|
|
1.IntroductionWith microfluidic/nanofluidic technology it is possible to operate on microscale or nanoscale liquids for controling or sensing purposes. With the microfabrication techniques initially rooted from the microelectromechanical systems (MEMS) technology, this field will gradually develop into a discipline itself.1, 2, 3 The fundamental techniques for microfluidic or optofluidic device fabrication originate from the semiconductor industry and are based on silicon or glass materials. With the development of polymer-based fabrication techniques, polymer-based microfluidic devices become more prevalent with the advantages of being economic and easy to fabricate, and having material versatility and good system compatibility. The early designs of the microfluidic controling systems were mostly monolithic miniaturized components such as microvalves, micropumps, and micromixers.4, 5, 6 With further development, whole microfluidic systems were realized, which were made up of multiple elements to achieve certain functions. These parts comprise either of the monolithic microfluidic components7 or of the microfluidic components with the external components/devices.8 Designs of those microfluidic devices, such as geometries and scales, have been modified and improved according to the applications for better performance. 9, 10, 11, 12, 13, 14, 15, 16 Further development requires more components to be coupled with the microfluidic system for increased system functionality. Optical components seem ideal, with the benefits of noncontact, fast response, compactness, high sensitivity, multiplex operation possibility, and so on. Self-contained microfluidic devices have generated impact in the point-of-care and global health.17, 18 However, in order to achieve real self-contained miniature devices, it is ultimately required to have highly integrated systems. This means lowering or even eradicating the dependence on macro, bulky, external devices to perform control, detection, or analysis functions, by the development and integration of the necessary functional microcomponents. Examples recently demonstrated are the implementation of lensless microscopes of sub-pixel resolving ability on microfluidic platforms for on-chip imaging or diagnosis.12, 13, 19 The term optofluidics was coined in 2003 at the California Institute of Technology in Pasadena to describe systems that combine optics and microfluidics/nanofluidics (or to say, optical microfluidics).20, 21 The interaction between light and fluid provides possibilities of both versatile microsystems and a wide spectrum of applications.9, 22, 23, 24, 25, 26 In the past decade, there has been a growing interest and development of optical microfluidics (optofluidics) and an explosion of publications in the field. Actually, we can classify all microfluidic devices to either controlers or sensors. Here, we would like to emphasis on the microfluidic sensors. After the review of fabrication techniques in Sec. 2, we will have a brief introduction of the existing microfluidic detection methods and techniques in Secs. 3, 4. This is followed by a review of optofluidic sensing techniques in Sec. 5. 2.Fabrication MethodsIn the early days, the fabrication of microfluidic devices mainly relied on techniques transferred from the conventional two-dimensional integrated circuit (IC) and silicon-based two- or three-dimensional MEMS processes. This includes photolithography, thin film metallization, and chemical etching. Later, glass based, glass-silicon, glass-polymer mixed microfluidic fabrication techniques, and devices started to garner more interest. 6, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 The glass materials were preferred partly for the biocompatibility toward the biomedical related applications40, 41, 42, 43, 44 and the ideal surface characteristics where high temperature or strong solvents should appear 45, 46, 47, 48, 49, 50, 51, 52, 53 (e.g., on-chip capillary electrophoresis-based operations). However, lack of optical transparency at interested wavelengths (for silicon), micromachining difficulties, and comparably high expenses for both silicon and glass materials have hampered their wider applications in microfluidics. Tremendous effort has been made to find alternative materials that are more cost-effective and easier for micromachining. With the development of related fabrication techniques in recent years, the polymer/plastic-based microfluidic systems has garnered more interest than its conventional competitors.54 In spite of comparatively weak bonding and structure deformation during device packaging processes, polymer materials still seem attractive due to the facts that: they are more economic compared with silicon and glasses, easier to be fabricated in/on, avoidance of high-temperature annealing and stringent cleaning, more system integration friendly (e.g., interconnections), and there exists a wider range of materials to be chosen for characteristics that are required for each specific application, such as good optical transparency, biocompatibility, and chemical or mechanical properties. Another important reason for the interest from both academia and industry on polymer microfluidic devices is the possibility of disposable microfluidic chips toward biomedical and clinical applications. These devices usually require low cost of fabrication, high volume production, good reproducibility, and versatility in design for a wide spectrum of specific applications. Current methods for fabrication of microfluidic devices include prototyping techniques (includes hot embossing,55, 56 injection molding,57, 58 and soft lithography59) and direct fabrication techniques such as laser photoablation or laser micromachining,60 photolithography/optical lithography61 and x-ray lithography62 (Figs. 1, 2). Table 1 presents the advantages and disadvantages of those techniques. Note that “photolithography” in this table refers to conventional photolithography that is mostly employed in IC industry for micrometer scale patterning. In fact, during the past decade photolithography techniques have progressed to achieve smaller feature patterning ability and have been coupled to various plastic/polymer-based techniques to better suit lab-on-a-chip applications. To date, most of the current soft lithography processes still rely on modern photolithography techniques for master template/mask fabrication. Consequently, the low resolution ability of soft lithography can be gradually improved with the high quality masks by modern photolithography. Sub-100-nm fabrication resolution can also be achieved by composite layers of stamps.63 Other techniques were also used to obtain the soft lithography masters with nanometer scale features below 5 nm: such as to replicate those features from single-walled carbon nanotubes64 or from crystal fractures65 as soft lithography masters. For photolithography made masters for the soft lithography process, the recently reported resolution limit has been pushed to around 20 nm by Li 66 Fig. 1Illustrations of the typical fabrication procedures by the prototyping techniques (hot embossing, inject molding, and soft lithography). 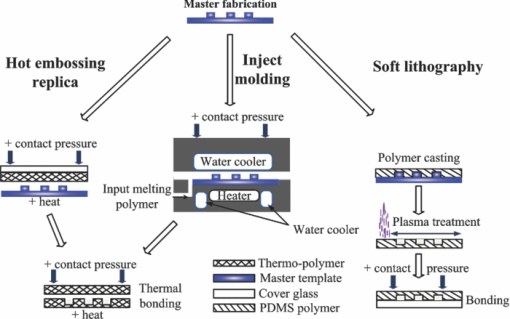 Fig. 2Illustrations of the typical fabrication procedures by the direct fabrication techniques: (a) laser micromachining, (b) photolithography/optical lithography, and (c) x-ray lithography. The “metal mask insertion” step in x-ray lithography is usually realized by electroplating. 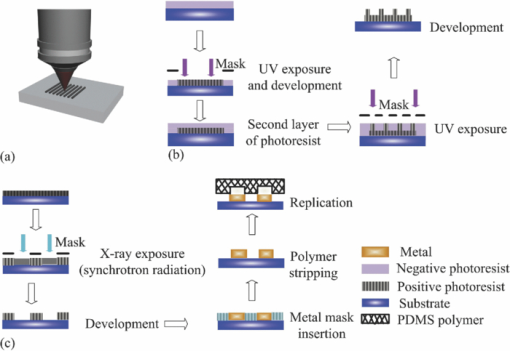 Fig. 7Density of active catalyst and flow map imaging by the hyperpolarization enhanced NMR spectroscopy reported by Pines’ group. MRI images [field of view (FOV) (x to z): 2.3 mm by 7.0 mm; pixel size: 20 μm by 60 μm] of a tightly packed catalyst bed (catalyst layer thickness is ∼5 mm) are shown. The picture shows the flow map in the xz plane with the use of polarized propane. The orientation of the arrows represents direction of the velocity, and their length represents its magnitude. The SNR of thermally polarized propylene was insufficient to generate a velocity map. The resolution of the flow map is intentionally decreased by retaining only one of every 16 arrows to avoid excessive overlap of the arrows (Ref. 116). 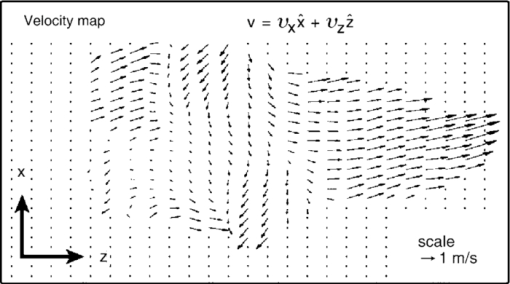 Table 1Microfluidic fabrication techniques.
3.Detection MethodsIntegrated microfluidic devices involve the large-scale integration of various microfluidic components, such as microvalves, microchannels, micropumps, microfluidic mixers, and other elements to handle and control fluids at the microscale.67 They are frequently used for biological, chemical, and biomedical analysis. There has already been a considerable amount of insightful review articles on related topics.67, 68, 69, 70, 71, 72 Various detection methods exist in the field of chemical, biological diagnosis, or analysis on microfluidic platforms. The detection methods in microfluidics can be classified into three major types: optical methods, 9, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89 electrochemical methods, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102 and mass spectrometry methods. 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113 Among these methods, optical and electrochemical methods are the most frequently utilized due to their selectivity and sensitivity. Other than the above major methods, approaches such as nuclear magnetic resonance (NMR) spectroscopy,114, 115, 116, 117, 118 magneto-resistive,119 and acoustical120, 121 are also coupled to microfluidics for sensing application. As demonstrated in Fig. 3, typical optical detection methods comprise the direct detection by monitoring the light properties including fluorescence,74, 81, 82, 83 absorbance,84, 85, 86 and luminescence-based87, 88, 89 methods, and the light property modulation detections such as surface plasmon resonance (SPR) detection.76, 80, 122 These methods usually involve techniques such as evanescent waves,10 SPR,76, 80, 122, 123 interferometry,75, 124, 125, 126, 127, 128 Raman spectrometry,77, 129, 130, 131 fiber optics,78 and optical waveguides.79, 132 Photonic crystals, optical cavity structures, and several optical techniques have also been reported to be integrated with the microfluidic system for sensing purposes, using one- and two-dimensional surface PCs (guided mode resonance filters),133, 134, 135, 136, or three-dimensional photonic crystals,137, 138 optical cavities,139, 140 whispering gallery mode resonators, 141, 142, 143, 144, 145, 146, 147 and optical tweezers for cell related monitoring or fluidic rheological measurements148, 149, 150 (Fig. 4). Fig. 3Typical optical methods. (a) Fluorescence (in this case, fluorescent resonance energy transfer/FRET), (b) absorbance, (c) luminescence, and (d) surface plasmon resonance-based optical detection methods. GFP: green fluorescent protein; YFP: yellow fluorescent protein; GS: glass substrate. 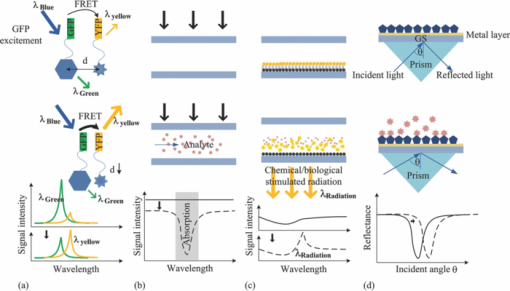 Fig. 4(a) Result of a three-dimensional photonic crystal optofluidic sensor for refractive index detection. The fluid refractive index in the microchannel above the photonic crystal was detected by the bandgap position shift. Transmittance spectrum of the whole system that reveals a bandgap around 4.3 μm (left inset). An illustration of the sensor (right inset and see Ref. 138). (b) Demonstration of the shear stress mapping inside a microfluidic device by optical tweezers (top sketch). Theoretical calculated results by the pure fluidic model and the experimental measured results of the fluid velocity and shear stress along y direction in the microfluidic device (bottom and see Ref. 150). 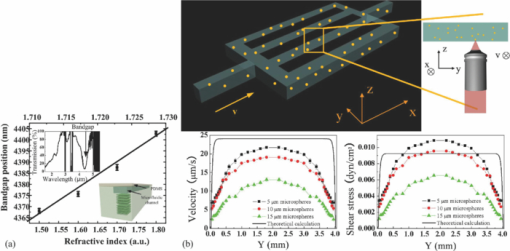 Electrochemical measurements are based on electrical property modulations of the analyte species that undergo redox reactions, and are usually employed for the detection of the electroactive species. They can be assigned to three categories: amperometries,90, 91, 92, 93, 94 potentiometries95, 96, 97 and conductometry98, 99, 100, 101, 102 measurements. The principles of these methods are demonstrated in Fig. 5. Amperometric detection is based on the fact that an applied voltammetric potential between a reference and a working electrode would cause the oxidation or reduction of the electroactive species in the vicinity, and an electrical current will be induced. Sensitive detection of the analyte(s) can be realized by the cyclic voltammogram(s) and current versus time curves of the electrode. In potentiometric detection, analyte detection is realized by monitoring the potential of an ion-selective electrode (usually a membrane) against a reference electrode. When selective ions pass through the membrane and a local equilibrium is established at the sensing interface, the resulting charge separation causes a potential between the working electrode and the reference electrode in relation to the species type and concentration. The principle of conductometric detection is that the conductivity of a zone is affected by the charged species in the zone. Different types of species would have their specific conductivity responses, which would also vary with different concentrations. It is the most commonly employed method in electrochemical measurements since it, in principle, can deal with all charged species of interest. The detection involves measuring the conductivity at a series of frequencies, both in conventional contacted conductivity detection101 and capacitively coupled contactless conductivity detection (C4D) methods such as potential gradient detection.102 Electrochemical detections are often used together with (capillary) electrophoresis operations (such as capillary electrophoresis separation) or in electrophoresis systems. Considering this, in this review the “electrochemical methods” actually include electrophoresis related work.92, 99, 101, 102 Fig. 5Three types of electrochemical detection methods for microfluidics: (a) amperometric, (b) potentiometric, and (c) conductometic (capacitively coupled conductivity detector) detection. 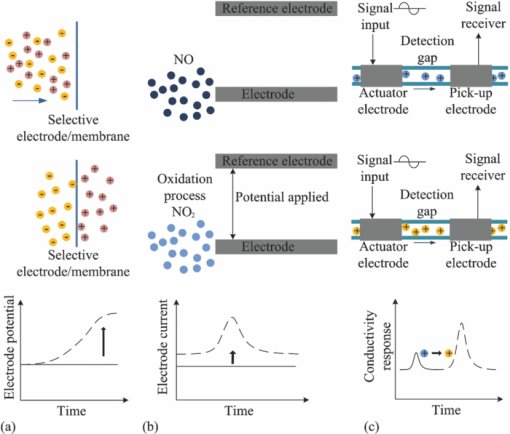 Mass spectrometry103 (MS) is able to perform highly selective detection by monitoring the trajectory of ions in electric and/or magnetic fields, which elucidate the mass and charge of the ions. Its most important application is in proteomic studies for protein separation and further identification from the fragmentation pattern of proteins. Identification after separation can be carried out in two ways. One is direct detection by combing through the database using the individual types of obtained protein(s). The more sophisticated way is carrying out tandem MS (Ref. 151) to get protein fragments/ions for sequence tagging. To date, several MS configurations have already been developed to be integrated with microfluidic devices for this application, for instance, electrospray ionization (ESI)-MS (Refs. 104, 105, 106) and matrix-assisted laser desorption ionization (MALDI)-MS.107, 108, 109 In 2006, another paradigm is ion trap mass spectrometry integrated with microfluidics for protein identification by Hardouin 111 Other systems were also reported, such as a chip-liquid chromatography (LC)-MS system for label-free profiling of human serum113 and a LC-ESI-MS system for multiple proteins detection from breast cancer cellular extract.112 The resolving ability of MS has recently been pushed down to the detection of a single molecular by Roukes's group in Caltech using a nanoelectromechanical system-based MS (NEMS-MS) (Fig. 6).110 It can be expected that in the future when NEMS-MS meets microfluidics, a much more effective microfluidic-MS platform could be realized to analyze biological or chemical species (e.g., proteins or nanoparticles) at a single molecular level with the ability to operate in multiplexing and parallel modes. Fig. 6First generation NEMS-MS system developed by Roukes's group (a), the NEMS mass spectrometry for a gold nanoparticles dispersion (b), and the real-time records of single-molecule adsorption events from their experiments (Ref. 110). 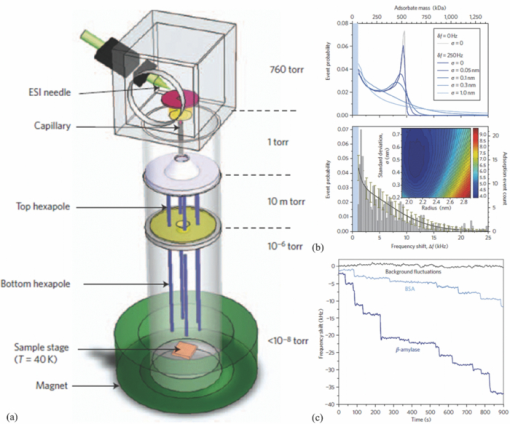 Besides protein separation and identification, MS has also been applied to the quantitative detection in protein expression in various states (especially in disease states). However, the miniaturization and high sensitivity requirements have currently been one of the most significant technological hurdles. Yet, microfluidics seems to hold great promises for the related technological breakthrough: The micrometer scale geometries and smaller platform of microfluidic devices meet the enhanced sensitivity and system miniaturization need; the multi-channel geometries in microfluidics enable high throughput processing and multiplexing ability. Considering these, to couple with microfluidics could be the ultimate lab-on-a-chip solution for MS toward quantitative proteome applications. To our knowledge, there has not been any further report on MS-based microfluidic sensing and yet a lot of interesting research remains to be carried out. Other than the three major categories, other methods such as the NMR spectroscopy115 have also been explored to be applied to microfluidic detection.43 It is a well-developed detection method in chemistry and life sciences, which employs the magnetic properties of nucleis or the chemical shift Zeeman effect and/or the Knight shift effect for detection purpose. NMR spectroscopy is able to detect biological and chemical analyte species such as proteins and nucleic acids. However, its application in microscale systems has been restricted by the low sensitivity of conventional NMR detection technique. Recently, this problem has been solved by hyperpolarization methods (e.g., to introduce the highly polarized para-hydrogen agent for signal enhancement). High resolution NMR for microfluidic systems was realized in 2007 by Pine's group in UC Berkley on the study of multi-phase flows and catalyst deactivation (Fig. 7).116 Besides direct detection, the Pines’ group also pioneered the remote monitor work of NMR-based microfluidic detections. In 2007, they reported the remote monitoring of spin coherence transfer in chemical transformations117 and a double-phase encoded remote detector of the fluid diffusion through membranes.118 This technique is readily applicable mostly in hydrogenation reaction-related detection and imaging, and might be extended for more applications in microfluidics. However, the limited reaction time scale/polarization lifetimes remains a key technological bottle-neck for NMR-based microfluidic analytical detection. Besides, similar to MS spectroscopy, another key hurdle is system miniaturization, in other words, to reduce the comparably large detector for better interface into microfluidics. In fact, not many related publications were reported afterward on NMR-based microfluidic detection. In spite of the technological challenges, NMR is still a competitive technique for microfluidic applications compared to optical or electrochemical methods: It does not have the optical accessibility issue for the region of interest (ROI) and the data acquisition time is much shorter, which makes time-resolved studies much easier; it has better detection pervasiveness than electrochemical methods that are limited to electroactive species. Hence, the solution to the weak NMR signal issue in microsystems, either by further signal enhancement or better detection instrumentations, will trigger a wider spectrum of lab-on-a-chip applications of NMR. 4.Sensing TechniquesIn Sec. 3 we discussed general detection methods in microfluidics. Here, we would like to emphasize the microfluidics/optofluidics sensing techniques. A general review is presented in Table 2. From Table 2 we can see that the different methods have corresponding advantages and limitations. Some of them can be coupled with each other either to achieve certain phenomena for detection or to enhance sensor ability. For instance, evanescent waves are utilized with interferometers, optical fibers, fluorescence induction, and optical waveguides, in order to further enhance the detection sensitivity (e.g., in the form of a fiber-optic evanescent-wave approach152 or a fiber-optic localized plasmon resonance sensor153). They are also widely used to induce surface plasmon resonance, which is essential in surface-enhanced Raman scattering.154 Cavities structures are used in localized plasmon resonance detections to improve sensing performance.155 Other detection methods were also actively studied for microfluidics, such as integrated optics components that can accommodate large arrays of compact optical channels and devices. One example is the laser induced fluorescence (LIF) detection, which is extensively used in microchip separations.156 Table 2Optofluidic sensing techniques.
5.OutlookThe past decade has witnessed the progresses in microfluidics: more microfluidic system prototypes, increased device complexity, and more fabrication and sensing techniques have been developed or improved. However, microfluidics sensors are still in a formative stage and hold tremendous opportunities to be applied to a wider spectrum of fields and applications. We could expect the next drive engine to microfluidics sensors development would be based on:
ReferencesA. Berg, H. G. Craighead, and
P.-D. Yang,
“From microfluidic applications to nanofluidic phenomena,”
Chem. Soc. Rev., 39 899
–900
(2010). https://doi.org/10.1039/C001349H Google Scholar
W.-C. Tian and
E. Finehout, Current and Future Trends in Microfluidics within Biotechnology Research, Springer, New York
(2008). Google Scholar
G. M. Whitesides,
“The origins and the future of microfluidics,”
Nature, 442 368
–373
(2006). https://doi.org/10.1038/nature05058 Google Scholar
A. D. Stroock, S. K. W. Dertinger, A. Ajdari, I. Mezic, H. A. Stone, and
G. M. Whitesides,
“Chaotic mixer for microchannels,”
Science, 295 647
–651
(2002). https://doi.org/10.1126/science.1066238 Google Scholar
B. Husband, M. Bu, A. G. R. Evans, and
T. Melvin,
“Investigation for the operation of an integrated peristaltic micropump,”
J. Micromech. Microeng., 14 S64
–S69
(2004). https://doi.org/10.1088/0960-1317/14/9/011 Google Scholar
M. A. Unger, H. P. Chou, T. Thorsen, A. Scherer, and
S. R. Quake,
“Monolithic microfabricated valves and pumps by multilayer soft lithography,”
Science, 288 113
–116
(2000). https://doi.org/10.1126/science.288.5463.113 Google Scholar
T. Thorsen, S. J. Maerkl, and
S. R. Quake,
“Microfluidic large-scale integration,”
Science, 298 580
–584
(2002). https://doi.org/10.1126/science.1076996 Google Scholar
S. Balslev, B. Bilenberg, O. Geschke, A. M. Jorgensen, A. Kristensen, J. P. Kutter, K. B. Mogensen, and
D. Snakenborg,
“Fully integrated optical system for lab-on-a-chip applications,”
89
–92
(2004). Google Scholar
A. M. Armani, R. P. Kulkarni, S. E. Fraser, R. C. Flagan, and
K. J. Vahala,
“Label-free, single-molecule detection with optical microcavities,”
Science, 317 783
–787
(2007). https://doi.org/10.1126/science.1145002 Google Scholar
D. J. Sirbuly, A. Tao, M. Law, R. Fan, and
P.-D. Yang,
“Multifunctional nanowire evanescent wave optical sensors,”
Adv. Mater., 19 61
–66
(2007). https://doi.org/10.1002/adma.200601995 Google Scholar
M. Krishnan, N. Mojarad, P. Kukura, and
V. Sandoghdar,
“Geometry-induced electrostatic trapping of nanometric objects in a fluid,”
Nature, 467 692
–695
(2010). https://doi.org/10.1038/nature09404 Google Scholar
X.-Q. Cui, L. M. Lee, X. Heng, W.-W. Zhong, P. W. Sternberg, D. Psaltis, and
C.-H. Yang,
“Lensless high-resolution on-chip optofluidic microscopes for Caenorhabditis elegans and cell imaging,”
Proc. Natl. Acad. Sci. U.S.A., 105 10670
–10675
(2008). https://doi.org/10.1073/pnas.0804612105 Google Scholar
G. Zheng, S. A. Lee, S. Yang, and
C.-H. Yang,
“Sub-pixel resolving optofluidic microscope for on-chip cell imaging,”
Lab Chip, 10 3125
–3129
(2010). https://doi.org/10.1039/c0lc00213e Google Scholar
S. Mandal and
D. Erickson,
“Nanoscale optofluidic sensor arrays,”
Opt. Express, 16 1623
–1631
(2008). https://doi.org/10.1364/OE.16.001623 Google Scholar
S.-M. Park, Y. S. Huh, K. Szeto, D. J. Joe, J. Kameoka, G. W. Coates, J. B. Edel, D. Erickson, and
H. G. Craighead,
“Rapid prototyping of nanofluidic systems using size-reduced electrospun nanofibers for biomolecular analysis,”
Small, 6 2420
–2426
(2011). https://doi.org/10.1002/smll.201000884 Google Scholar
J.-L. Li, D. Day, and
M. Gu,
“Design of a compact microfludic device for controllable cell distribution,”
Lab Chip, 10 3054
–3057
(2010). https://doi.org/10.1039/c0lc00090f Google Scholar
P. Yager, T. Edwards, E. Fu, K. Helton, K. Nelson, M. R. Tam, and
B. H. Weigl,
“Microfluidic diagnostic technologies for global public health,”
Nature, 442 412
–418
(2006). https://doi.org/10.1038/nature05064 Google Scholar
S. J. Moon, H. O. Keles, A. Ozcan, A. Khademhosseini, E. Haeggstrom, D. Kuritzkes, and
U. Demirci,
“Integrating microfluidics and lensless imaging for point-of-care testing,”
Biosens. Bioelectron., 24 3208
–3214
(2009). https://doi.org/10.1016/j.bios.2009.03.037 Google Scholar
S. Seo, T.-W. Su, D. K. Tseng, A. Erlinger, and
A. Ozcan,
“Lensfree holographic imaging for on-chip cytometry and diagnostics,”
Lab Chip, 9 777
–787
(2009). https://doi.org/10.1039/b813943a Google Scholar
V. R. Horowitz, D. D. Awschalom, and
S. Pennathur,
“Optofluidics: field or technique?,”
Lab Chip, 8 1856
–1863
(2008). https://doi.org/10.1039/B816416A Google Scholar
Y. Fainman, L. Lee, D. Psaltis, and
C.-H. Yang, Optofluidics: Fundamentals, Devices, and Applications, McGraw-Hill, New York
(2009). Google Scholar
A. Groisman, M. Enzelberger, and
S. R. Quake,
“Microfluidic memory and control devices,”
Science, 300 955
–958
(2003). https://doi.org/10.1126/science.1083694 Google Scholar
J. W. Hong, V. Studer, G. Hang, W. F. Anderson, and
S. R. Quake,
“A nanoliter scale nucleic acid processor with parallel architecture,”
Nat. Biotech., 22 435
–439
(2004). https://doi.org/10.1038/nbt951 Google Scholar
B. Tian, T. Cohen-Karni, Q. Qing, X.-J. Duan, P. Xie, and
C. M. Lieber,
“Three-dimensional, flexible nanoscale field-effect transistors as localized bioprobes,”
Science, 319 830
–834
(2010). https://doi.org/10.1126/science.1192033 Google Scholar
X. Tao, A. Lee, W. Limapichat, D. A. Dougherty, and
R. MacKinnon,
“A gating charge transfer center in voltage sensors,”
Science, 328 67
–73
(2010). https://doi.org/10.1126/science.1185954 Google Scholar
M. Yang, C.-W. Li, and
J. Yang,
“Cell docking and on-chip monitoring of cellular reactions with a controlled concentration gradient on a microfluidic device,”
Anal. Chem., 74 3991
–4001
(2002). https://doi.org/10.1021/ac025536c Google Scholar
S. C. Terry, J. H. Jerman, and
J. B. Angell,
“A gas chromatographic air analyzer fabricated on a silicon wafer,”
IEEE Trans. Electron Devices, 26 1880
–1886
(1979). https://doi.org/10.1109/T-ED.1979.19791 Google Scholar
S. J. Harrison, K. Fluri, K. Seiler, Z.-H. Fan, C. S. Effenhauser, and
A. Manz,
“Micromachining a miniaturized capillary electrophoresis-based chemical analysis system on a chip,”
Science, 261 895
–897
(1993). https://doi.org/10.1126/science.261.5123.895 Google Scholar
N. F. Raley, J. C. Davidson, and
J. W. Balch,
“Examination of glass-silicon and glass-glass bonding techniques for microfluidic systems,”
Proc. SPIE, 2639 41
–45
(1995). https://doi.org/10.1117/12.221298 Google Scholar
M. U. Kopp, A. J. D. Mello, and
A. Manz,
“Chemical amplification: continuous-flow PCR on a chip,”
Science, 280 1046
–1048
(1998). https://doi.org/10.1126/science.280.5366.1046 Google Scholar
M. Stjernstrom and
J. Roeraade,
“Method for fabrication of microfluidic systems in glass,”
J. Micromech. Microeng., 8 33
–38
(1998). https://doi.org/10.1088/0960-1317/8/1/006 Google Scholar
N. Bings, C. Wang, C. Skinner, K. Colyer, D. Harrison, J. Li, and
P. Thibault,
“Microfluidic devices connected to glass capillaries with minimal dead volume,”
Anal. Chem., 71 3292
–3296
(1999). https://doi.org/10.1021/ac981419z Google Scholar
J. M. Ruano, V. Benoit, J. S. Aitchison, and
J. M. Cooper,
“Flame hydrolysis deposition of glass on silicon for the integration of optical and microfluidic devices,”
Anal. Chem., 72 1093
–1097
(2000). https://doi.org/10.1021/ac9906983 Google Scholar
W. H. Grover, A. M. Skelley, C. N. Liu, E. T. Lagally, and
R. A. Mathies,
“Monolithic membrane valves and diaphragm pumps for practical large-scale integration into glass microfluidic devices,”
Sens. Actuators B, 89 315
–323
(2003). https://doi.org/10.1016/S0925-4005(02)00468-9 Google Scholar
Z.-J. Jia, Q. Fang, and
Z.-L. Fang,
“Bonding of glass microfluidic chips at room temperatures,”
Anal. Chem., 76 5597
–5602
(2004). https://doi.org/10.1021/ac0494477 Google Scholar
Y. Cheng, K. Sugioka, and
K. Midorikawa,
“Microfluidic laser embedded in glass by three-dimensional femtosecond laser microprocessing,”
Opt. Lett., 29 2007
–2009
(2004). https://doi.org/10.1364/OL.29.002007 Google Scholar
M.-Q. Bu, T. Melvin, G. J. Ensell, J. S. Wilkinson, and
A. G. R. Evans,
“A new masking technology for deep glass etching and its microfluidic application,”
Sens. Actuators B, 115 476
–482
(2004). https://doi.org/10.1016/j.sna.2003.12.013 Google Scholar
P. B. Allen and
D. T. Chiu,
“Calcium-assisted glass-to-glass bonding for fabrication of glass microfluidic devices,”
Anal. Chem., 80 7153
–7157
(2008). https://doi.org/10.1021/ac801059h Google Scholar
P. Vulto, T. Huesgen, B. Albrecht, and
G. A. Urban,
“A full-wafer fabrication process for glass microfluidic chips with integrated electroplated electrodes by direct bonding of dry film resist,”
J. Micromech. Microeng., 19 077001
(2009). https://doi.org/10.1088/0960-1317/19/7/077001 Google Scholar
P. C. H. Li and
D. J. Harrison,
“Transport, manipulation, and reaction of biological cells on-chip using electrokinetic effects,”
Anal. Chem., 69 1564
–1568
(1997). https://doi.org/10.1021/ac9606564 Google Scholar
L. C. Waters, S. C. Jacobson, N. Kroutchinina, J. Khandurina, R. S. Foote, and
J. M. Ramsey,
“Microchip device for cell lysis, multiplex PCR amplification, and electrophoretic sizing,”
Anal. Chem., 70 158
–162
(1998). https://doi.org/10.1021/ac970642d Google Scholar
T. Ichiki, T. Ujiie, S. Shinbashi, T. Okuda, and
Y. Horiike,
“Immunoelectrophoresis of red blood cells performed on microcapillary chips,”
Electrophoresis, 23 2029
–2034
(2002). https://doi.org/10.1002/1522-2683(200207)23:13<2029::AID-ELPS2029>3.0.CO;2-R Google Scholar
H. Lee, E. Sun, D. Ham, and
R. Weissleder,
“Chip-NMR biosensor for detection and molecular analysis of cells,”
Nat. Med., 14 869
–874
(2008). https://doi.org/10.1038/nm.1711 Google Scholar
B.-Y. Qu, Z.-Y. Wu, F. Fang, Z.-M. Bai, D.-Z. Yang, and
S.-K. Xu,
“A glass microfluidic chip for continuous blood cell sorting by a magnetic gradient without labeling,”
Anal. Bioanal. Chem., 392 1317
–1324
(2008). https://doi.org/10.1007/s00216-008-2382-4 Google Scholar
S. C. Jacobson, L. B. Koutny, R. Hergenroder, A. W. Moore, and
J. M. Ramsey,
“Microchip capillary electrophoresis with an integrated postcolumn reactor,”
Anal. Chem., 66 3472
–3476
(1994). https://doi.org/10.1021/ac00092a027 Google Scholar
Z.-H. Liang, N. Chiem, G. Ocvirk, T. Tang, K. Fluri, and
D. J. Harrison,
“Microfabrication of a planar absorbance and fluorescence cell for integrated capillary electrophoresis devices,”
Anal. Chem., 68 1040
–1046
(1996). https://doi.org/10.1021/ac950768f Google Scholar
K. Fluri, G. Fitzpatrick, N. Chiem, and
D. J. Harrison,
“Integrated capillary electrophoresis devices with an efficient postcolumn teactor in planar quartz and glass chips,”
Anal. Chem., 68 4285
–4290
(1996). https://doi.org/10.1021/ac9604090 Google Scholar
S. C. Jacobson, C. T. Culbertson, J. E. Daler, and
J. M. Ramsey,
“Microchip structures for submillisecond electrophoresis,”
Anal. Chem., 70 3476
–3480
(1998). https://doi.org/10.1021/ac980349t Google Scholar
T. Ujiie, T. K. Ichiki, and
Y. Horiike,
“Fabrication of quartz microcapillary electrophoresis chips using plasma etching,”
Jpn. J. Appl. Phys., 39 3677
–3682
(2000). https://doi.org/10.1143/JJAP.39.3677 Google Scholar
T. M. H. Lee, I.-M. Hsing, A. I. K. Lao, and
M. C. Carles,
“A miniaturized DNA amplifier: its application in traditional chinese medicine,”
Anal. Chem., 72 4242
–4247
(2000). https://doi.org/10.1021/ac000384b Google Scholar
Y.-Z. Deng, H.-W. Zhang, and
J. Henion,
“Chip-based quantitative capillary electrophoresis/mass spectrometry determination of drugs in human plasma,”
Anal. Chem., 73 1432
–1439
(2001). https://doi.org/10.1021/ac0010328 Google Scholar
N. Gottschlich, S. C. Jacobson, C. T. Culbertson, and
J. M. Ramsey,
“Two-dimensional electrochromatography/capillary electrophoresis on a microchip,”
Anal. Chem., 73 2669
–2674
(2001). https://doi.org/10.1021/ac001019n Google Scholar
F. Omasu, Y. Nakano, and
T. Ichiki,
“Measurement of the electrophoretic mobility of sheep erythrocytes using microcapillary chips,”
Electrophoresis, 26 1163
–1167
(2005). https://doi.org/10.1002/elps.200410182 Google Scholar
R. Mukhopadhyay,
“When PDMS isn't the best,”
Anal. Chem., 79 3248
–3253
(2007). https://doi.org/10.1021/ac071903e Google Scholar
H. Becker and
U. Heim,
“Hot embossing as a method for the fabrication of polymer high aspect ratio structures,”
Sens. Actuators, A, 83 130
–135
(2000). https://doi.org/10.1016/S0924-4247(00)00296-X Google Scholar
S.-Z. Qi, X.-Z. Liu, S. Ford, J. Barrows, G. Thomas, K. Kelly, A. McCandless, K. Lian, J. Goettert, and
S. A. Soper,
“Microfluidic devices fabricated in poly(methyl methacrylate) using hot-embossing with integrated sampling capillary and fiber optics for fluorescence detection,”
Lab Chip, 2 88
–95
(2002). https://doi.org/10.1039/b200370h Google Scholar
R.-D. Chien,
“Micromolding of biochip devices designed with microchannels,”
Sens. Actuators, A, 128 238
–247
(2006). https://doi.org/10.1016/j.sna.2006.02.029 Google Scholar
U. M. Attia, S. Marson, and
J. R. Alcock,
“Micro-injection moulding of polymer microfluidic devices,”
Microfluid. Nanofluid., 7 1
–28
(2009). https://doi.org/10.1007/s10404-009-0421-x Google Scholar
J. C. McDonald, D. C. Duffy, J. R. Anderson, D. T. Chiu, H.-K. Wu, O. J. A. Schueller, and
G. M. Whitesides,
“Fabrication of microfluidic systems in poly(dimethylsiloxane),”
Electrophoresis, 21 27
–40
(1999). https://doi.org/10.1002/(SICI)1522-2683(20000101)21:1<27::AID-ELPS27>3.0.CO;2-C Google Scholar
J. Rossier, F. Reymond, and
P. E. Michel,
“Polymer microfluidic chips for electrochemical and biochemical analyses,”
Electrophoresis, 23 858
–867
(2002). https://doi.org/10.1002/1522-2683(200203)23:6<858::AID-ELPS858>3.0.CO;2-3 Google Scholar
H. Cao, J. O. Tegenfeldt, R. H. Austin, and
S. Y. Chou,
“Gradient nanostructures for interfacing microfluidics and nanofluidics,”
Appl. Phys. Lett., 81 3058
–3060
(2002). https://doi.org/10.1063/1.1515115 Google Scholar
T. Mappes, S. Achenbach, and
J. Mohr,
“X-ray lithography for devices with high aspect ratio polymer submicron structures,”
Microelectron. Eng., 84 1235
–1239
(2007). https://doi.org/10.1016/j.mee.2007.01.154 Google Scholar
T. W. Odom, J. C. Love, D. B. Wolfe, K. E. Paul, and
G. M. Whitesides,
“Improved pattern transfer in soft lithography using composite stamps,”
Langmuir, 18 5314
–5320
(2002). https://doi.org/10.1021/la020169l Google Scholar
F. Hua, Y.-G. Sun, A. Gaur, M. A. Meitl, L. Bilhaut, L. Rotkina, J.-F. Wang, P. Geil, M. Shim, and
J. A. Rogers,
“Polymer imprint lithography with molecular-scale resolution,”
Nano Lett., 4 2467
–2471
(2004). https://doi.org/10.1021/nl048355u Google Scholar
Q.-B. Xu, B. T. Mayers, M. Lahav, D. V. Vezenov, and
G. M. Whitesides,
“Approaching zero: using fractured crystals in metrology for replica molding,”
J. Am. Chem. Soc., 127 854
–855
(2005). https://doi.org/10.1021/ja043436d Google Scholar
Z.-W. Li, Y.-N. Gu, L. Wang, H.-X. Ge, W. Wu, Q.-F. Xia, C.-S. Yuan, Y.-F. Cheng, B. Cui, and
R. S. Williams,
“Hybrid nanoimprint-soft lithography with sub-15 nm resolution,”
Nano Lett., 9 2306
–2310
(2009). https://doi.org/10.1021/nl9004892 Google Scholar
D. J. Beebe, G. A. Mensing, and
G. M. Walker,
“Physics and applications of microfluids in biology,”
Annu. Rev. Biomed. Eng., 4 261
–286
(2002). https://doi.org/10.1146/annurev.bioeng.4.112601.125916 Google Scholar
H. A. Stone, A. D. Stroock, and
A. Ajdari,
“Engineering flows in small devices: microfluidics toward a lab-on-a-chip,”
Annu. Rev. Fluid Mech., 36 381
–411
(2004). https://doi.org/10.1146/annurev.fluid.36.050802.122124 Google Scholar
L. Baharudin,
“Microfluidics: fabrications and applications,”
Instru. Sci. Tech., 36 222
–230
(2008). https://doi.org/10.1080/10739140701850993 Google Scholar
K. B. Mogensen, H. Klank, and
J. P. Kutter,
“Recent developments in detection for microfluidic systems,”
Electrophoresis, 25 3498
–3512
(2004). https://doi.org/10.1002/elps.200406108 Google Scholar
A. Q. Liu, H. J. Huang, L. K. Chin, Y. F. Yu, and
X. C. Li,
“Label-free detection with micro optical fluidic systems (MOFS): a review,”
Anal. Bioanal. Chem., 391 2243
–2452
(2008). https://doi.org/10.1007/s00216-008-1878-2 Google Scholar
A. C. Siegel, S. K. Y. Tang, C. A. Nijhuis, M. Hashimoto, S. T. Phillips, M. D. Dickey, and
G. M. Whitesides,
“Co-fabrication: a strategy for building multi-component microsystems,”
Acc. Chem. Res., 43 518
–528
(2010). https://doi.org/10.1021/ar900178k Google Scholar
D. J. Bornhop and
K. Swinney,
“Detection in capillary electrophoresis: a review,”
Electrophoresis, 21 1239
–1250
(2000). https://doi.org/10.1002/(SICI)1522-2683(20000401)21:7<1239::AID-ELPS1239>3.0.CO;2-6 Google Scholar
D. Ross, M. Gaitan, and
L. E. Locascio,
“Temperature measurement in microfluidic systems using a temperature-dependent fluorescent dye,”
Anal. Chem., 73 4117
–4123
(2001). https://doi.org/10.1021/ac010370l Google Scholar
P. Dumais, C. L. Callender, J. P. Noad, and
C. J. Ledderhof,
“Integrated optical sensor using a liquid-core waveguide in a Mach-Zehnder interferometer,”
Opt. Express, 16 18164
–18172
(2008). https://doi.org/10.1364/OE.16.018164 Google Scholar
I. T. Kim and
K. D. Kihm,
“Label-free visualization of microfluidic mixture concentration fields using SPR reflectance imaging,”
Exp. Fluids, 41 905
–916
(2006). https://doi.org/10.1007/s00348-006-0210-1 Google Scholar
T. Park, S. Lee, G. H. Seong, J. Choo, E. K. Lee, Y. S. Kim, W. H. Ji, S. Y. Hwang, D. G. Gweon, and
S. Lee,
“Highly sensitive signal detection of duplex dye-labelled DNA oligonucleotides in a PDMS microfluidic chip: confocal surface-enhanced Raman spectroscopic study,”
Lab Chip, 5 437
–442
(2005). https://doi.org/10.1039/b414457k Google Scholar
V. Lien and
F. Vollmer,
“Microfluidic flow rate detection based on integrated optical fiber cantilever,”
Lab Chip, 7 1352
–1356
(2007). https://doi.org/10.1039/b706944h Google Scholar
D. A. Chang-Yen and
B. K. Gale,
“An integrated optical oxygen sensor fabricated using rapid-prototyping techniques,”
Lab Chip, 3 297
–301
(2003). https://doi.org/10.1039/b305358j Google Scholar
D. Sinton, R. Gordon, and
A. G. Brolo,
“Nanohole arrays in metal films as optofluidic elements: progress and potential,”
Microfluid. Nanofluid., 4 107
–116
(2008). https://doi.org/10.1007/s10404-007-0221-0 Google Scholar
W. K. Ridgeway, E. Seitaridou, R. Phillips, and
J. R. Williamson,
“RNA-protein binding kinetics in an automated microfluidic reactor,”
Nucleic Acids Res., 37 e142
(2009). https://doi.org/10.1093/nar/gkp733 Google Scholar
J. G. Santiago, S. Wereley, C. D. Meinhart, D. J. Beebe, and
R. J. Adrian,
“A micro-particle image velocimetry system,”
Exp. Fluids, 25 316
–319
(1998). https://doi.org/10.1007/s003480050235 Google Scholar
M. G. Olsen, J. M. Bauer, and
D. J. Beebe,
“Particle imaging technique for measuring the deformation rate of hydrogel microstructures,”
Appl. Phys. Lett., 76 3310
–3312
(2000). https://doi.org/10.1063/1.126635 Google Scholar
G. Minas, J. S. Martins, J. C. Ribeiro, R. F. Wolffenbuttel, and
J. H. Correia,
“Biological microsystem for measuring uric acid in biological fluids,”
Sens. Actuators, A, 110 33
–38
(2004). https://doi.org/10.1016/j.sna.2003.10.049 Google Scholar
M. P. Duggan, T. McCreedy, and
J. W. Aylott,
“A non-invasive analysis method for on-chip spectrophotometric detection using liquid-core waveguiding within a 3D architecture,”
Analyst (Cambridge, U.K.), 128 1336
–1340
(2003). https://doi.org/10.1039/b309869a Google Scholar
N. J. Petersen, K. B. Mogensen, and
J. P. Kutter,
“Performance of an in-plane detection cell with integrated waveguides for UV/Vis absorbance measurements on microfluidic separation devices,”
Electrophoresis, 23 3528
–3536
(2002). https://doi.org/10.1002/1522-2683(200210)23:20<3528::AID-ELPS3528>3.0.CO;2-5 Google Scholar
B. Filanoski, S. K. Rastogi, E. Cameron, N. N. Mishra, W. Maki, and
G. Maki,
“A novel homogeneous bioluminescence resonance energy transfer element for biomolecular detection with CCD camera or CMOS device,”
Luminescence, 23 22
–27
(2008). https://doi.org/10.1002/bio.1011 Google Scholar
O. Hofmann, P. Miller, P. Sullivan, S. T. Jones, J. C. deMello, D. D. C. Bradley, and
A. J. deMello,
“Thin-film organic photodiodes as inte- grated detectors for microscale chemiluminescence assays,”
Sens. Actuators B, 106 878
–884
(2005). https://doi.org/10.1016/j.snb.2004.10.005 Google Scholar
A. M. Jorgensen, K. B. Mogensen, J. P. Kutter, and
O. Geschke,
“A biochemical microdevice with an integrated chemiluminescence detector,”
Sens. Actuators B, 90 1
–3
(2003). https://doi.org/10.1016/S0925-4005(03)00016-9 Google Scholar
J.-Z. Zhu, Z.-Q. Zhu, Z.-S. Lai, R. Wang, X.-M. Guo, X.-Q. Wu, G.-X. Zhang, Z.-R. Zhang, Y.-T. Wang, Z.-Y. Chen,
“Planar amperometric glucose sensor based on glucose oxidase immobilized by chitosan film on prussian blue layer,”
Sensors, 2 127
–136
(2002). https://doi.org/10.3390/s20400127 Google Scholar
W. Cha, Y.-C. Tung, M. E. Meyerhoff, and
S. Takayama,
“Patterned electrode-based amperometric gas sensor for direct nitric oxide detection within microfluidic devices,”
Anal. Chem., 82 3300
–3305
(2010). https://doi.org/10.1021/ac100085w Google Scholar
J. C. Fanguy and
C. S. Henry,
“The analysis of uric acid in urine using microchip capillary electrophoresis with electrochemical detection,”
Electrophoresis, 23 767
–773
(2002). https://doi.org/10.1002/1522-2683(200203)23:5<767::AID-ELPS767>3.0.CO;2-8 Google Scholar
X. X. Cai, N. Klauke, A. Glidle, P. H. Cobbold, G. L. Smith, and
J. M. Cooper,
“Ultra-low-volume, real-time measurements of lactate from the single heart cell using microsystems technology,”
Anal. Chem., 74 908
–914
(2002). https://doi.org/10.1021/ac010941+ Google Scholar
N. Ohgami, S. Upadhyay, A. Kabata, K. Morimoto, H. Kusakabe, and
H. Suzuki,
“Determination of the activities of glutamic oxaloacetic transaminase and glutamic pyruvic transaminase in a microfluidic system,”
Biosens. Bioelectron., 22 1330
–1336
(2007). https://doi.org/10.1016/j.bios.2006.06.007 Google Scholar
H. Suzuki and
Y. Matsugi,
“Microfabricated flow system for ammonia and creatinine with an air-gap structure,”
Sens. Actuators B, 98 101
–111
(2004). https://doi.org/10.1016/j.snb.2003.08.018 Google Scholar
R. H. Liu, J.-N. Yang, R. Lenigk, J. Bonanno, and
P. Grodzinski,
“Self-contained, fully integrated biochip for sample preparation, polymerase chain reaction amplification, and DNA microarray detection,”
Anal. Chem., 76 1824
–1831
(2004). https://doi.org/10.1021/ac0353029 Google Scholar
I. Grabowska, M. Sajnoga, M. Juchniewicz, M. Chudy, A. Dybko, and
Z. Brzozka,
“Microfluidic system with electrochemical and optical detection,”
Microelectron. Eng., 84 1741
–1743
(2007). https://doi.org/10.1016/j.mee.2007.01.248 Google Scholar
M. Galloway, W. Stryjewski, A. Henry, S. M. Ford, S. Llopis, R. L. McCarley, and
S. A. Soper,
“Contact conductivity detection in poly(methyl methacylate)-based microfluidic devices for analysis of mono- and polyanionic molecules,”
Anal. Chem., 74 2407
–2415
(2002). https://doi.org/10.1021/ac011058e Google Scholar
M. Zuborova, Z. Demianova, D. Kaniansky, M. Masar, and
B. Stanislawski,
“Zone electrophoresis of proteins on a poly(methyl methacrylate) chip with conductivity detection,”
J. Chromatogr. A, 990 179
–188
(2003). https://doi.org/10.1016/S0021-9673(02)01805-8 Google Scholar
C. L. Lago, H. D. T. Silva, C. A. Neves, J. G. A. Brito-Neto, and
J. A. F. Silva,
“A dry process for production of microfluidic devices based on the lamination of laser-printed polyester films,”
Anal. Chem., 75 3853
–3858
(2003). https://doi.org/10.1021/ac034437b Google Scholar
X.-H. Huang, R. N. Zare, S. Sloss, and
A. G. Ewing,
“End-column detection for capillary zone electrophoresis,”
Anal. Chem., 63 189
–192
(1991). https://doi.org/10.1021/ac00002a020 Google Scholar
Z.-Y. Wu, F. Fang, J. Josserand, and
H. H. Girault,
“On-column conductivity detection in capillary-chip electrophoresis,”
Electrophoresis, 28 4612
–4619
(2007). https://doi.org/10.1002/elps.200700456 Google Scholar
E. Hoffmann and
V. Stroobant, Mass Spectrometry: Principles and Application, 3rd ed.Wiley, New York
(2007). Google Scholar
G. A. Schultz, T. N. Corso, S. J. Prosser, and
S. Zhang,
“A fully integrated monolithic microchip electrospray device for mass spectrometry,”
Anal. Chem., 72 4058
–4063
(2000). https://doi.org/10.1021/ac000325y Google Scholar
L. Licklider, X. Q. Wang, A. Desai, Y. C. Tai, and
T. D. Lee,
“A micromachined chip-based electrospray source for mass spectrometry,”
Anal. Chem., 72 367
–375
(2000). https://doi.org/10.1021/ac990967p Google Scholar
J. Kameoka, R. Orth, B. Ilic, D. Czaplewski, T. Wachs, and
H. G. Craighead,
“An electrospray ionization source for integration with microfluidics,”
Anal. Chem., 74 5897
–5901
(2002). https://doi.org/10.1021/ac020396s Google Scholar
T. Miliotis, S. Kjellstrom, J. Nilsson, T. Laurell, L. E. Edholm, and
G. Marko-Varga,
“Capillary liquid chromatography interfaced to matrix-assisted laser desorption/ionization time-of-flight mass spectrometry using an on-line coupled piezoelectric flow-through microdispenser,”
J. Mass Spectrom., 35 369
–377
(2000). https://doi.org/10.1002/(SICI)1096-9888(200003)35:3<369::AID-JMS944>3.0.CO;2-N Google Scholar
J. Liu, K. Tseng, B. Garcia, C. B. Lebrilla, E. Mukerjee, S. Collins, and
R. Smith,
“Electrophoresis separation in open microchannels. A method for coupling electrophoresis with MALDI-MS,”
Anal. Chem., 73 2147
–2151
(2001). https://doi.org/10.1021/ac001326t Google Scholar
M. L. Mok, L. Hua, J. B. Phua, M. K. Wee, and
N. S. Sze,
“Capillary isoelectric focusing in pseudo-closed channel coupled to matrix assisted laser desorption/ioization mass spectrometry for protein analysis,”
Analyst (Cambridge, U.K.), 129 109
–110
(2004). https://doi.org/10.1039/b309610f Google Scholar
A. K. Naik, M. S. Hanay, W. K. Hiebert, X. L. Feng, and
M. L. Roukes,
“Towards single-molecule nanomechanical mass spectrometry,”
Nat. Nanotechnol., 4 445
–450
(2009). https://doi.org/10.1038/nnano.2009.152 Google Scholar
J. Hardouin, M. Duchateau, R. Joubert-Caron, and
M. Caron,
“Usefulness of an integrated microfluidic device (HPLC-Chip-MS) to enhance confidence in protein identification by proteomics,”
Rapid Commun. Mass Spectrom., 20 3236
–3244
(2006). https://doi.org/10.1002/rcm.2725 Google Scholar
J. M. Armenta, A. A. Dawoud, and
L. M. Lazar,
“Microfluidic chips for protein differential expression profiling,”
Electrophoresis, 30 1145
–1156
(2009). https://doi.org/10.1002/elps.200800653 Google Scholar
P. Horvatovich, N. I. Govorukhina, T. H. Reijmers, A. G. J. van der Zee, F. Suits, and
R. Bischoff,
“Chip-LC-MS for label-free profiling of human serum,”
Electrophoresis, 28 4493
–4505
(2007). https://doi.org/10.1002/elps.200600719 Google Scholar
A. Webb,
“Nuclear magnetic resonance of mass-limited samples using small RF coils,”
Anal. Bioanal. Chem., 388 525
–528
(2007). https://doi.org/10.1007/s00216-007-1178-2 Google Scholar
G. A. Webb, Nuclear Magnetic Resonance, The Royal Society of Chemistry, Cambridge
(2003). Google Scholar
L.-S. Bouchard, S. R. Burt, M. S. Anwar, K. V. Kovtunov, I. V. Koptyug, and
A. Pines,
“NMR imaging of catalytic hydrogenation in microreactors with the use of para-hydrogen,”
Science, 319 442
–445
(2008). https://doi.org/10.1126/science.1151787 Google Scholar
M. S. Anwar, C. Hilty, C. Chu, L.-S. Bouchard, K. L. Pierce, and
A. Pines,
“Spin Coherence Transfer in Chemical Transformations Monitored by Remote Detection NMR,”
Anal. Chem., 79 2806
–2811
(2007). https://doi.org/10.1021/ac062327+ Google Scholar
V.-V. Telkki, C. Hilty, S. Garcia, E. Harel, and
A. Pines,
“Quantifying the diffusion of a fluid through membranes by double phase encoded remote detection magnetic resonance imaging,”
J. Phys. Chem. B, 111 13929
–13936
(2007). https://doi.org/10.1021/jp076760e Google Scholar
M. Mujika, S. Arana, E. Castano, M. Tijero, R. Vilares, and
J. M. Ruano-Lopez,
“Magnetoresisitve immunosensor for the detection of escherichia coli O157:H7,”
Biosens. Bioelectron., 24 1253
–1258
(2009). https://doi.org/10.1016/j.bios.2008.07.024 Google Scholar
O. Tamarin, S. Comeau, C. Dejous, D. Moynet, D. Riebre, and
J. Beziam,
“Design of a bacteriophage model using love acoustic waves,”
Biosens. Bioelectron., 18 755
–763
(2003). https://doi.org/10.1016/S0956-5663(03)00022-8 Google Scholar
B. Godber, S. J. Kevin, K. S. J. Thompson, M. Rehak, Y. Uludag, S. Kelling, M. Sleptsov, M. Frogley, K. Wiehler, C. Whalen, and
J. M. Cooper,
“Direct quantification of analyte concentration by resonant acoustic profiling,”
Clin. Chem., 51 1962
–1972
(2005). https://doi.org/10.1373/clinchem.2005.053249 Google Scholar
A. D. Leebeeck, L. K. S. Kumar, V. D. Lange, D. Sinton, R. Gordon, and
A. G. Frolo,
“On-chip surface-based detection with nanohole arrays,”
Anal. Chem., 79 4094
–4100
(2007). https://doi.org/10.1021/ac070001a Google Scholar
E. Ouellet, C. Lausted, T. Lin, C. W. T. Yang, L. Hood, and
E. T. Lagally,
“Parallel microfluidic surface plasmon resonance imaging arrays,”
Lab Chip, 10 581
–588
(2010). https://doi.org/10.1039/b920589f Google Scholar
R. G. Heideman and
P. V. Lambeck,
“Remote opto-chemical sensing with extreme sensitivity: design, fabrication and performance of a pigtailed integrated optical phase-modulated Mach-Zehnder interferometer system,”
Sens. Actuators B, 61 100
–127
(1999). https://doi.org/10.1016/S0925-4005(99)00283-X Google Scholar
J. A. Chediak, Z. S. Lou, J. Seo, N. Cheung, L. P. Lee, and
T. D. Sands,
“Heterogeneous integration of CdS filters with GaN LEDs for fluorescence detection microsystems,”
Sens. Actuators, A, 111 1
–7
(2004). https://doi.org/10.1016/j.sna.2003.10.015 Google Scholar
S. H. Hsu and
Y. T. Huang,
“A novel Mach-Zehnder interferometer based on dual-ARROW structures for sensing applications,”
J. Lightwave Technol., 23 4200
–4206
(2005). https://doi.org/10.1109/JLT.2005.859435 Google Scholar
H. Berney and
K. Oliver,
“Dual polarization interferometry size and density characterization of DNA immobilization and hybridization,”
Biosens. Bioelectron., 21 618
–626
(2005). https://doi.org/10.1016/j.bios.2004.12.024 Google Scholar
B. Lillis, M. Manning, H. Berney, E. Hurley, A. Mathewson, and
M. M. Sheehan,
“Dual polarisation interferometry characterisation of DNA immobilisation and hybridisation detection on a silanised support,”
Biosens. Bioelectron., 21 1459
–1467
(2006). https://doi.org/10.1016/j.bios.2005.06.009 Google Scholar
K. R. Strehle, D. Cialla, P. Rosch, T. Henkel, M. Kohler, and
J. Popp,
“A reproducible surface-enhanced Raman spectroscopy approach. Online SERS measurements in a segmented microfluidic system,”
Anal. Chem., 79 1542
–1547
(2007). https://doi.org/10.1021/ac0615246 Google Scholar
B. D. Piorek, S. J. Lee, J. G. Santiago, M. Moskovits, S. Banerjee, and
C. D. Meinhart,
“Free-surface microfluidic control of surface-enhanced Raman spectroscopy for the optimized detection of airborne molecules,”
Proc. Natl. Acad. Sci. U.S.A, 104 18898
–18901
(2007). https://doi.org/10.1073/pnas.0708596104 Google Scholar
L. Tong, M. Righini, M. U. Gonzalez, R. Quidant, and
M. Kall,
“Optical aggregation of metal nanoparticles in a microfluidic channel for surface-enhanced Raman scattering analysis,”
Lab Chip, 9 193
–195
(2008). https://doi.org/10.1039/b813204f Google Scholar
M. I. Rudenko, M. R. Holmes, P. Measor, D. W. Deamer, A. R. Hawkins, and
H. Schmidt,
“Planar electro-optofluidic chip: integration of nanopore with optofluidics,”
(2010). Google Scholar
D. Erickson, T. Rockwood, T. Emery, A. Scherer, and
D. Psaltis,
“Nanofluidic tuning of photonic crystal circuitry,”
Opt. Lett., 31 59
–61
(2006). https://doi.org/10.1364/OL.31.000059 Google Scholar
C. J. Choi and
B. T. Cunningham,
“A 96-well microplate incorporating a replica molded microfluidic network integrated with photonic crystal biosensors for high throughput kinetic biomolecular interaction analysis,”
Lab Chip, 7 550
–556
(2007). https://doi.org/10.1039/b618584c Google Scholar
L. L. Chan, S. L. Gosangari, K. L. Watkin, and
B. T. Cunningham,
“Label-free imaging of cancer cells using photonic crystal biosensors and application to cytotoxicity screening of a natural compound library,”
Sens. Actuators B, 132 418
–425
(2007). https://doi.org/10.1016/j.snb.2007.10.027 Google Scholar
B. R. Schudel, C. J. Choi, B. T. Cunningham, and
P. J. A. Kenis,
“Microfluidic chip for combinatorial mixing and screening of assays,”
Lab Chip, 9 1676
–1680
(2009). https://doi.org/10.1039/b901999e Google Scholar
H. J. Kim, S. Kim, H. Jeon, J. Ma, S. H. Choi, S. Lee, C. Ko, and
W. Park,
“Fluorescence amplification using colloidal photonic crystal platform in sensing dye-labeled deoxyribonucleic acids,”
Sens. Actuators B, 124 147
–152
(2007). https://doi.org/10.1016/j.snb.2006.12.024 Google Scholar
J. Wu, D. Day, and
M. Gu,
“A microfluidic refractive index sensor based on an integrated three-dimensional photonic crystal,”
Appl. Phys. Lett., 92 071108
(2008). https://doi.org/10.1063/1.2840700 Google Scholar
M. Loncar, A. Scherer, and
Y. M. Qiu,
“Photonic crystal laser sources for chemical detection,”
Appl. Phys. Lett., 82 4648
–4650
(2003). https://doi.org/10.1063/1.1586781 Google Scholar
M. L. Adams, M. Loncar, A. Scherer, and
Y. M. Qiu,
“Microfluidic integration of porous photonic crystal nanolasers for chemical sensing,”
IEEE J. Sel. Areas Commun., 23 1348
–1354
(2005). https://doi.org/10.1109/JSAC.2005.851192 Google Scholar
N. M. Hanumegowda, I. M. White, H. Oveys, and
X. Fan,
“Label-free protease sensors based on optical microsphere resonators,”
Sens. Lett., 3 315
–319
(2005). https://doi.org/10.1166/sl.2005.044 Google Scholar
M. Noto, M. Koshsima, D. Keng, I. Teraoka, V. Kolchenko, and
S. Arnold,
“Molecular weight dependence of a whispering gallery mode biosensor,”
Appl. Phys. Lett., 87 223901
(2005). https://doi.org/10.1063/1.2137902 Google Scholar
N. M. Hanumegowda, C. J. Stica, B. C. Patel, I. M. White, and
X. Fan,
“Refractometric sensors based on microsphere resonators,”
Appl. Phys. Lett., 87 201107
(2005). https://doi.org/10.1063/1.2132076 Google Scholar
Y. Lin, V. S. Ilchenko, J. Nadeau, and
L. Maleki,
“Biochemical detection with optical whispering-gallery resonators,”
Proc. SPIE, 6452 64520U
(2007). https://doi.org/10.1117/12.716591 Google Scholar
S. L. Westcott, J. Zhang, R. K. Shelton, N. M. K. Bruce, S. Gupta, S. L. Keen, J. W. Tillman, L. B. Wald, B. N. Strecker, A. T. Rosenberger, R. R. Davidson, W. Chen, K. G. Donovan, and
J. V. Hryniewicz,
“Broadband optical absorbance spectroscopy using a whispering gallery mode microsphere resonator,”
Rev. Sci. Instrum., 79 033106
(2008). https://doi.org/10.1063/1.2894307 Google Scholar
S. Arnold, R. Ramjit, D. Keng, V. Kolchenko, and
I. Teraoka,
“Microparticle photophysics illuminates viral bio-sensing,”
Faraday Discuss, 137 65
–83
(2008). https://doi.org/10.1039/b702920a Google Scholar
D. Keng, S. R. McAnanama, I. Teraoka, and
S. Arnold,
“Resonance fluctuations of a whispering gallery mode biosensor by particles undergoing Brownian motion,”
Appl. Phys. Lett., 91 103902
(2007). https://doi.org/10.1063/1.2778351 Google Scholar
E. Eriksson, J. Scrimgeour, J. Enger, and
M. Goksor,
“Holographic optical tweezers combined with a microfluidic device for exposing cells to fast environmental changes,”
Proc. SPIE, 6592 65920P
(2007). https://doi.org/10.1117/12.721859 Google Scholar
H. Mushfique, J. Leach, H. Yin, R. D. Leonardo, M. J. Padgett, and
J. M. Cooper,
“3D mapping of microfluidic flow in laboratory-on-a-chip structures using optical tweezers,”
Anal. Chem., 80 4237
–4240
(2008). https://doi.org/10.1021/ac8002006 Google Scholar
J. Wu, D. Day, and
M. Gu,
“Shear stress mapping in microfluidic devices by optical tweezers,”
Opt. Express, 18 7611
–7616
(2010). https://doi.org/10.1364/OE.18.007611 Google Scholar
D. F. Hunt, J. R. Yates III, J. Shabanowitz, S. Winston, and
C. R. Hauer,
“Protein sequencing by tandem mass spectrometry,”
Proc. Natl. Acad. Sci. U.S.A, 83 6233
–6237
(1986). https://doi.org/10.1073/pnas.83.17.6233 Google Scholar
S.-F. Cheng and
L.-K. Chau,
“Colloidal gold-modified optical fiber for chemical and biochemical sensing,”
Anal. Chem., 75 16
–21
(2003). https://doi.org/10.1021/ac020310v Google Scholar
Y. Chuang, C.-Y. Lee, S.-H. Lu, S.-C. Wang, L.-K. Chau, and
W.-H. Hsieh,
“Using ac-field-induced electro-osmosis to accelerate biomolecular binding in fiber-optic sensing chips with microstructures,”
Anal. Chem., 82 1123
–1127
(2010). https://doi.org/10.1021/ac902682k Google Scholar
D. Hou, S. Maheshwari, and
H.-C. Chang,
“Rapid bioparticle concentration and detection by combining a discharge driven vortex with surface enhanced Raman scattering,”
Biomicrofluidics, 1 014106
(2007). https://doi.org/10.1063/1.2710191 Google Scholar
R. Ameling, L. Langguth, M. Hentschel, M. Mesch, P. V. Braun, and
H. Giessen,
“Cavity-enhanced localized plasmon resonance sensing,”
Appl. Phys. Lett., 97 253116
(2010). https://doi.org/10.1063/1.3530795 Google Scholar
K. Uchiyama, H. Nakajima, and
T. Hobo,
“Detection methods for microchip separations,”
Anal. Bioanal. Chem., 379 375
–382
(2004). https://doi.org/10.1007/s00216-004-2616-z Google Scholar
S. H. I. Yeung, T. S. Seo, C. A. Crouse, S. A. Greenspoon, T. N. Chiesl, J. D. Ban, and
R. A. Mathies,
“Fluorescence energy transfer-labeled primers for high-performance forensic DNA profiling,”
Electrophoresis, 29 2251
–2259
(2008). https://doi.org/10.1002/elps.200700772 Google Scholar
R. T. Ranasinghe and
T. Brown,
“Ultrasensitive fluorescence-based methods for nucleic acid detection: towards amplification-free genetic analysis,”
Chem. Commun. (Cambridge), 47 3717
–3735
(2011). https://doi.org/10.1039/c0cc04215c Google Scholar
R. Bruck, E. Melnik, P. Muellner, R. Hainberger, and
M. Lammerhofer,
“Integrated polymer-based Mach-Zehnder interferometer label-free streptavidin biosensor compatible with injection molding,”
Biosens. Bioelectron., 26 3832
–3837
(2011). https://doi.org/10.1016/j.bios.2011.02.042 Google Scholar
P. Estrela, D. Paul, Q.-F. Song, L. K. J. Stadler, L. Wang, E. Huq, J. J. Davis, P. K. Ferrigno, and
P. Migliorato,
“Label-free sub-picomolar protein detection with field-effect transistors,”
Anal. Chem., 82 3531
–3536
(2010). https://doi.org/10.1021/ac902554v Google Scholar
T. Endo, D. Ikeda, Y. Yawakami, Y. Yanagida, and
T. Hatsuzawa,
“Fabrication of core-shell structured nanoparticle layer substrate for excitation of localized surface plasmon resonance and its optical response for DNA in aqueous conditions,”
Anal. Chim. Acta, 661 200
–205
(2010). https://doi.org/10.1016/j.aca.2009.12.022 Google Scholar
D. Choi, T. Kang, H. Cho, Y. Choi, and
L. P. Lee,
“Additional amplifications of SERS via an optofluidic CD-based platform,”
Lab Chip, 9 239
–243
(2009). https://doi.org/10.1039/B812067F Google Scholar
W. Liang, Y. Huang, Y. Xu, R. K. Lee, and
A. Yariv,
“Highly sensitive fiber Bragg grating refractive index sensors,”
Appl. Phys. Lett., 86 151122
(2009). https://doi.org/10.1063/1.1904716 Google Scholar
C. Markos, W. Yuan, K. Vlachos, G. E. Town, and
O. Bang,
“Label-free biosensing with high sensitivity in dual-core microstructured polymer optical fibers,”
Opt. Express, 19 7790
–7798
(2010). https://doi.org/10.1364/OE.19.007790 Google Scholar
I. M. White, H. Oveys, and
X.-D. Fan,
“Liquid-core optical ring-resonator sensors,”
Opt. Lett., 31 1319
–1321
(2006). https://doi.org/10.1364/OL.31.001319 Google Scholar
|

