|
|
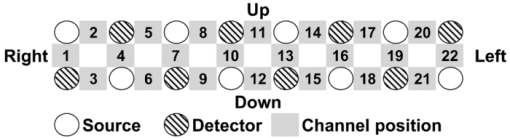
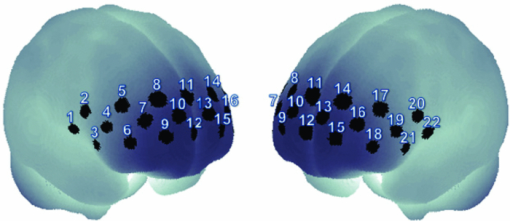
1.IntroductionOne of the common symptoms of autism,1, 2 Asperger syndrome,1, 3 schizophrenia, and depression is a lack of social skill. To alleviate these mental disorders on the basis of a neuroscience approach, it is important to investigate the relationship between social skills and brain function.4 Social skills, in general, include adaptability of one's own behavior to a social context, reading the atmosphere or others’ intentions, communication skills, and ethical sense.5, 6 Most of these social skills are acquired through interactions with others. To study the social-brain function, it is therefore meaningful to investigate interacting brains of multiple participants. Although the “social brain”7, 8, 9, 10 has been studied by many researchers, it is mostly measured in only one brain at a time by using various modalities, such as electroencephalography (EEG),11, 12 positron-emission tomography,13, 14, 15 single-photon-emission computed tomography,16 functional magnetic-resonance imaging (fMRI),17, 18, 19, 20, 21 and functional near-infrared spectroscopy (fNIRS).22, 23, 24, 25, 26 Consequently, little is known about how social functions are processed in interacting brains of multiple participants. There are various ways to study two or more brains at once. We used a wearable-optical-topography (WOT) system based on near-infrared spectroscopy (NIRS)27, 28, 29 because this system can simultaneously measure the brain activities of two or more people who are interacting in a natural manner, namely, during face-to-face communication. An NIRS system measures the changes in cerebral blood volume by radiating weak visible or near-infrared light into the head and detecting the transmitted light from another position.30, 31, 32, 33 On the basis of NIRS, optical topography (OT),34, 35 which uses multiple light sources and detectors, obtains two-dimensional topographical images of the changes in cerebral blood volume. OT systems have been widely used for research and clinical purposes,36, 37, 38, 39, 40 especially for measuring the brain activity of infants and children,25, 41, 42, 43, 44, 45 because they have a high level of safety46, 47 and require few constraints. For simultaneously measuring of multiple participants’ brains (i.e., hyperscanning) and thereby investigating the relationship between brain activity and social interaction, some researchers used EEG,48, 49, 50, 51 which is less expensive and has a higher temporal resolution than NIRS. The spatial resolution of EEG, however, remains lower than that of NIRS.52, 53 Others used fMRI,54, 55 which has a higher spatial resolution than NIRS and EEG, but it is difficult for participants to interact in a natural manner in an fMRI environment. To measure multiple participants’ brain activity during face-to-face interaction and to demonstrate the effectiveness of simultaneous NIRS measurement, we used a wearable NIRS system in this study. It has been reported that when people interact or cooperate during common social-interaction tasks, a specific brain area activates.18, 48 For example, in an EEG study, the medial prefrontal region was activated in conflict situations during the “prisoner's dilemma” task (one common social-interaction task),48 and in an fMRI study, during the “trust and reciprocity” game, the medial prefrontal region of highly cooperative participants was significantly more activated during human–human interactions than during human–computer interactions.18 It is therefore reasonable to suppose that the relationship between spatial brain-activity patterns of two people is effective in the investigation of brain functions related to social interactions. In the present study, considering that the medial prefrontal region has been reported to be activated during social interaction, we focused our attention on activity in the prefrontal-cortex region during a cooperative task as a form of social process. To investigate this region, we proposed an analytical method using spatiotemporal activation patterns of multiple participants. If the relationship between the analyzed brain-activity data of two participants in a natural attitude and the “quality of cooperation” were obtained by using simultaneous NIRS measurement and data analysis of multiple participants, this methodology would become an important tool in research on social cognition. The aim of this study was to investigate the relationship between multiparticipants’ coinstantaneous brain-activation signals and cooperative-task performance by simultaneous NIRS measurement and to show the significance of the simultaneous measurement of two or more brains. A WOT system was used to investigate interactive brain activities during the cooperative task (namely, a cooperative button-pressing) because it is suitable for measuring the prefrontal cortex of multiple participants interacting in a natural manner.27, 28, 29 2.Materials and Methods2.1.ParticipantsTwelve healthy adults (six pairs), nine males and three females, between 25 and 50 years old (mean ± SD: 37.5 ± 8.0) participated as volunteer subjects in this study. All participants provided written informed consent after a complete explanation of the study. The experiments were approved by the Ethics Committee of Hitachi, Ltd. 2.2.Near-Infrared-Spectroscopy MeasurementA WOT system, the same one used in our previous study,29 was used for measuring cerebral blood-volume change of the 12 participants. The system generally measures the changes in the product of hemoglobin (Hb) concentration (C) and effective optical path length (L), called simply “Hb change (ΔCL)” here, in human-brain tissue, such as the cerebral cortex. The unit of Hb change (in mM times millimeter) is molar concentration (in mM = mmol/l) multiplied by optical path length (in millimeters). Two kinds of Hb change, oxygenated (oxy-) and deoxygenated (deoxy-), are obtained by using two different absorption-coefficient spectra as well as absorption data for at least two wavelengths according to the modified Beer–Lambert law.34, 56 The WOT system does not have optical fibers, which may limit the participant's range of movement, and enables simultaneous measurement of multiple participants by using a personal computer through a wireless local area network (LAN). The details of the WOT system are described previously.27, 28, 29 The WOT system is small, light, and covers the entire forehead. The probe positions of the light sources and detectors and the channel positions of the WOT system are shown in Fig. 1. The channel position is defined as the center point between each light source and detector. The WOT probes were placed on each participant's head so that the bottom line of the probes was on the participant's eyebrow line. 2.3.Interparticipant Variability of Channel PositionsTo evaluate the variability of the measurement positions on all the participants, the probe positions were measured with a three-dimensional digitizer on the basis of the international 10–20 system,57 namely, the standard method for electrode placement in electroencephalography, and the measurement positions on the scalp were converted to those on the brain in the Montreal Neurological Institute (MNI) coordinate system by the probabilistic-determination method.58, 59, 60 Figure 2 shows the measurement positions averaged among the 12 participants. For the 12 participants, Table 1 lists the means and standard deviations of the channel positions in regard to each axis. Channel 5 had the maximal square sum of the standard deviations in the three directions. The standard deviations of the channel-5 positions in regard to the three axes were 4.7 mm on the x-axis, 8.5 mm on the y-axis, and 7.0 mm on the z-axis. Because these deviations were much smaller than the 30-mm probe intervals, the difference and variability of the positions of the measurement channels for different participants could be ignored and the data of a channel for one participant was compared to those of the same channel for the other participants. Fig. 10Mean and standard error of intertime intervals for low- and high-covariance groups. Black squares represent the mean value, and error bars represent the standard errors of the normalized inter-time intervals. 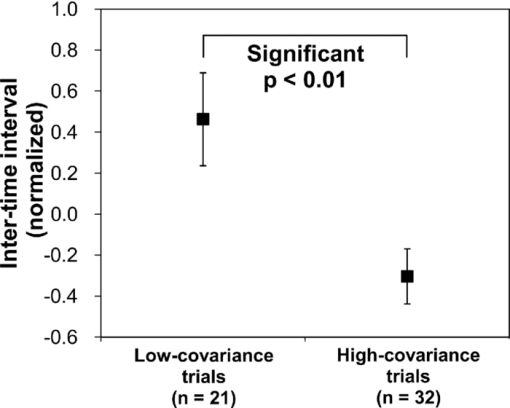 Table 1Mean and standard deviation (SD) of channel positions for 12 participants.
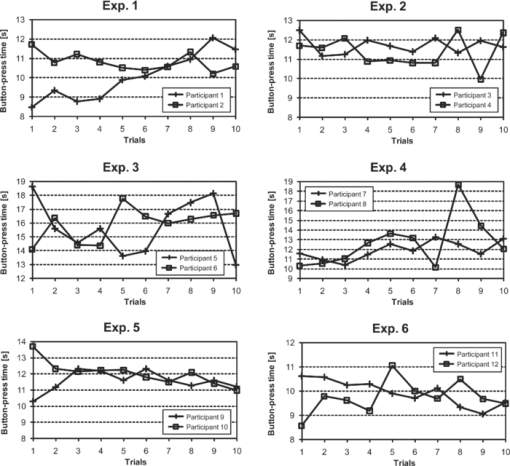
2.4.Measurement of Activation in mPFCIt has been reported that the depth of light penetration from the surface of the brain in adult humans is several centimeters,61 depending on the thickness of superficial tissue.62 Some researchers reported to have measured parts of relatively deep areas by noninvasive NIRS imaging. For example, NIRS imaging was used to measure the anterior-dorsal region of the medial prefrontal cortex (mPFC) regions of adults,63 the prefrontal region (which is a similar region to the mPFC region in adults) of four-month-old infants,64 and anterior orbitofrontal cortex regions of mothers and infants.26 Mitchell 19 reported the MNI coordinates of a part of the mPFC region that is related to social cognition as (X = 9 mm, Y = 54 mm, Z = 36 mm). This position is near the superficial layer of the brain and close to channel 11 in the WOT system (see Table 1). The superficial part of the mPFC region is therefore assumed to be within the optical paths of an NIRS imaging system with 30-mm source-detector spacing. 2.5.Experimental SetupFigure 3 illustrates the experimental setup used to simultaneously measure the brain activity two participants by using the WOT system. Two participants sat face-to-face across a table. Each pair of participants wore WOT probes connected to portable boxes for measurement control, signal processing, and wireless communication. The portable boxes were controlled via a wireless-LAN access point by a personal computer (PC) for a WOT controller. Fig. 3Experimental setup. (a) Face-to-face arrangement of two participants across a table. (b) System diagram of simultaneous measurement of two participants’ brain activity by using the WOT system. The two WOTs are controlled via wireless LAN by a PC, and the timing of auditory stimulation and marker input are controlled via a stimulation-presentation PC (SP-PC). The button-press time of each participant is recorded by the SP-PC. 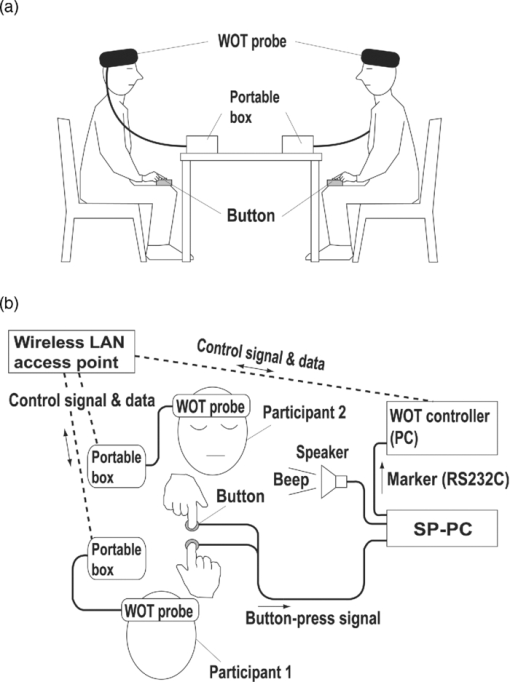 The stimulation-presentation PC (SP-PC) presented auditory cues and feedback sounds to the participants. The feedback sounds were presented a few seconds after he or she pressed the button (connected to the SP-PC) with different tones for each participant. When the auditory cue to start counting is given and when the first button is pressed by one of the participants in each pair (hereafter, first “button-press time”), the SP-PC sends a marker signal to the WOT controller and records the button-press times of each pair of participants. 2.6.Cooperative Button-Press TaskEach pair of participants performed the following cooperative button-press task while the changes in their cerebral blood volume were measured by the WOT system. Figure 4 shows the time sequence of this task. At the start of the task, the two participants closed their eyes and put their index finger on a button on a PC keyboard. In each trial, they were told to press their button after counting 10 s in their mind and were also told to adjust their button-press times to make them as synchronized as possible. The auditory cue to start the time counting was given by a 1000-Hz beep sound (duration 200 ms) emitted from a speaker. Fig. 4Time sequence of cooperative task. The sequence of a control task was the same for each trial (except the feedback sounds were not given). Δt represents the inter-time interval between button presses. 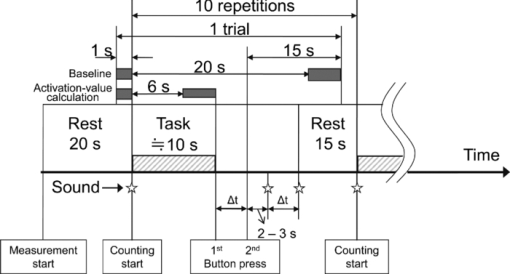 In the cooperative task, the “intertime interval” (namely, the time between the button-press timings of the two participants) was fed back to them by two short beep sounds (with two participant-dependent sound pitches) presented a few seconds after each button press. A 1600-Hz beep (50-ms duration) indicated the timing of one participant, and an 800-Hz beep (50-ms duration) indicated that of the other participant. The time delay between the button presses and the feedback sounds was between 2 and 3 s; it varied across the trials but was the same for both participants. Each participant thus knew the intertime interval between the button presses of the participants in each pair and who of them was faster after each trial. The time each button was pressed was recorded by the SP-PC. In the control task, no feedback sound was given to the participants; therefore, it was a simple time-production task as used in a previous NIRS study.65 Ten trials (with 15-s rest periods between trials) for each task were performed. The time resolution of the recorder for the button-press time was within 10 ms. The order of the two task conditions (i.e., cooperative and control) among the six pairs was counterbalanced because an unbalanced order could influence the analytical results. Six experiments (Exps. 1–6), including cooperative and control tasks, were conducted on six pairs of participants in total. To eliminate the effects of the brain function that were not the focus of this study and to control the experimental conditions, the two participants of each pair kept their eyes closed during the entire experiment. 2.7.Data AnalysisThe oxygenated (oxy-) and deoxygenated (deoxy-) hemoglobin changes were calculated by using the absorbance change of 754- and 830-nm light according to the modified Beer–Lambert law.34, 56 To reject any artifacts induced by the heartbeat or fast body movements, the oxy- and deoxy-Hb changes were processed with a 0.8-Hz low-pass filter and smoothed with a convolution of a Gaussian function with a full width at half maximum of 2 s. Subsequently, all the time-course data were separated into 10-trial data by using a task-onset marker in the data. As a result, each trial began 1 s before each auditory cue to start the counting, and ended 15 s after the second (the latter of two) button press (Fig. 4). To reduce systemic slow oscillations, the trial data were baseline corrected by linear-function fitting using the data set obtained during the baseline periods shown in Fig. 4. The baseline periods are the time periods from 1 s before the auditory cue (counting start) to the auditory cue and from 20 s after the auditory cue to 15 s after the button-press time of the participant who pressed his or her button later than his or her counterpart. The oxy-Hb changes of the control task (which were averaged over 10 trials) were subtracted from those of the cooperative task for each trial. Because the time period of each trial varies from trial to trial, the time period of each control-task trial was adjusted to that of each cooperative-task trial with task-onset timing in line. Because the timings of the button presses under the cooperative and control conditions commonly do not match, the data recorded after button presses cannot be simply compared in a time series. After the subtraction, the activation value (AV) for each channel was therefore calculated by using the data obtained during the rest and counting periods as Eq. 1a[TeX:] \documentclass[12pt]{minimal}\begin{document}\begin{equation} {\rm AV} = \left| {\frac{{\mu _{\rm c} - \mu _{\rm r} }}{{s\sqrt {{1 / {n_{\rm c} }} + {1 / {n_{\rm r} }}} }}} \right|, \end{equation}\end{document}Eq. 1b[TeX:] \documentclass[12pt]{minimal}\begin{document}\begin{equation} s=\sqrt{\frac{(n_c-1)V_c+(n_r-1)V_r}{n_c+n_r-2}}. \end{equation}\end{document}Figure 5 shows the “used” channels, “unused” channels, and “interpolated” channels in the WOT system. Linear-interpolated channels were generated by averaging oxy-Hb change of adjoining used channels. In other words, mean values of oxy-Hb change at each time point were calculated by using adjoining used channels for the interpolated channels. The number of adjoining used channels was varied from two to four in accordance with channel position (see Fig. 5). In total, 33 channels (including both the used and interpolated channels) were therefore analyzed. The effect of using the interpolated channels is to enhance the effective activated area of the prefrontal cortex and, consequently, make the covariance between AV maps higher even when the activated areas of two participants’ brains were located at different (but nearby) channel positions. Fig. 5Used, unused, and interpolated channels. In the figure “ch” indicates channel. In total, 33 channels (including both the used and interpolated channels) were analyzed. 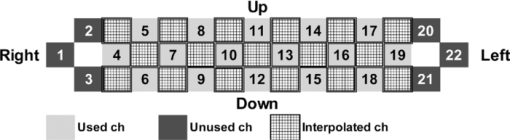 AVs for the used and interpolated channels were calculated by using the data set composed of the baseline and activation periods shown in Fig. 4; that is, 1 s before the start of counting was taken as the baseline period, and the time period from the 6-s point after the start of counting until the first button press was taken as the activation period. The AVs of the two participants in each trial are called AV1(i, c) (i = 1, 2, …, 10; c = 1, 2, …, 33) and AV2(i, c) (i = 1, 2, …, 10; c = 1, 2, …, 33), where i indicates trial number, and c indicates the analyzed channel number including interpolated channels. Covariance r(i) (i = 1, 2, …, 10) between AV1 and AV2 in each trial was calculated from Eqs. 2, 3a, 3b, and r(i) of each pair was normalized to zero-average and unit standard deviation among trials, Eq. 2[TeX:] \documentclass[12pt]{minimal}\begin{document}\begin{equation} r(i) = \sum\limits_{c = 1}^{33} {\phi _1 (i,c)\phi _2 (i,c)}, \end{equation}\end{document}Eq. 3a[TeX:] \documentclass[12pt]{minimal}\begin{document}\begin{equation} \phi _1 (i,c) = {\rm AV}_1 (i,c) - \frac{1}{{33}}\sum\limits_{c = 1}^{33} {{\rm AV}_1 (i,c)}, \end{equation}\end{document}Eq. 3b[TeX:] \documentclass[12pt]{minimal}\begin{document}\begin{equation} \phi _2 (i,c) = {\rm AV}_2 (i,c) - \frac{1}{{33}}\sum\limits_{c = 1}^{33} {{\rm AV}_2 (i,c)}. \end{equation}\end{document}The ten-trial data of all the pairs were divided into two groups: one included the trials in which the normalized covariances of the AV maps were positive (referred to hereafter as “high-covariance trials”), and the other included those in which the normalized covariances were negative (referred to hereafter as “low-covariance trials”). To investigate the relationship between the normalized covariance of the brain activations of two participants and the normalized intertime intervals between the two button presses of each pair, a Wilcoxon rank-sum tests using the intertime intervals of the two groups (i.e., the high- and low-covariance trials) was conducted. We confirmed in advance that at least one of the two groups did not have a normal distribution using both coefficient of skewness and coefficient of kurtosis. The covariances and intertime intervals for the first trial in the cooperative task were not used because it was assumed that neither participant would be able to anticipate the button-press time of the other in the first trial; that is, the participants would not be able to cooperate with one another before getting feedback. Data processing and statistical analyses of the NIRS signals were performed using Matlab Version 7.4 (The MathWorks, Inc., Natick, MA). 3.Results3.1.Behavioral ResultsFigure 6 plots the behavioral results, namely, button-press time, for each trial in the cooperative task performed in Exps. 1–6. The horizontal axis represents trial number, and the vertical axis represents button-press time. In the eighth trial of Exp. 4, a behavioral error occurs in the results for participant 8, who said after the experiment that she missed pressing the button in the instructed way by mistake. The result for the eighth trial of Exp. 4 was therefore not used in the following analysis. Figure 7 plots the mean intertime intervals between button presses among all the pairs for each trial. The error bars represents standard deviations. The intertime interval of the first trial is the longest of all the trials and greatly decreases at the second trial. From the second to tenth trials, the mean intertime intervals do not greatly change. Table 2 lists the means and standard deviations of the button-press times of all the participants participating in the control task. In the control task, because there was no explicit interaction between any of the paired participants and no feedback in each trial, it was expected that the participants would behave in the same way in all trials. Only the means and standard deviations of the button-press times were therefore calculated. Table 2Mean and standard deviation (SD) of button-press time during a control task.
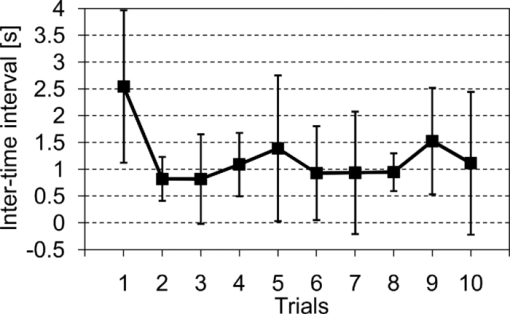
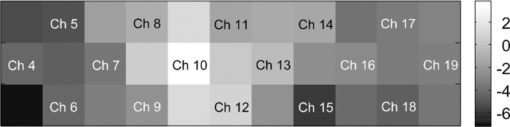
In Exps. 1, 3, and 6, the cooperative task was performed first, after which the control task was performed. In contrast, in Exps. 2, 4, and 5, the order of the tasks was opposite. The intertime intervals between the button presses of each pair performing the control task under both task-order conditions were not significantly different (Student's t-test, p > 0.2). It is thus considered that the behavioral results for the control task were not influenced by the order of the two tasks. 3.2.NIRS DataFigure 8 shows the trial-averaged oxy-Hb time-course data for participant 1 (Exp. 1), measured with a WOT system, as representative data. The horizontal axis represents time, and the vertical axis represents oxy-Hb change (ΔCL). The start time of counting is t = 1 s. The trial-averaged oxy-Hb change obtained in the control task was subtracted from the data obtained at each time point for oxy-Hb in the cooperative task. The cooperative-minus-control data was thus obtained. The trial-averaged control-task data were calculated by using data from all the trials because there was no feedback sound in the control task. The cooperative-minus-control data of trials 2–10 were averaged to provide the data plotted in Fig. 8. Fig. 8Trial-averaged oxy-Hb time-course data for participant 1 (Exp. 1) measured with a WOT system. The data of all channels (including both the used and interpolated channels) are shown. The horizontal axis represents time, and the vertical axis represents the oxy-Hb change. The start time of counting is t = 1 s. 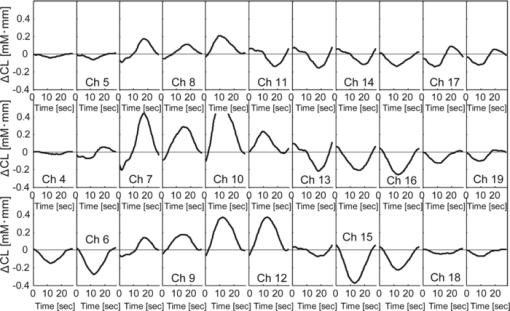 According to Fig. 8, during the counting period, oxy-Hb change in the middle and right-side prefrontal area increases, whereas that at both ends of the prefrontal area decreases. The spatial patterns of the changes in oxy-Hb change vary according to the participant. In the case of many of the participants, however, it is very common for the middle frontal area to be activated. Figure 9 shows a gray-scale map of AVs (at each analyzed channel) averaged from data of all the participants in trials 2–10. In Fig. 9, the color density of each element in the map represents the AV in the gray-scale bar. It is clear that the AVs in the middle frontal area are higher on average. 3.3.Relationship between Covariance of Two AV Maps and Intertime Intervals between Button PressesThe trials, except for the first trials, were divided into two groups: one including the trials in which the normalized covariance of the AV maps had a positive value (high-covariance trials), and one including those with a negative value (low-covariance trials). To compare the performance of the two groups, a Wilcoxon rank-sum test using the inter-time intervals of the two groups was conducted. Figure 10 plots the mean and standard error of the inter-time intervals of the low- and high-covariance groups; the numbers of data of those groups are 21 and 32, respectively. The error bars represent the standard errors of normalized intertime interval data. It was found that the intertime intervals of the high-covariance trials are significantly shorter than those of the low-covariance trials (p < 0.01). When only the control-task data were used for calculating covariances, no relationship between normalized covariances and intertime intervals was obtained (Wilcoxon rank-sum test, p > 0.4). The number of combinations for selecting two people out of the 12 participants is 12 C 2 = 66 patterns in total. The number of combinations of paired participants in this study is six patterns; thus, the number of combinations of unpaired participants is 60 patterns in total. To confirm the pair specificity, the spatiotemporal covariances of the 60 unpaired participants were analyzed in the same way. This analysis found that there was not a significant difference between the intertime intervals of the higher- and lower-covariance groups (Wilcoxon rank-sum test, p > 0.6). These results show that when the spatiotemporal brain-activity patterns of the two participants were more synchronous, the time interval between their button-press times became shorter. 4.Discussion4.1.Adjusted Button-Press Time in Cooperative TaskAccording to the behavioral results (Fig. 6), during the cooperative task, each participant seems to have adjusted his or her button-press time in each trial. In the ninth trial of Exp. 1, the fourth and ninth trials of Exp. 2, fifth and tenth trials of Exp. 3, seventh trial of Exp. 4, and eighth trial of Exp. 6, however, the intertime interval increased, possibly because of overadjusting. The effect of overadjusting is that the order of the button presses of the participants is inverted and the intertime interval becomes larger than the last one. The overadjusting trials are in the second half of the ten trials, except for the fourth trial of Exp. 2. That trend might reflect a break in concentration or the limited stability of the 10-s time-production task. 4.2.Relationship between Brain-Activation Patterns and Task PerformanceThe relationship between the synchronized pattern of the activated area during the counting period and cooperative-task performance was investigated. To investigate the brain activities under a cooperative condition, the data obtained during counting period before button presses was used. It was found that when the spatial brain-activity patterns during the counting period of the two participants were more synchronous, the intertime interval between their button-press times became shorter. This result cannot be contaminated by a motion artifact because the data recorded after button presses was not used in the analysis. The possible reasons to explain this result are presented in the following. It has been reported that when a human performs a branching task, which involves the process of integrating working memory with attention-resource allocation, the frontopolar prefrontal cortex is activated.66 The cooperative task used in this study possibly requires two simultaneous mental processes: time counting and thinking about the timing of the button press in order to adjust it to that of the other person. It is therefore possible that the frontopolar prefrontal cortices of the two participants’ brains during this cooperative task might be synchronously activated because they perform a kind of branching task at the same time. The difference between the cooperative task and control task consisted of whether or not feedback was provided to the participants. It is possible that providing the participants with feedback made them think about each other. During the cooperative task, each participant might consider the adjusting precision and feedback gain (slight or drastic adjustment, which may be dependent on individuals) of another participant in order to prevent overadjusting. Even if these processes might not be actual cooperation or communication, they are important in inferring thinking or future activities of others. It has been reported that when people “mentalize,” namely, think about other people's intentions, the mPFC region is activated.15, 67, 68, 69 If adjusting the timing to other people during the cooperative task can be considered a kind of mentalizing process, then the AV map shown in Fig. 9 might reflect the activation of the superficial mPFC region. 4.3.Effects of Motor Planning and Intrinsic FluctuationIt has been reported that the lateral prefrontal cortex plays a key role in preparing or planning an intended movement or action.70, 71, 72 The cooperative and control tasks used in this study include a common motor-planning process, that is, planning to press a button at certain time. The effect of the motor-planning function in regard to the difference between the spatiotemporal activity patterns during the cooperative and control tasks was therefore considered negligible. Another possibility accounting for the high cooperative-task performances (i.e., short intertime intervals) with high covariances between the two participants’ brains might be the influence of intrinsic fluctuations within cortical systems.73 However, because the covariances of the AV maps obtained in the cooperative and control tasks are significantly different in terms of the relationship with the task performance, it is reasonable to assume that the results reflect the influence of the cooperative behavior of the two participants. 4.4.LimitationsIn each experiment, neither the degree of interhuman relations between any two participants nor their personality characteristics regarding adaptability or cooperativeness was controlled. Moreover, all of the participants recruited in this study already knew each other. The cooperative-task performance might change in accordance with factors that are affected by interhuman relationships, such as whether the participants know each other and the personalities of the participants. These factors should be investigated further. In addition, the following four limitations exist, in principle, when the oxy- and deoxy-Hb signals obtained from a participant are compared to those from another participant. The first one is the difference in the structure (thickness of scalp, skull, and gray/white matter) of the head,74 which causes a difference in penetration depth and path length of the near-infrared light.75 If the structural details of the head were measured by x-ray computed tomography or MRI scanner, the Hb-change signal could be more precisely quantified by using the simulated optical path length.76 The second limitation is the variation of probe positions. Figure 2 shows that the probe positions for each participant vary slightly. To reduce the effects of difference in the activated positions when the AV maps of two participants are compared, interpolated channels (in addition to the measurement channels) were used. It was thus possible to robustly calculate the covariance of the AV maps and analyze the relation between the two brains of a participant pair. The standard deviations of the measurement positions on the participants who participated in this study are within 10 mm, which is regarded as being sufficiently small for an OT study using a 30-mm source-detector distance. If the optodes were placed on the scalp on the basis of the probabilistic registration59 using three-dimensional position data, the accuracy of interparticipant comparison would be better. The third limitation is a diverse brain-activation response induced by a participant-dependent hemodynamic response. An analytical method that takes account of the phase difference between NIRS signals by using a Hilbert transform77 could improve the results presented here. 5.ConclusionTo investigate the relationship between mutually interactive brain-activation signals and cooperative-task performance, six pairs of participants took part in trials in which each pair of participants were instructed to count 10 s in their mind after hearing an auditory cue and then simultaneously press a button. The obtained brain-activity data of two participants were analyzed by calculating the covariance of AV maps. It was found that the synchronized activation patterns of the brains of the two participants of each pair during counting before the button presses is associated with the intertime interval between the two button presses of each pair when the participants tried to adjust their button-press timings to each other. This result cannot be explained in terms of a motion artifact; instead, it suggests that there is a relationship between synchronization of the brain activities and cooperative-task performance. It is concluded that the spatiotemporal coherence of the NIRS signals between the paired participants is associated with their performance of a cooperative activity. The effect of interhuman relationships and personality on the NIRS signals should be further investigated. It was demonstrated that NIRS hyperscanning can be used to evaluate human interaction. Human-brain functions related to social skills should thus be progressively studied by taking an approach using coinstantaneous spatiotemporal activation patterns of two or more participants. A WOT system, which measures multiple brains in a shared space in natural manners, will contribute to a new research field of interbrain interaction in everyday life. AcknowledgmentsWe thank Dr. Atsushi Maki, Dr. Takusige Katura, Dr. Akiko Obata, Dr. Hirokazu Tanaka, and Ryuta Aoki for their helpful comments on our research. We also thank Dr. Kazuo Saitoh for contributing to our fruitful discussions and Dr. Haruo Takeda for his overall support. ReferencesS. Baron-Cohen, A. M. Leslie, and
U. Frith,
“Does the autistic child have a ‘theory of mind,”
Cognition, 21
(1), 37
–46
(1985). https://doi.org/10.1016/0010-0277(85)90022-8 Google Scholar
E. Fombonne,
“The Prevalence of Autism,”
JAMA, 289
(1), 87
–89
(2003). https://doi.org/10.1001/jama.289.1.87 Google Scholar
S. Baron-Cohen, H. A. Ring, S. Wheelwright, E. T. Bullmore, M. J. Brammer, A. Simmons, and
S. C. Williams,
“Social intelligence in the normal and autistic brain: an fMRI study,”
Eur. J. Neurosci., 11
(6), 1891
–1898
(1999). https://doi.org/10.1046/j.1460-9568.1999.00621.x Google Scholar
R. Hari and
M. V. Kujala,
“Brain basis of human social interaction: from concepts to brain imaging,”
Physiol. Rev., 89
(2), 453
–479
(2009). https://doi.org/10.1152/physrev.00041.2007 Google Scholar
J. Blair, A. A. Marsh, E. Finger, K. S. Blair, and
J. Luo,
“Neuro-cognitive systems involved in morality,”
Philos. Explor., 9
(1), 13
–27
(2006). https://doi.org/10.1080/13869790500492359 Google Scholar
R. Aoki, T. Funane, and
H. Koizumi,
“Brain science of ethics: present status and the future,”
Mind Brain Educ., 4
(4), 188
–195
(2010). https://doi.org/10.1111/j.1751-228X.2010.01098.x Google Scholar
M. S. Gazzaniga, The Social Brain: Discovering the Networks of the Mind, Basic Books, New York
(1985). Google Scholar
L. Brothers,
“The social brain: a project for integrating primate behavior and neurophysiology in a new domain,”
Concepts Neurosci., 1 27
–51
(1990). Google Scholar
C. D. Frith,
“The social brain,”
Philos. Trans. R. Soc. Lond. B, 362
(1480), 671
–678
(2007). https://doi.org/10.1098/rstb.2006.2003 Google Scholar
N. Fujii, S. Hihara, and
A. Iriki,
“Dynamic social adaptation of motion-related neurons in primate parietal cortex,”
PLoS ONE, 2
(4), e397
(2007). https://doi.org/10.1371/journal.pone.0000397 Google Scholar
C. H. Cheung, H. J. Rutherford, L. C. Mayes, and
J. C. McPartland,
“Neural responses to faces reflect social personality traits,”
Soc. Neurosci., 5
(4), 351
–359
(2010). https://doi.org/10.1080/17470911003597377 Google Scholar
L. Gao, J. Xu, B. Zhang, L. Zhao, A. Harel, and
S. Bentin,
“Aging effects on early-stage face perception: an ERP study,”
Psychophysiology, 46
(5), 970
–983
(2009). https://doi.org/10.1111/j.1469-8986.2009.00853.x Google Scholar
V. Goel, J. Grafman, N. Sadato, and
M. Hallett,
“Modeling other minds,”
Neuroreport, 6
(13), 1741
–1746
(1995). https://doi.org/10.1097/00001756-199509000-00009 Google Scholar
P. C. Fletcher, F. Happé, U. Frith, S. C. Baker, R. J. Dolan, R. S. J. Frackowiak, and
C. D. Frith,
“Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension,”
Cognition, 57
(2), 109
–128
(1995). https://doi.org/10.1016/0010-0277(95)00692-R Google Scholar
H. L. Gallagher, A. I. Jack, A. Roepstorff, and
C. D. Frith,
“Imaging the intentional stance in a competitive game,”
Neuroimage, 16
(3 Pt 1), 814
–821
(2002). https://doi.org/10.1006/nimg.2002.1117 Google Scholar
S. Baron-Cohen, H. Ring, J. Moriarty, B. Schmitz, D. Costa, and
P. Ell,
“Recognition of mental state terms: clinical findings in children with autism and a functional neuroimaging study of normal adults,”
Br. J. Psychiatry, 165
(5), 640
–649
(1994). https://doi.org/10.1192/bjp.165.5.640 Google Scholar
H. Fukui, T. Murai, J. Shinozaki, T. Aso, H. Fukuyama, T. Hayashi, and
T. Hanakawa,
“The neural basis of social tactics: an fMRI study,”
Neuroimage, 32
(2), 913
–920
(2006). https://doi.org/10.1016/j.neuroimage.2006.03.039 Google Scholar
K. McCabe, D. Houser, L. Ryan, V. Smith, and
T. Trouard,
“A functional imaging study of cooperation in two-person reciprocal exchange,”
Proc. Natl. Acad. Sci. USA, 98
(20), 11832
–11835
(2001). https://doi.org/10.1073/pnas.211415698 Google Scholar
J. P. Mitchell, M. R. Banaji, and
C. N. Macrae,
“General and specific contributions of the medial prefrontal cortex to knowledge about mental states,”
Neuroimage, 28
(4), 757
–762
(2005). https://doi.org/10.1016/j.neuroimage.2005.03.011 Google Scholar
M. B. Schippers, A. Roebroeck, R. Renken, L. Nanetti, and
C. Keysers,
“Mapping the information flow from one brain to another during gestural communication,”
Proc. Natl. Acad. Sci. USA, 107
(20), 9388
–9393
(2010). https://doi.org/10.1073/pnas.1001791107 Google Scholar
G. J. Stephens, L. J. Silbert, and
U. Hasson,
“Speaker-listener neural coupling underlies successful communication,”
Proc. Natl. Acad. Sci. USA, 107
(32), 14425
–14430
(2010). https://doi.org/10.1073/pnas.1008662107 Google Scholar
S. Lloyd-Fox, A. Blasi, A. Volein, N. Everdell, C. E. Elwell, and
M. H. Johnson,
“Social perception in infancy: a near infrared spectroscopy study,”
Child Dev., 80
(4), 986
–999
(2009). https://doi.org/10.1111/j.1467-8624.2009.01312.x Google Scholar
T. Grossmann and
M. H. Johnson,
“Selective prefrontal cortex responses to joint attention in early infancy,”
Biol. Lett., 6
(4), 540
–543
(2010). https://doi.org/10.1098/rsbl.2009.1069 Google Scholar
M. Suda, Y. Takei, Y. Aoyama, K. Narita, T. Sato, M. Fukuda, and
M. Mikuni,
“Frontopolar activation during face-to-face conversation: an in situ study using near-infrared spectroscopy,”
Neuropsychologia, 48
(2), 441
–447
(2010). https://doi.org/10.1016/j.neuropsychologia.2009.09.036 Google Scholar
T. Grossmann, R. Oberecker, S. P. Koch, and
A. D. Friederici,
“The developmental origins of voice processing in the human brain,”
Neuron, 65
(6), 852
–858
(2010). https://doi.org/10.1016/j.neuron.2010.03.001 Google Scholar
Y. Minagawa-Kawai, S. Matsuoka, I. Dan, N. Naoi, K. Nakamura, and
S. Kojima,
“Prefrontal activation associated with social attachment: facial-emotion recognition in mothers and infants,”
Cereb. Cortex, 19
(2), 284
–292
(2009). https://doi.org/10.1093/cercor/bhn081 Google Scholar
H. Atsumori, M. Kiguchi, A. Obata, H. Sato, T. Katura, K. Utsugi, T. Funane, and
A. Maki,
“Development of a multi-channel, portable optical topography system,”
3362
–3364
(2007). Google Scholar
H. Atsumori, M. Kiguchi, A. Obata, H. Sato, T. Katura, T. Funane, and
A. Maki,
“Development of wearable optical topography system for mapping the prefrontal cortex activation,”
Rev. Sci. Instrum., 80
(4), 043704
(2009). https://doi.org/10.1063/1.3115207 Google Scholar
H. Atsumori, M. Kiguchi, T. Katura, T. Funane, A. Obata, H. Sato, T. Manaka, M. Iwamoto, A. Maki, H. Koizumi, and
K. Kubota,
“Noninvasive imaging of prefrontal activation during attention-demanding tasks performed while walking using a wearable optical topography system,”
J. Biomed. Opt., 15
(4), 046002
(2010). https://doi.org/10.1117/1.3462996 Google Scholar
F. F. Jöbsis,
“Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters,”
Science, 198
(4323), 1264
–1267
(1977). https://doi.org/10.1126/science.929199 Google Scholar
B. Chance, Z. Zhuang, C. UnAh, C. Alter, and
L. Lipton,
“Cognition-activated low-frequency modulation of light absorption in human brain,”
Proc. Natl. Acad. Sci. USA., 90
(8), 3770
–3774
(1993). https://doi.org/10.1073/pnas.90.8.3770 Google Scholar
Y. Hoshi and
M. Tamura,
“Detection of dynamic changes in cerebral oxygenation coupled to neuronal function during mental work in man,”
Neurosci. Lett., 150
(1), 5
–8
(1993). https://doi.org/10.1016/0304-3940(93)90094-2 Google Scholar
A. Villringer, J. Planck, C. Hock, L. Schleinkofer, and
U. Dirnagl,
“Near infrared spectroscopy (NIRS): a new tool to study hemodynamic changes during activation of brain function in human adults,”
Neurosci. Lett., 154
(1-2), 101
–104
(1993). https://doi.org/10.1016/0304-3940(93)90181-J Google Scholar
A. Maki, Y. Yamashita, Y. Ito, E. Watanabe, Y. Mayanagi, and
H. Koizumi,
“Spatial and temporal analysis of human motor activity using noninvasive NIR topography,”
Med. Phys., 22
(12), 1997
–2005
(1995). https://doi.org/10.1118/1.597496 Google Scholar
H. Koizumi, Y. Yamashita, A. Maki, T. Yamamoto, Y. Ito, H. Itagaki, and
R. P. Kennan,
“Higher-order brain function analysis by trans-cranial dynamic near-infrared spectroscopy imaging,”
J. Biomed. Opt., 4
(4), 403
–413
(1999). https://doi.org/10.1117/1.429959 Google Scholar
E. Watanabe, A. Maki, F. Kawaguchi, K. Takashiro, Y. Yamashita, H. Koizumi, and
Y. Mayanagi,
“Non-invasive assessment of language dominance with near-infrared spectroscopic mapping,”
Neurosci. Lett., 256
(1), 49
–52
(1998). https://doi.org/10.1016/S0304-3940(98)00754-X Google Scholar
H. Sato, T. Takeuchi, and
K. L. Sakai,
“Temporal cortex activation during speech recognition: an optical topography study,”
Cognition, 73
(3), B55
–66
(1999). https://doi.org/10.1016/S0010-0277(99)00060-8 Google Scholar
Y. Minagawa-Kawai, K. Mori, I. Furuya, R. Hayashi, and
Y. Sato,
“Assessing cerebral representations of short and long vowel categories by NIRS,”
Neuroreport, 13
(5), 581
–584
(2002). https://doi.org/10.1097/00001756-200204160-00009 Google Scholar
A. Obata, K. Morimoto, H. Sato, A. Maki, and
H. Koizumi,
“Acute effects of alcohol on hemodynamic changes during visual stimulation assessed using 24-channel near-infrared spectroscopy,”
Psychiatry Res., 123
(2), 145
–152
(2003). https://doi.org/10.1016/S0925-4927(03)00063-5 Google Scholar
T. Suto, M. Fukuda, M. Ito, T. Uehara, and
M. Mikuni,
“Multichannel near-infrared spectroscopy in depression and schizophrenia: cognitive brain activation study,”
Biol. Psychiatry, 55
(5), 501
–511
(2004). https://doi.org/10.1016/j.biopsych.2003.09.008 Google Scholar
G. Taga, Y. Konishi, A. Maki, T. Tachibana, M. Fujiwara, and
H. Koizumi,
“Spontaneous oscillation of oxy- and deoxy- hemoglobin changes with a phase difference throughout the occipital cortex of newborn infants observed using non-invasive optical topography,”
Neurosci. Lett., 282
(1–2), 101
–104
(2000). https://doi.org/10.1016/S0304-3940(00)00874-0 Google Scholar
G. Taga, K. Asakawa, A. Maki, Y. Konishi, and
H. Koizumi,
“Brain imaging in awake infants by near-infrared optical topography,”
Proc. Natl. Acad. Sci. USA., 100
(19), 10722
–10727
(2003). https://doi.org/10.1073/pnas.1932552100 Google Scholar
M. Pena, A. Maki, D. Kovacic, G. Dehaene-Lambertz, H. Koizumi, F. Bouquet, and
J. Mehler,
“Sounds and silence: an optical topography study of language recognition at birth,”
Proc. Natl. Acad. Sci. USA., 100
(20), 11702
–11705
(2003). https://doi.org/10.1073/pnas.1934290100 Google Scholar
T. Nakano, H. Watanabe, F. Homae, and
G. Taga,
“Prefrontal cortical involvement in young infants’ analysis of novelty,”
Cereb. Cortex, 19
(2), 455
–463
(2009). https://doi.org/10.1093/cercor/bhn096 Google Scholar
F. Homae, H. Watanabe, T. Otobe, T. Nakano, T. Go, Y. Konishi, and
G. Taga,
“Development of global cortical networks in early infancy,”
J. Neurosci., 30
(14), 4877
–4882
(2010). https://doi.org/10.1523/JNEUROSCI.5618-09.2010 Google Scholar
Y. Ito, R. P. Kennan, E. Watanabe, and
H. Koizumi,
“Assessment of heating effects in skin during continuous wave near infrared spectroscopy,”
J. Biomed. Opt., 5
(4), 383
–390
(2000). https://doi.org/10.1117/1.1287730 Google Scholar
M. Kiguchi, N. Ichikawa, H. Atsumori, F. Kawaguchi, H. Sato, A. Maki, and
H. Koizumi,
“Comparison of light intensity on the brain surface due to laser exposure during optical topography and solar irradiation,”
J. Biomed. Opt., 12
(6), 062108
(2007). https://doi.org/10.1117/1.2804152 Google Scholar
F. Babiloni, L. Astolfi, F. Cincotti, D. Mattia, A. Tocci, A. Tarantino, M. Marciani, S. Salinari, S. Gao, A. Colosimo, and
F. De Vico Fallani,
“Cortical activity and connectivity of human brain during the prisoner's dilemma: an EEG hyperscanning study,”
4953
–4956
(2007). Google Scholar
E. Tognoli, J. Lagarde, G. C. DeGuzman, and
J. A. Kelso,
“The phi complex as a neuromarker of human social coordination,”
Proc. Natl. Acad. Sci. USA., 104
(19), 8190
–8195
(2007). https://doi.org/10.1073/pnas.0611453104 Google Scholar
G. Dumas, J. Nadel, R. Soussignan, J. Martinerie, and
L. Garnero,
“Inter-brain synchronization during social interaction,”
PLoS One, 5
(8), e12166
(2010). https://doi.org/10.1371/journal.pone.0012166 Google Scholar
F. De Vico Fallani, V. Nicosia, R. Sinatra, L. Astolfi, F. Cincotti, D. Mattia, C. Wilke, A. Doud, V. Latora, B. He, and
F. Babiloni,
“Defecting or not defecting: how to “read” human behavior during cooperative games by EEG measurements,”
PLoS One, 5
(12), e14187
(2010). https://doi.org/10.1371/journal.pone.0014187 Google Scholar
R. N. Aslin and
J. Mehler,
“Near-infrared spectroscopy for functional studies of brain activity in human infants: promise, prospects, and challenges,”
J. Biomed. Opt., 10
(1), 11009
(2005). https://doi.org/10.1117/1.1854672 Google Scholar
R. Sitaram, H. Zhang, C. Guan, M. Thulasidas, Y. Hoshi, A. Ishikawa, K. Shimizu, and
N. Birbaumer,
“Temporal classification of multichannel near-infrared spectroscopy signals of motor imagery for developing a brain-computer interface,”
Neuroimage, 34
(4), 1416
–1427
(2007). https://doi.org/10.1016/j.neuroimage.2006.11.005 Google Scholar
P. R. Montague, G. S. Berns, J. D. Cohen, S. M. McClure, G. Pagnoni, M. Dhamala, M. C. Wiest, I. Karpov, R. D. King, N. Apple, and
R. E. Fisher,
“Hyperscanning: Simultaneous fMRI during Linked Social Interactions,”
NeuroImage, 16
(4), 1159
–1164
(2002). https://doi.org/10.1006/nimg.2002.1150 Google Scholar
D. N. Saito, H. C. Tanabe, K. Izuma, M. J. Hayashi, Y. Morito, H. Komeda, H. Uchiyama, H. Kosaka, H. Okazawa, Y. Fujibayashi, and
N. Sadato,
“Stay tuned”: inter-individual neural synchronization during mutual gaze and joint attention,”
Front. Integr. Neurosci., 4
(127), 1
–12
(2010). https://doi.org/10.3389/fnint.2010.00127 Google Scholar
D. T. Delpy, M. Cope, P. van der Zee, S. Arridge, S. Wray, and
J. Wyatt,
“Estimation of optical pathlength through tissue from direct time of flight measurement,”
Phys. Med. Biol., 33
(12), 1433
–1442
(1988). https://doi.org/10.1088/0031-9155/33/12/008 Google Scholar
H. H. Jasper,
“The ten twenty electrode system of the International Federation,”
Electroencephalogr. Clin. Neurophysiol., 10 371
–375
(1958). Google Scholar
M. Okamoto, H. Dan, K. Sakamoto, K. Takeo, K. Shimizu, S. Kohno, I. Oda, S. Isobe, T. Suzuki, K. Kohyama, and
I. Dan,
“Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10-20 system oriented for transcranial functional brain mapping,”
Neuroimage, 21
(1), 99
–111
(2004). https://doi.org/10.1016/j.neuroimage.2003.08.026 Google Scholar
A. K. Singh, M. Okamoto, H. Dan, V. Jurcak, and
I. Dan,
“Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI,”
Neuroimage, 27
(4), 842
–851
(2005). https://doi.org/10.1016/j.neuroimage.2005.05.019 Google Scholar
D. Tsuzuki, V. Jurcak, A. K. Singh, M. Okamoto, E. Watanabe, and
I. Dan,
“Virtual spatial registration of stand-alone fNIRS data to MNI space,”
Neuroimage, 34
(4), 1506
–1518
(2007). https://doi.org/10.1016/j.neuroimage.2006.10.043 Google Scholar
Y. Fukui, Y. Ajichi, and
E. Okada,
“Monte Carlo prediction of near-infrared light propagation in realistic adult and neonatal head models,”
Appl. Opt., 42
(16), 2881
–2887
(2003). https://doi.org/10.1364/AO.42.002881 Google Scholar
E. Okada and
D. T. Delpy,
“Near-Infrared Light Propagation in an Adult Head Model. II. Effect of Superficial Tissue Thickness on the Sensitivity of the Near-Infrared Spectroscopy Signal,”
Appl. Opt., 42
(16), 2915
–2922
(2003). https://doi.org/10.1364/AO.42.002915 Google Scholar
H. Yasui, K. Takamoto, E. Hori, S. Urakawa, Y. Nagashima, Y. Yada, T. Ono, and
H. Nishijo,
“Significant correlation between autonomic nervous activity and cerebral hemodynamics during thermotherapy on the neck,”
Auton. Neurosci., 156
(1-2), 96
–103
(2010). https://doi.org/10.1016/j.autneu.2010.03.011 Google Scholar
T. Grossmann, M. H. Johnson, S. Lloyd-Fox, A. Blasi, F. Deligianni, C. Elwell, and
G. Csibra,
“Early cortical specialization for face-to-face communication in human infants,”
Proc. Biol. Sci., 275
(1653), 2803
–2811
(2008). https://doi.org/10.1098/rspb.2008.0986 Google Scholar
T. Soshi, K. Kuriyama, S. Aritake, M. Enomoto, A. Hida, M. Tamura, Y. Kim, and
K. Mishima,
“Sleep deprivation influences diurnal variation of human time perception with prefrontal activity change: a functional near-infrared spectroscopy study,”
PLoS One, 5
(1), e8395
(2010). https://doi.org/10.1371/journal.pone.0008395 Google Scholar
E. Koechlin, G. Basso, P. Pietrini, S. Panzer, and
J. Grafman,
“The role of the anterior prefrontal cortex in human cognition,”
Nature, 399
(6732), 148
–151
(1999). https://doi.org/10.1038/20178 Google Scholar
R. Adolphs,
“Cognitive neuroscience of human social behaviour,”
Nat. Rev. Neurosci., 4
(3), 165
–178
(2003). https://doi.org/10.1038/nrn1056 Google Scholar
U. Frith and
C. D. Frith,
“Development and neurophysiology of mentalizing,”
Philos. Trans. R. Soc. Lond. B Biol. Sci., 358
(1431), 459
–473
(2003). https://doi.org/10.1098/rstb.2002.1218 Google Scholar
D. M. Amodio and
C. D. Frith,
“Meeting of minds: the medial frontal cortex and social cognition,”
Nat. Rev. Neurosci., 7
(4), 268
–277
(2006). https://doi.org/10.1038/nrn1884 Google Scholar
E. Hoshi and
J. Tanji,
“Area-selective neuronal activity in the dorsolateral prefrontal cortex for information retrieval and action planning,”
J. Neurophysiol., 91
(6), 2707
–2722
(2004). https://doi.org/10.1152/jn.00904.2003 Google Scholar
H. Mushiake, N. Saito, K. Sakamoto, Y. Itoyama, and
J. Tanji,
“Activity in the Lateral Prefrontal Cortex Reflects Multiple Steps of Future Events in Action Plans,”
Neuron, 50
(4), 631
–641
(2006). https://doi.org/10.1016/j.neuron.2006.03.045 Google Scholar
J. Tanji and
E. Hoshi,
“Role of the lateral prefrontal cortex in executive behavioral control,”
Physiol. Rev., 88
(1), 37
–57
(2008). https://doi.org/10.1152/physrev.00014.2007 Google Scholar
M. D. Fox, A. Z. Snyder, J. L. Vincent, and
M. E. Raichle,
“Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior,”
Neuron, 56
(1), 171
–184
(2007). https://doi.org/10.1016/j.neuron.2007.08.023 Google Scholar
A. T. Uthman, N. H. Al-Rawi, A. S. Al-Naaimi, A. S. Tawfeeq, and
E. H. Suhail,
“Evaluation of frontal sinus and skull measurements using spiral CT scanning: an aid in unknown person identification,”
Forensic Sci. Int., 197
(1), 124 e121–127
(2010). https://doi.org/10.1016/j.forsciint.2009.12.064 Google Scholar
A. Duncan, J. H. Meek, M. Clemence, C. E. Elwell, L. Tyszczuk, M. Cope, and
D. Delpy,
“Optical pathlength measurements on adult head, calf and forearm and the head of the newborn infant using phase resolved optical spectroscopy,”
Phys. Med. Biol., 40
(2), 295
–304
(1995). https://doi.org/10.1088/0031-9155/40/2/007 Google Scholar
Y. Hoshi, M. Shimada, C. Sato, and
Y. Iguchi,
“Reevaluation of near-infrared light propagation in the adult human head: implications for functional near-infrared spectroscopy,”
J. Biomed. Opt., 10
(6), 064032
(2005). https://doi.org/10.1117/1.2142325 Google Scholar
V. Toronov, M. A. Franceschini, M. Filiaci, S. Fantini, M. Wolf, A. Michalos, and
E. Gratton,
“Near-infrared study of fluctuations in cerebral hemodynamics during rest and motor stimulation: temporal analysis and spatial mapping,”
Med. Phys., 27
(4), 801
–815
(2000). https://doi.org/10.1118/1.598943 Google Scholar
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||