with the same wavelength, intensity, and irradiation time promoted the same biological effect.1 In the last decade, some researchers have demonstrated the applicability of light-emitting diode (LED) therapy in experimental and clinical studies demonstrating cellular photobiomodulative effectiveness similar to that of low-intensity laser.2, 3, 4, 5, 6 The reason for the increase of research seeking to compare the effects of phototherapy using LEDs instead of laser is that, unlike wavelength, coherence of a light is not a major factor in the biomodulative effect.7 Thus, LEDs have great potential for action, besides being cheaper than lasers.6 Phototherapy is painless, noninvasive, and when correctly administered, has no known side effects.3 However, for its incorporation into clinical practice, more well-designed studies are necessary to be conducted with the objective of defining optimal parameters for its application. That will result in better clinical outcomes, increasing patients’ quality of life, and proving the absence of side effects of light on the proposed parameters at either the clinical or the cellular level. Another factor that needs more attention is the development and evaluation of new sources of light at lower cost for such treatment other than low intensity laser, which is a factor that could contribute significantly to the spread of phototherapy. Thus, it is appropriate to carry out a study to evaluate the effects of therapy with low-intensity laser and LEDs on cell viability through its application in fibroblasts cultured under nutritional stress similar to that experienced by fibroblasts in vivo following tissue injury and therefore of clinical relevance. 2.Materials and Methods2.1.Cell CultureBalb/c 3T3 cells [American Type Culture Collection (ATCC)] were cultured in Dulbecco's modified eagle's medium (DMEM), at 37 °C, in a humid atmosphere containing 5% CO2. The experimental cultures were prepared by inoculation of cells in disposable 96 well plates. Before irradiation, cells were kept for 6 h in the culture medium to allow them time to adhere to the base of the plates’ wells; then it was replaced by 5% fetal bovine serum (FBS) supplemented culture medium (exception for the control group: 10% fetal bovine serum). Samples from each group were taken for mitochondrial and lysosomal activity analysis 24, 48, and 72 h after the first irradiation. 2.2.Laser and LED IrradiationThe phototherapy devices used were: an aluminum gallium indium phosphorus (AlGaInP) low-intensity laser (Twinflex II, MMOptics Ltda, São Carlos, SP, Brazil), with one diode probe emitting at a visible red wavelength (660 nm) and 40 mW power, and the other probe emitting at an infrared wavelength (780 nm) and 50 mW power [Fig. 1a] and one LED device (prototype of the Center for Research in Optics and Photonics of the Institute of Physics of São Carlos/University of São Paulo, São Carlos, SP, Brazil) emitting noncoherent polarized red light with a visible red wavelength (637 ± 15 nm) and 40 mW power developed specifically for this study [Fig. 1b]. Fig. 1Phototherapy devices used in the study. (a) Low-intensity laser device (Twinflex II, MMOptics, São Carlos, SP, Brazil); (b) LED prototype (CePOF/IFSC/USP, São Carlos, SP, Brazil).  All cell viability assays were performed in 96-well plates, seeding Balb/c 3T3 cells (in the fifth passage) with a density of 5 × 102 cells/well. Prior to laser and LED irradiation, the plates were wrapped in a mask made out of black cardboard with holes located in the position of the wells of the experimental groups. Each hole was individually sealed by a hatch, also in black cardboard, that allowed only the bore of the well that was being irradiated to remain open, while all the others were kept in the dark. The holes in the mask had the diameter of 0.5 cm2, while the laser and LED spot had the diameter of 0.4 cm2. Low-intensity laser and LED irradiation were made through the transparent bottom of the 96 wells plate, keeping the distance between the light beam and the cell monolayer constant at 1 mm. Therefore, the radiation passed directly to the cell monolayer via the plate base without traveling through the culture medium reaching the cells, following the methodology adopted previously.8, 9 The power of the laser and LED devices was measured with the Lasercheck PowerMeter (Coherent Inc., Santa Clara, California) prior to each application. At the application time, plates were positioned so that the devices’ probes were totally in (punctual) contact with the bottom of the wells. After phototherapy, the culture medium was removed in the experimental pre-determined times (24, 48, and 72 h) and the cells were washed with PBS-A. Then the cell viability assays were performed as outlined below. 2.3.Experimental GroupsAfter incubation in a CO2 oven, eight groups were prepared, as presented in Table 1. Table 1Experimental groups and corresponding irradiation parameters.
Each group had eight replicates. All samples were subjected to the same environmental conditions such as light, humidity, temperature, and time outside the incubator. Group 7 (negative control) and group 8 (positive control), both nonirradiated groups, were subjected to the same experimental conditions of the irradiated groups except by the irradiation itself. Each irradiated well had an unirradiated well adjacent to it, the separation limiting treatment to a single well. 2.4.Mitochondrial Activity AnalysisThe mitochondrial activity was assessed by incubation of the cells in a solution of 0.5 mg MTT/mL of DMEM without FBS for 4h, at 37oC. After removing the solution, the insoluble pigment reduced intracellularly was extracted with 1 ml of ethanol/well, stirring gently for 10 minutes at room temperature. Then, the absorbance of the alcoholic extract from each well was measured at 570 nm in a fluorescence reader (Fluostar Óptima- BMG LABTECH, Offenburg, Germany) using the program MARS Data Analysis Software. The mitochondrial function of the viable cells following each treatment was calculated. 2.5.Lysosomal Activity AnalysisFrom a stock solution of 0.4% neutral red (NR), a solution of 50 μg/ml neutral red in DMEM was obtained. The resulting solution remained in the oven at 37°C overnight for the crystals precipitation, and then filtered through a Millipore filter (0.22 μ). The cells were treated with this solution (50 μg/mL) and the plates incubated for 3 h at 37 °C to allow uptake of the dye by the lysosomes of viable cells. They were washed with PBS-Ca+2, and the dye extracted in a solution of ethanol 50% and 1% acetic acid by placing 1 ml of the solution/well. Then, it was determined the absorbance of the extracts at 540 nm in the same fluorescence reader and program. The lysosomal activity of the viable cells following each treatment was calculated. 2.6.Statistical AnalysisObtained data do not follow a normal distribution, making it impossible for comparisons of means. Thus, for evaluation, independent groups were compared using the median and nonparametric Kruskal–Wallis test to evaluate differences between groups. To evaluate the interaction between the variables, the analysis of variance of the general linear model was used. Statistical analysis was performed with a significance level of 5%. 3.ResultsCalculations of data obtained by MTT reduction are summarized in Fig. 2. It was observed that there is a statistically significant difference between the medians of the tests using the MTT reduction for the groups, as shown in Fig. 2a. Using a range nonparametric test, it was observed with 95% of confidence that the group in which the cell culture was irradiated with the LED is statistically different from the groups irradiated with red laser and infrared laser. Fig. 2Mitochondrial activity analysis acquired after performing MTT reduction. (a) Box-plots comparing data obtained by MTT reduction by intervention groups. (b) Box-plots comparing data obtained by MTT reduction by exposure time. (c) Box-plots comparing data obtained by MTT reduction by experimental period. (d) Interaction between the means obtained by the MTT reduction for the intervention groups and for the experimental periods (24 and 72 h). 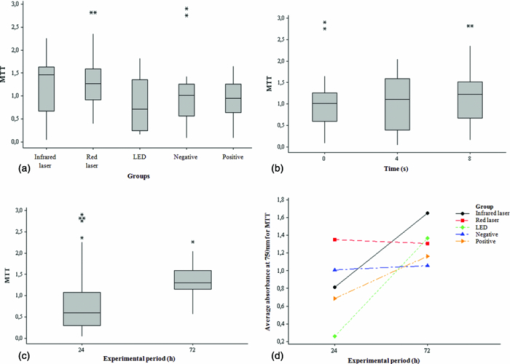 A trend of MTT reduction increase can be seen as the irradiation time, and consequently, the total energy irradiated increased from 0 to 4 s and 8 s. This increase, however, is not statistically significant [Fig. 2b]. Data presented in Fig. 2c show that there is a statistically significant difference between the medians of the tests performed using the MTT reduction for the experimental periods of 24 and 72 h. Note that, on average, when the trial period is considered separately, changing from 24 to 72 h, there is an increase in the values of MTT reduction, and this increase is statistically significant (p < 0.001). It is also noted that, on average, the individual values obtained by MTT reduction in the group irradiated with red laser is the highest (p < 0.001), and values of the group irradiated with LED are the lowest (p < 0.001). The interaction between the averages for the experimental periods of 24 and 72 h and the intervention groups (infrared laser, red laser, LED, positive control, and negative control) is illustrated in Fig. 2d. This effect was statistically significant for the 72 h period with the infrared laser (p = 0.001) and LED (p < 0.001). In other words, there was an average increase in the values of MTT reduction, and the red laser did not cause a statistically significant decrease with the period of 72 h (p = 0.137). It should be noted that in this case the period of 24 h and the negative control group were considered as reference. As with the data obtained by MTT reduction, the values found by the capture of NR, do not follow a normal distribution, making it impossible for comparisons of means. Thus, for evaluation, independent groups were again compared using the median and nonparametric Kruskal–Wallis test to evaluate differences between groups. Statistical analysis was performed with a significance level of 5% (p ≤ 0.05) (Fig. 3). Fig. 3Lysosomal activity analysis acquired after performing neutral red assays. (a) Box-plots comparing data obtained by the uptake of neutral red by the intervention groups. (b) Box-plots comparing data obtained by the uptake of neutral red by the exposure time. (c) Box-plots comparing data obtained by the uptake of neutral red by the experimental period. (d) Interaction between the means obtained by neutral red uptake for the intervention group and for the experimental periods (24, 48, and 72 h). 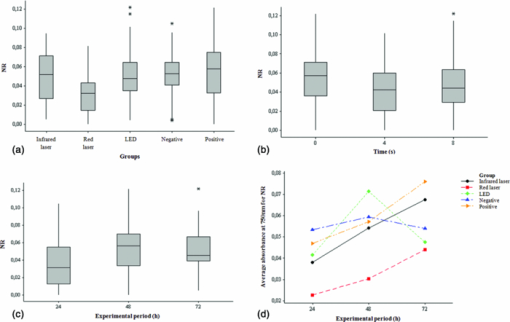 Also, in this cell viability test, statistically significant differences were observed between the median values for the considered groups [Fig. 3a]. Using a range nonparametric test, it was observed with 95% confidence that the group in which the cell culture was irradiated with laser at the red wavelength is statistically different from the groups irradiated with infrared laser and LED and positive and negative control groups. A statistically significant decrease in the median values of neutral red uptake is observed as the exposure time increases from 0 to 4 s. A decrease is also observed between 0 and 8 s, but it is not statistically significant. A slight, not statistically significant, increase is also seen when the exposure time and the irradiated energy increases from 4 to 8 s [Fig. 3b]. A statistically significant difference between the medians of testing for the experimental periods is observed when going from 24 to 48 and 72 h [Fig. 3c]. It should be noted that on average, when the trial period is considered separately, changing from 24 to 48 and 72 h, there is an increase in the values of the capture of neutral red, and this increase is statistically significant (p < 0.001). It was also noted that, on average, the individual values obtained by the neutral red uptake in the group irradiated with red laser are the lowest (p < 0.001). As previously mentioned, although an increase is observed between the interactions, this increase is not statistically significant. Only the interaction at 48 h with the group irradiated with the LED was statistically significant (p = 0.001), meaning that there is an average increase in the capture of neutral red. The interaction between the averages for the experimental periods of 24, 48, and 72 h, and for the intervention groups (infrared laser, red laser, LED, positive control, and negative control) is illustrated in Fig. 3d. Again, the 24 h period and the negative control group were considered as reference. 4.DiscussionSince the introduction of photobiomodulation in medicine, the effectiveness and applicability of a variety of light sources to treat various medical conditions has been extensively investigated, both in vitro and in vivo. Many results, however, are remarkably contradictory. Some reasons for the contradictions are the wide range of indications for phototherapy, the number of different light parameters used, the impossibility of measuring the likely post-irradiation effects with the necessary objectivity, and even the lack of theoretical understanding about this intervention.10 Nevertheless, low-intensity lasers and LEDs are well accepted therapeutic tools to treat infected, ischemic, and hypoxic wounds, and other changes in soft tissue.11 Other light sources have been proposed as alternatives to laser, mainly because of lower cost, but the LED-based devices have been considered the main alternative. Although LED is not a monochromatic source, it emits in more restricted bands compared with conventional bulbs.12 In addition, LED light therapy has been considered of nonsignificant risk by the FDA and was approved for use in humans.4 Therapy with low intensity light using regions of the spectrum ranging from the far-red to near-infrared (630 to 1000 nm) modulates many cellular functions4 and has been demonstrated to be effective in the process of biologic stimulus.11 The positive effects of photobiomodulation include acceleration of healing and even improvement on the recovery of ischemic heart lesion.11 It was postulated that in the cellular level, phototherapy can modulate cell adhesion and synthesis of collagen and procollagen, promote angiogenesis, and stimulate macrophages and lymphocytes and modulate the proliferation of fibroblasts by improving energy metabolism in the mitochondria.4 Many studies highlight the effect of low-intensity lasers on the mitochondria13, 14 and on some signaling molecules (c-fos, cyclin A, cyclin E, and cyclin D1) of the mitosis.13 Our results seem to confirm these studies since we noticed a positive effect (increased viability, measured indirectly by the MTT, and neutral red tests) in the treated fibroblasts. Since the phototherapy biomodulator effect acts by balancing cells that are unbalanced, it is understood that if cells are in the ideal condition for proliferation, phototherapy will have little or no positive effect.8, 9, 15 Thus, to simulate in vivo stress conditions, cells were grown in nutritional deficit. It was observed in this study that, considering the median of the tests using the MTT reduction, the difference between the groups was statistically significant, and those irradiated with the infrared laser had the highest score. The similarity of the red laser and LED groups’ results, which used similar phototherapy parameters, suggests that the coherence of light was a factor that did not interfere with MTT reduction. Finally, the fact that the three irradiated groups presented better results than the positive and negative control groups after 72 h allows one to infer that photobiomodulation produced with different light sources showed higher cell survival, and possibly cell proliferation than noninter-vention. This conclusion was based on the fact that when cells are grown in culture medium supplemented with low concentrations of fetal serum (culture medium with nutritional deficit), its growth rate is decreased.8, 9, 15 However, in this study only the positive control group had ideal supplementation; all other groups were supplemented with 5% fetal bovine serum, or with nutritional deficit. However, all irradiated groups showed greater fibroblastic survival and possibly cell proliferation than the positive control group. This result is consistent with previous studies8, 9 which showed that the application of low-intensity laser is able to increase the growth rate of epithelial cells under poor nutritional conditions. However, in the study of Eduardo,8 laser irradiation could not induce cell cultures to achieve their potential cell growth rate for the same strain grown under normal nutritional status. In the present study, the cultures irradiated with laser and LED exceeded the growth of cells of the positive control group grown under normal nutrient conditions. A similar result was also found by Vinck 10 that analyzed the proliferation of fibroblasts after irradiation with laser and LED at wavelengths of 950, 660, and 570 nm. In this work, besides the MTT reduction assay, cell viability was also assessed by the ability of lysosomes to absorb the neutral red dye. Among the groups grown in nutritional deficit, the infrared laser group showed the highest score, followed by the negative control group and the red laser and LED groups. These results resembled even more the results found by Eduardo,8 in which none of the groups cultured under nutritional deficit reached the cell proliferation obtained by the nonirradiated control group grown under normal conditions of nutrition. That work, however, used MTT reduction to measure cell prolif-eration. The higher rate of MTT reduction after phototherapy compared to that found by the neutral red uptake may be another sign of increased mitochondrial activity related to photobiomodulation, as described by other studies.4, 16 Another significant similarity with the work of Eduardo 8 is that the improvement found in cell survival and possibly also cell proliferation after phototherapy, apparently does not depend on the wavelength applied. However, there was a more significant trend of growth rate increase when the infrared laser (780 nm) was used. In this study, in the two cell viability tests performed, the infrared laser was shown to be the intervention that increased the most the activity of mitochondria and lysosomes, resulting in a higher cell survival and possibly cell proliferation. Other studies support these results demonstrating an increase in cell proliferation with irradiation with low-intensity lasers, especially the red and near-infrared.13 The results of the cell viability tests using two different methodologies suggest that the cell survival and possibly proliferation of fibroblasts grown in adverse conditions is more dependent on the wavelength and power than on the coherence of light, similar to results of previous studies.12 It is important to note that phototherapy held under the used parameters in this study did not show any negative effect on fibroblast survival in vitro and may even have stimulated cell growth under nutritional deficit. This information is extremely valuable for the clinical application of phototherapy as cellular survival followed by proliferation is a major factor in wound healing,9 and there is an increasing need for safe and effective therapeutic interventions for the treatment of chronic wounds.11 5.ConclusionsAnalyzing the results of this work and considering the parameters and protocol of phototherapy used, it can be concluded that phototherapy stimulated the viability of fibroblasts cultured under nutritional deficit. The increase was greatest in the group irradiated with infrared laser (780 nm, 50 mW). AcknowledgmentsThe authors thank Professor Carmen Verissima, from the Laboratory of Signal Transduction of the Institute of Biology, University of Campinas, for the provision of the cell line used in this study. They also thank CePOF for the provision of the LED prototype, FAPEMAT (Mato Grosso Research Support Foundation) which supported this study by the processes 696/2006 and 884/2007, and FAPESP (São Paulo State Research Support Foundation) which supported this study by the process 2007/08367-0. They thank Flávia A. Oliveira, Adriana A. Matos, Camila Slompo, and Carla A. Damante for the valuable assistance in the conduction of the technical work. ReferencesT. I. Karu, O. A. Tiphlova, V. S. Letokhov, and
V. V. Lobko,
“Stimulation of E.coli growth by laser and incoherent red light,”
Il Nuovo Cimento D, 2 1138
–1144
(1983). https://doi.org/10.1007/BF02457148 Google Scholar
H. T. Whelan, J. F. Connelly, B. D. Hodgson, L. Barbeau, A. C. Post, G. Bullard, E. V. Buchmann, M. Kane, N. T. Whelan, A. Warwick, and
D. Margolis,
“NASA light-emitting diodes for the prevention of oral mucositis in pediatric bone marrow transplant patients,”
J. Clin. Laser Med. Surg., 20 319
–324
(2002). https://doi.org/10.1089/104454702320901107 Google Scholar
L. Corti, V. Chiarion-Sileni, S. Aversa, A. Ponzoni, R. D’Arcais, S. Pagnutti, D. Fiore, and
G. Sotti,
“Treatment of Chemotherapy-induced oral mucositis with light-emitting diode,”
Photomed. Laser Surg., 24 207
–213
(2006). https://doi.org/10.1089/pho.2006.24.207 Google Scholar
K. D. Desmet, D. A. Paz, J. J. Corry, J. T. Eells, M. T. T. Wong-Riley, M. M. Henry, E. V. Buchmann, M. P. Connelly, J. V. Dovi, H. L. Liang, D. S. Henshel, R. L. Yeager, D. S. Millsap, J. Lim, L. J. Gould, R. Das, M. Jett, B. D. Hodgson, D. Margolis, and
H. T. Whelan,
“Clinical and experimental applications of nir-led photobiomodulation,”
Photomed. Laser Surg., 24 121
–128
(2006). https://doi.org/10.1089/pho.2006.24.121 Google Scholar
R. L. Yeager, D. A. Oleske, R. A. Sanders, J. B. Watkins III, J. T. Eells, and
D. S. Henshel,
“Melatonin as a principal component of red light therapy,”
Med. Hypotheses, 69 372
–376
(2007). https://doi.org/10.1016/j.mehy.2006.12.041 Google Scholar
N. T. Sacono, C. A. S. Costa, V. S. Bagnato, and
F. C. B. Abreu-e-Lima,
“Light-emitting diode therapy in chemotherapy-induced mucositis,”
Lasers Surg. Med., 40 625
–633
(2008). https://doi.org/10.1002/lsm.20677 Google Scholar
K. C. Smith,
“Laser (and LED) therapy is phototherapy,”
Photomed. Laser Surg., 23 78
–80
(2005). https://doi.org/10.1089/pho.2005.23.78 Google Scholar
F. P. Eduardo, D. U. Mehnert, T. A. Monezi, D. M. Zezell, M. M. Schubert, C. P. Eduardo, and
M. M. Marques,
“Cultured epithelial cells response to phototherapy with low intensity laser,”
Lasers Surg. Med., 39 365
–372
(2007). https://doi.org/10.1002/lsm.20481 Google Scholar
L. H. Azevedo, F. P. Eduardo, M. S. Moreira, C. P. Eduardo, and
M. M. Marques,
“Influence of different power densities of LILT on cultured human fibroblast growth: A pilot study,”
Lasers Med. Sci., 21 86
–89
(2006). https://doi.org/10.1007/s10103-006-0379-9 Google Scholar
E. M. Vinck, B. J. Cagnie, M. J. Cornelissen, H. A. Declercq, and
D. C. Cambier,
“Increased fibroblast proliferation induced by light emitting diode and low power laser irradiation,”
Lasers Med. Sci., 18 95
–99
(2003). https://doi.org/10.1007/s10103-003-0262-x Google Scholar
H. T. Whelan, E. V. Buchmann, N. T. Whelan, S. G. Turner, V. Cevenin, H. Stinson, R. Ignatius, T. Martin, J. Cwiklinski, G. A. Meyer, B. Hodgson, L. Gould, M. Kane, G. Chen, and
J. Caviness,
“NASA light emitting diode medical applications from deep space to deep sea,”
Space Tech. Appl. Intl. Forum, AIP Conf. Proceedings, 552 35
–45
(2001). https://doi.org/10.1063/1.1357902 Google Scholar
A. V. Corazza, J. Jorge, C. Kurachi, and
V. S. Bagnato,
“Photobiomodulation on the angiogenesis of skin wounds in rats using different light sources,”
Photomed. Laser Surg, 25 102
–106
(2007). https://doi.org/10.1089/pho.2006.2011 Google Scholar
X. Gao and
D. Xing,
“Molecular mechanisms of cell proliferation induced by low power laser irradiation,”
J. Biomed. Sci., 16 4
(2009). https://doi.org/10.1186/1423-0127-16-4 Google Scholar
P. Kaššák, T. Przygodzki, D. Habodászová, M. Bryszewska, and
L. Šikurová,
“Mitochondrial alterations induced by 532 nm laser irradiation,”
Gen. Physiol. Biophys., 24 209
–220
(2005). https://doi.org/10.1186/1423-0127-16-4 Google Scholar
C. A. Damante, G. de Micheli, I. S. Feist, S. P. H. Miyagi, and
M. M. Marques,
“Effect of laser phototherapy on the release of fibroblast growth factors by human gingival fibroblasts,”
Lasers Med. Sci, 24 885
–891
(2009). https://doi.org/10.1007/s10103-008-0582-y Google Scholar
T. I. Karu,
“Cellular mechanisms of low power laser therapy: new questions,”
Lasers in Medicine, Surgery, Dentistry, Urology, And Veterinary, 79
–99 European Medical Laser Association, Locarno, Switzerland
(2003). Google Scholar
|

