|
|
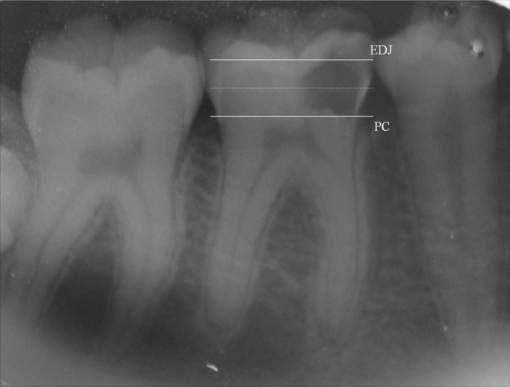
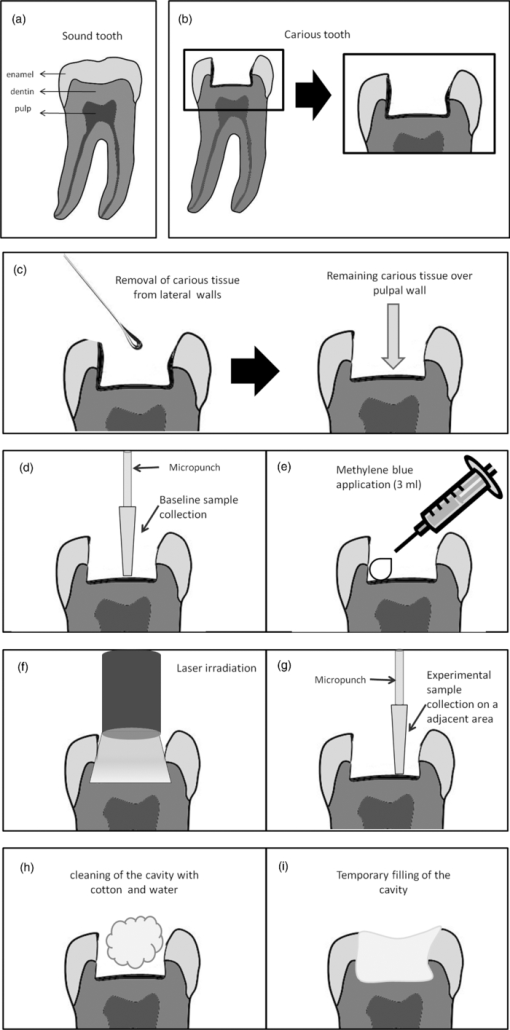
1.IntroductionCaries is an infectious bacterial disease that results in the localized destruction of dental hard tissues. The disease starts with an imbalance between the processes of remineralization and demineralization on the enamel surface. The reasons are an enhance in bacterial metabolism and the consequent increase in acid production, primarily mediated by the ingestion of sucrose.1 The traditional treatment of advanced carious lesions commonly involves the removal of all demineralized dentin using rotary burs until sound dentin forms the entire pulpal floor. The intention is to ensure the elimination of all remaining micro-organisms, to prevent a possible recurrence of caries and pulpal injury. However, this procedure is not always successful and some micro-organisms may remain even after the removal of all softened dentin.2 The main risk of this approach is the unnecessary wear of dental tissues and the possible exposure of the pulp, particularly in young patients. The inner layer of the carious lesion, adjacent to sound dentin, is partially decalcified and still contains cariogenic species, although it also leaves the original dentinal tubule structure and sound collagen fibers partially preserved. It has been shown that dentin can even remineralize to a certain degree.3 Thus, an alternative to the removal of decalcified dentinal tissues is to kill the bacteria and preserve the affected dentin, so that the remineralization can occur.4 Photodynamic antimicrobial chemotherapy (PACT) has been shown to be a promising antibacterial treatment.5 The therapy involves the association of a photosensitizer agent and light of a specific wavelength, resulting in the generation of cytotoxic species in the presence of oxygen such as singlet oxygen and free radicals.6 Already established as a potential treatment for cancer, photodynamic therapy has once again aroused interest in the field of antimicrobial chemotherapy, with new studies demonstrating the considerable advantages of its use.7 Previous studies have revealed that PACT is capable of killing oral bacteria in cultures, plaque scrapings, and biofilms. 4, 8, 9, 10, 11, 12, 13 Some in vitro studies have already demonstrated that PACT may be effective against bacteria involved in caries development.14 Burns 15 showed that PACT could eliminate cariogenic species when a bacterial suspension was treated with the photosensitizer toluidine blue (TB) before irradiation with red laser light. Later, it was demonstrated that the lethal photosensitization of S. mutans is possible even when the bacteria is embedded in a collagen matrix or when the laser light passes through the demineralized dentin slices, simulating the carious lesion.4, 16 Additional studies have sought to reproduce clinical conditions and obtained favorable results with regard to PACT and its effects on the decontamination of ex vivo carious dentin4, 17 or caries lesions produced both in vitro 18 and in situ.19 PACT may represent an efficient and less invasive approach to the treatment of deep carious lesions. Since no previous clinical study has been reported and the antimicrobial effects of PACT are usually more efficient in vitro than in vivo,4 the real effect of this type of therapy needs to be tested in a clinical setting. The aim of this investigation was to evaluate the effectiveness of PACT in bacterial reduction for the treatment of deep dentinal carious lesions. 2.Subjects and Methods2.1.Subject SelectionHealthy patients of both genders were selected from the dental clinic of the School of Dentistry, University of São Paulo, Brazil, with ages ranging from 8 to 25 years. Calculation of statistical significance was performed using the paired t-test for the paired samples (Biostat 4.0, α = 0.05% e β = 0.15%), and the minimum sample size was estimated in 22 children. Written informed consent was obtained from each patient or from the responsible parent or guardian in cases involving minors 18 years or younger. The possible discomforts, risks, and benefits were fully explained to the patients or to their guardians. The research protocol was conducted in accordance with the Declaration of Helsinki and Brazilian Human Research Law (Res 196/96), and it was approved by the Ethics Committee of University of São Paulo, Brazil (# 01/08). Patients included in the study were required to present at least one permanent molar with an active deep carious lesion limited to the occlusal surface and without pulpal involvement. Carious lesions were standardized by means of clinical and radiographic examinations performed before the procedures, with the aid of a measuring probe calibrated in millimeters. Lesion depths had to extend beyond the inner half of the dentin (i.e., between the enamel-dentin junction and the pulp chamber, as shown in Fig. 1). Exclusion criteria were 1. the use of any antibiotics during the study period or within six months prior to its initiation and 2. signs or symptoms of irreversible pulp inflammation. After clinical and radiographic examinations, 26 total teeth were selected from 23 patients. A sample of carious dentin was collected from each tooth both before and after PACT comprising baseline and experimental samples, respectively, for the microbiological analysis. The teeth were then temporarily restored with glass ionomer cement (Maxxion, FMG, Joinville, Brazil). 2.2.Clinical ProceduresAfter local anesthesia with 2% lidocaine with a vasoconstrictor (DFL, Petropolis, Brazil) and after isolation with a rubber dam, demineralized dentin, and enamel were removed from only the lateral walls of the carious lesions to promote the satisfactory adhesion of the restorative material [Figs. 2a, 2b]. These procedures were performed with a sharp excavator (N 19) and a spherical carbide bur (KG Sorensen, São Paulo, Brazil) set at slow rotation. Thus, carious dentinal layers were maintained over the pulpal wall of the cavities [Fig. 2c]. The collection of baseline carious dentin samples from the pulpal wall was carried out using a sterile 1 mm diameter micropunch [Fig. 2d] (Richter, São Paulo, Brazil) on its active segment. The penetration depth of the micropunch in the carious lesions was calibrated between measurements in different patients and between repeated measurements in the same patient by means of a 0.5 mm mark on its active point.20 Thus, the collected samples had diameters of 1 mm, depths of 0.5 mm and average weights of 0.059 mg. The samples were immediately transferred to the transport medium VMGA III (Viable Medium of Götenbörg Anaerobic)21 containing glass beads. 2.3.Photosensitizer and Light SourceMethylene blue (MB) was used as the photosensitizer agent, which was prepared with distilled water to obtain a final concentration of 0.01% (100 mg/L, 268 uM) (Fórmula & Ação, São Paulo, Brazil). The low power laser used was a diode laser (InGaAIP – Indium Gallium Aluminum Phosphide) with 660 nm wavelength, spot size of 0.028 cm2, and fixed output power of 100 mW. The parameters adopted were: energy density of 320 J cm−2, time exposure of 90 s, and total energy of 9 J. The output power was quantified with a power meter (Newport Corp., California) before the irradiations. 2.4.Photodynamic Antimicrobial TherapyAfter the baseline dentin collection, the photosensitizer MB was applied on carious tissue with an insulin injection syringe [Fig. 2e]. A volume of approximately three mL was used to fill each lesion. The carious tissue was maintained in contact with MB during a pre-irradiation time of five minutes. Laser beam was then perpendicularly positioned to the occlusal surfaces of the teeth and irradiations proceeded in one single point on the center of each cavity [Fig. 2f]. After the irradiation, experimental samples were collected from an area adjacent to that of the baseline sample collection [Fig. 2g]. This precaution was taken to minimize regional variations in the microbial population. The dentinal samples were then inserted in the transport medium VMGA III. Clinical procedures were performed by only one trained researcher to standardize the data collection. Teeth were then cleaned with a cotton swab and thoroughly washed with distilled water until the photosensitizer was completely removed [Fig. 2h]. Glass ionomer cement (Maxxion, FGM, Joinville, Brazil) was used for temporary restorations [Fig. 2f]. 2.5.Microbiological ProceduresThe flasks containing the samples in the VMGA III media were homogenized for two minutes in a vortex (Fisher Scientific, New York, USA) to break out the aggregates of bacteria. Immediately after homogenization, six decimal dilutions were carried out (1:10, 1:100, 1:1,000, 1:10,000, 1:100,000, and 1:1,000,000). In each dilution, three aliquots of 25 μl were plated onto Brucella blood agar, Mitis Salivarius agar supplemented with 15% sucrose and 0.2 units of Bacitracin ml−1 (MSSB), and Rogosa SL agar containing 0.13% glacial acetic acid (RSL). Brucella blood agar was used to determine total viable counts and plates were incubated in an anaerobic cabinet at 35°C for seven days, in an atmosphere of 85% N2, 10% CO2, and 5% H2. Mitis Salivarius agar supplemented with sucrose and bacitracin (MSSB) was used to count mutans streptococci and the plates were incubated at 37°C for 48 h in a candle jar. Rogosa SL agar (RSL) was used to count Lactobacillus spp. and the plates were aerobically incubated at 37°C for 48 h. After the incubation period, colony-forming units (CFU) per plate were counted, and reduction was calculated between the samples taken before and after the application of photodynamic therapy for each medium studied. 2.6.Statistical AnalysisFirst, the data distribution was assessed using D’Agostino's K-squared test. Statistical analyses comparing the baseline data and the experimental sample data were conducted using the Wilcoxon signed-rank test and comparisons between the groups were analyzed via the Mann-Whitney test at 5% significance level. 3.ResultsFrom March 2008 to May 2009, 165 patients were analyzed in the dental clinic of the School of Dentistry, University of São Paulo. Among them, 23 presented with active dentin carious lesions matching the inclusion criteria, resulting in 26 lesions for the study. Patients were followed up from the beginning of the study and no patient reported any pain or sensitivity after the clinical procedures. Statistical analyses showed significant differences in the count of CFU before and after PACT for the three groups of bacteria tested. The CFU reductions found after PACT on the tested micro-organisms are presented in Table 1. The therapy promoted a mean log reduction of 1.38 (p < 0.0001) for mutans streptococci, 0.93 (p < 0.0001) for Lactobacillus spp., and 0.91 (p < 0.0001) for total viable bacteria. No statistically significant differences were observed regarding the level of bacterial reduction among the test micro-organisms (p > 0.05) (Table 1). PACT was seen to promote similar levels of reduction in all tested bacteria. Table 1Bacterial count after PACT (qualitative and log results) – averages and standard deviations. [Different letters correspond to a significant statistic difference (p < 0.05).]
4.DiscussionEven though controversies persist regarding how much tissue must be removed to arrest the caries process, the literature appears to discourage the excessive removal of dentin over the pulpal surface, supporting the idea of minimally invasive procedures.22, 23 According to this concept, it is favorable to maintain a layer of partly demineralized dentin underneath a filling material to preserve pulpal tissue vitality, especially to encourage the reparative process of tubular sclerosis and tertiary dentin formation. It has already been demonstrated that oral bacteria organized in biofilms can be susceptible to PACT.8, 11, 13 Wilson 24 verified that a substantial reduction in the bacterial count was achieved when plaque samples obtained from volunteers were treated with toluidine blue O (TBO) or phthalocyanine and exposed to red light. Analyses through confocal laser scanning microscopy of multi-species biofilm cultured from saliva samples and treated with TBO showed bacterial reductions of 97.4% after irradiation with a low power laser.11 Moreover, transmission electron microscopy has confirmed that a photosensitizer can be absorbed by the biomass found in natural oral plaque biofilms.25 However, it remains unclear as to the depths to which the photosensitizer can penetrate carious dentin. Lethal photosensitization probably occurs predominantly in the outer layers of biofilm and carious tissue.11 This fact could occur due to the inability of the photosensitizer to spread into the inner layers or the inability of the light to be totally transmitted. These factors may explain the results obtained in the present study, because significant bacterial reductions of mutans streptococci (78.07%), Lactobacillus spp. (78.0%), and total viable count (76.03%) occurred, although the reductions were lower than those generally observed with in vitro studies in which the substrate is less complex. Pre-irradiation exposure time seems to be an important factor for photosensitizer diffusion through the tissue. In our study, the photosensitizer was left in contact with the dentin for five minutes before laser irradiation.13 Muller already demonstrated in vitro the minimal effect of PACT in the viability of micro-organisms when MB was applied for only 60 s in contact with the biofilm and removed prior to the laser irradiation. It is known that high concentrations of dyes can induce the phenomenon of self-quenching, reducing the amount of light that actually reaches the bacteria and induces the generation of reactive oxygen species. This effect may have interfered in the effectiveness of PACT in our study, which warrants new studies with lower dye concentrations. However, because the degree of photodamage is dependent upon the dye concentration and the intensity and fluence of the laser light, a higher concentration was chosen mainly because of the complexity of the substrate. This concentration of MB has been clinically used for the treatment of herpes simplex labialis, accelerating the healing process.26 Even though many studies have shown that PACT is an effective antimicrobial technique, most were performed with bacteria in an aqueous suspension, which is different from those conditions found in the oral cavity. It has been demonstrated that interposing 150-μm dentin slices between the laser light source and the bacteria leads to a reduction of 50% in the power density of the light source, although substantial kills were obtained.16 This effect may have occurred in our study, restricting the penetration of the photosensitizer and light transmission through the dentinal substrate.11, 16 For that reason, increased energy density was chosen in order to overcome such issues in the present study. In addition, it is important and clinically convenient to have short exposure times and, therefore, the use of greater power density may represent an advantage. Several previous reports have demonstrated that different light sources and photosensitizers can be combined to promote the bactericidal effect. The dental plaque disclosing agent erythrosin was considered a potential photosensitizer for the treatment of S. mutans biofilms grown in vitro when combined with a white light source.27 The complete elimination of S. mutans in a planktonic culture was as well demonstrated when it was previously treated with different concentrations of rose bengal combined with a held photopolymerizer (400 to 500 nm) or TBO combined with a light emitting diode (LED) (600 to 670 nm).28, 29 Considering that longer wavelengths enable the deeper penetration of light into the tissues,30 the association between a blue dye and a red light source was preferred. Methylene blue has shown significant phototoxicity in different types of oral bacteria involved in periodontal diseases and endodontic infections, among others.10, 31, 32 Because this photosensitizer has an intrinsic positive charge, it can efficiently bind to both Gram-positive and -negative bacteria.32, 33 Comparing the effectiveness of MB and TBO as lethal photosensitizers for Gram-positive and Gram-negative micro-organisms, it has been verified that both are capable of eradicating all micro-organisms to some extent under red laser light.10 Nevertheless, the efficiency of the therapy can vary according to the genus of bacteria. Gram-negative bacteria were shown to be more resistant to the therapy due to the greater complexity of their cytoplasmic membranes.7 Although a variety of studies have found S. mutans and Lactobacillus spp. to be the major micro-organisms involved in caries development, there may be large variations and changes within the lesion environment at advanced stages of caries34, 35 and Gram-negative species may also occur.36 For that reason, this study analyzed the effect of PACT on total viable bacteria, involving both Gram-negative and -positive strains, which was demonstrated to be equally successful. It is possible that this result is related to the high dose of energy that was applied. Statistical analysis (Table 1) demonstrated a wide standard deviation for all of the groups, although this variation is expected when working with bacteria and particularly involving clinical experiments. The present study was conducted in the clinical setting using the photosensitizer MB and a red light laser to promote dentin decontamination. The obtained results appear to be relevant for further clinical studies. The findings are in accordance with Giust,18 who demonstrated that PACT was effective in the decontamination of carious bovine dentin that was artificially induced, using a light-emitting diode light source and two different photosensitizers. The association of TBO and LED also resulted in a significant decrease in the viability of total streptococci, mutans streptococci, lactobacilli, and total micro-organisms on dentinal caries produced in situ.19 These data are in agreement with the present study. Although the achieved antimicrobial effect appears to be limited, it may still be considered a clinically relevant outcome and agrees with others clinical studies.37 The literature encourages the maintenance of a layer of “affected dentin” over the pulpal wall in order to avoid pulp exposure. In this way, any immediate bacterial reduction obtained for dentin decontamination would increase the chances of treatment success and it is expected that further reduction occurs over time if a filling material is properly placed.38 The aim of this study was to treat teeth with deep carious lesion while avoiding the risk of pulpal exposure and to arrest the carious process by favoring the repair of involved tissues. The results of this first clinical study demonstrate the potential clinical use of PACT for the treatment of caries. Also, our findings might encourage new research efforts to develop clinical protocols to make PACT feasible in clinical practice. New studies could be conducted with the goal of assessing different parameters for PACT (e.g., dye concentrations and exposure times) to improve its effectiveness. AcknowledgmentsThe authors wish to express their gratitude to FAPESP–Fundação de Amparo à Pesquisa do Estado de São Paulo-for the financial support (Grant 08/54903-3) and DMC Equipamentos, for providing the laser used in this study. ReferencesJ. van Houte,
“Role of micro-organisms in caries etiology,”
J. Dent. Res., 73
(3), 672
–681
(1994). https://doi.org/10.1177/00220345940730031301 Google Scholar
A. Lager, E. Thornqvist, and
D. Ericson,
“Cultivatable bacteria in dentine after caries excavation using rose-bur or carisolv,”
Caries Res., 37
(3), 206
–211
(2003). https://doi.org/10.1159/000070446 Google Scholar
T. Fusayama,
“Two layers of carious dentin; diagnosis and treatment,”
Oper. Dent., 4
(2), 63
–70
(1979). Google Scholar
J. A. Williams, G. J. Pearson, M. J. Colles, and
M. Wilson,
“The photo-activated antibacterial action of toluidine blue O in a collagen matrix and in carious dentine,”
Caries Res., 38
(6), 530
–536
(2004). https://doi.org/10.1159/000080582 Google Scholar
M. Wainwright,
“Photodynamic antimicrobial chemotherapy (PACT),”
J. Antimicrob. Chemother., 42
(1), 13
–28
(1998). https://doi.org/10.1093/jac/42.1.13 Google Scholar
T. J. Dougherty, C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan, and
Q. Peng,
“Photodynamic therapy,”
J. Natl. Cancer Inst., 90
(12), 889
–905
(1998). https://doi.org/10.1093/jnci/90.12.889 Google Scholar
G. Jori, C. Fabris, M. Soncin, S. Ferro, O. Coppellotti, D. Dei, L. Fantetti, G. Chiti, and
G. Roncucci,
“Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications,”
Lasers Surg. Med., 38
(5), 468
–481
(2006). https://doi.org/10.1002/lsm.20361 Google Scholar
I. C. Zanin, M. M. Lobo, L. K. Rodrigues, L. A. Pimenta, J. F. Hofling, and
R. B. Goncalves,
“Photosensitization of in vitro biofilms by toluidine blue O combined with a light-emitting diode,”
Eur. J. Oral Sci., 114
(1), 64
–69
(2006). https://doi.org/10.1111/j.1600-0722.2006.00263.x Google Scholar
D. Metcalf, C. Robinson, D. Devine, and
S. Wood,
“Enhancement of erythrosine-mediated photodynamic therapy of Streptococcus mutans biofilms by light fractionation,”
J. Antimicrob. Chemother., 58
(1), 190
–192
(2006). https://doi.org/10.1093/jac/dkl205 Google Scholar
M. N. Usacheva, M. C. Teichert, and
M. A. Biel,
“Comparison of the methylene blue and toluidine blue photobactericidal efficacy against gram-positive and gram-negative microorganisms,”
Lasers Surg. Med., 29
(2), 165
–173
(2001). https://doi.org/10.1002/lsm.1105 Google Scholar
J. F. O’Neill, C. K. Hope, and
M. Wilson,
“Oral bacteria in multi-species biofilms can be killed by red light in the presence of toluidine blue,”
Lasers Surg. Med., 31
(2), 86
–90
(2002). https://doi.org/10.1002/lsm.10087 Google Scholar
J. A. Williams, G. J. Pearson, M. J. Colles, and
M. Wilson,
“The effect of variable energy input from a novel light source on the photoactivated bactericidal action of toluidine blue O on Streptococcus Mutans,”
Caries Res., 37
(3), 190
–193
(2003). https://doi.org/10.1159/000070443 Google Scholar
I. C. Zanin, R. B. Goncalves, A. B. Junior, C. K. Hope, and
J. Pratten,
“Susceptibility of Streptococcus mutans biofilms to photodynamic therapy: an in vitro study,”
J. Antimicrob. Chemother., 56
(2), 324
–330
(2005). https://doi.org/10.1093/jac/dki232 Google Scholar
M. Wilson, J. Dobson, and
W. Harvey,
“Sensitization of oral bacteria to killing by low-power laser radiation,”
Curr. Microbiol., 25
(2), 77
–81
(1992). https://doi.org/10.1007/BF01570963 Google Scholar
T. Burns, M. Wilson, and
G. J. Pearson,
“Sensitisation of cariogenic bacteria to killing by light from a helium-neon laser,”
J. Med. Microbiol., 38
(6), 401
–405
(1993). https://doi.org/10.1099/00222615-38-6-401 Google Scholar
T. Burns, M. Wilson, and
G. J. Pearson,
“Effect of dentine and collagen on the lethal photosensitization of Streptococcus mutans,”
Caries Res., 29
(3), 192
–197
(1995). https://doi.org/10.1159/000262068 Google Scholar
R. Ng, F. Singh, D. A. Papamanou, X. Song, C. Patel, C. Holewa, N. Patel, V. Klepac-Ceraj, C. R. Fontana, R. Kent, T. C. Pagonis, P. P. Stashenko, and
N. S. Soukos,
“Endodontic photodynamic therapy ex vivo,”
J. Endod., 37
(2), 217
–222
(2011). https://doi.org/10.1016/j.joen.2010.10.008 Google Scholar
J. S. Giusti, L. Santos-Pinto, A. C. Pizzolito, K. Helmerson, E. Carvalho-Filho, C. Kurachi, and
V. S. Bagnato,
“Antimicrobial photodynamic action on dentin using a light-emitting diode light source,”
Photomed. Laser Surg., 26
(4), 281
–287
(2008). https://doi.org/10.1089/pho.2007.2149 Google Scholar
J. P. Lima, M. A. Sampaio, F. M. Borges, A. H. Teixeira, C. Steiner-Oliveira, M. Nobre Dos Santos, L. K. Rodrigues, and
I. C. Zanin,
“Evaluation of the antimicrobial effect of photodynamic antimicrobial therapy in an in situ model of dentine caries,”
Eur. J. Oral Sci., 117
(5), 568
–574
(2009). https://doi.org/10.1111/j.1600-0722.2009.00662.x Google Scholar
S. L. Pinheiro, M. R. Simionato, J. C. Imparato, and
M. Oda,
“Antibacterial activity of glass-ionomer cement containing antibiotics on caries lesion microorganisms,”
Am. J. Dent., 18
(4), 261
–266
(2005). Google Scholar
A. J. Moller,
“Microbiological examination of root canals and periapical tissues of human teeth. Methodological studies,”
Odontol. Tidskr., 74
(5), 1
–380
(1966). Google Scholar
E. J. Mertz-Fairhurst, K. M. Call-Smith, G. S. Shuster, J. E. Williams, Q. B. Davis, C. D. Smith, R. A. Bell, J. D. Sherrer, D. R. Myers, P. K. Morse, Thomas A. Garman, and
Victor E. Della-Giustina,
“Clinical performance of sealed composite restorations placed over caries compared with sealed and unsealed amalgam restorations,”
J. Am. Dent. Assoc., 115
(5), 689
–694
(1987). Google Scholar
D. N. Ricketts, E. A. Kidd, N. Innes, and
J. Clarkson,
“Complete or ultraconservative removal of decayed tissue in unfilled teeth,”
Cochrane Database Syst. Rev, 3 CD003808
(2006). https://doi.org/10.1002/14651858.CD003808.pub2. Google Scholar
M. Wilson, T. Burns, J. Pratten, and
G. J. Pearson,
“Bacteria in supragingival plaque samples can be killed by low-power laser light in the presence of a photosensitizer,”
J. Appl. Bacteriol., 78
(5), 569
–574
(1995). https://doi.org/10.1111/j.1365-2672.1995.tb03101.x Google Scholar
S. Wood, B. Nattress, J. Kirkham, R. Shore, S. Brookes, J. Griffiths, and
C. Robinson,
“An in vitro study of the use of photodynamic therapy for the treatment of natural oral plaque biofilms formed in vivo,”
J. Photochem. Photobiol., B, 50
(1), 1
–7
(1999). https://doi.org/10.1016/S1011-1344(99)00056-1 Google Scholar
J. Marotti, A. C. Aranha, P. Eduardo Cde, and
M. S. Ribeiro,
“Photodynamic therapy can be effective as a treatment for herpes simplex labialis,”
Photomed. Laser Surg., 27
(2), 357
–363
(2009). https://doi.org/10.1089/pho.2008.2268 Google Scholar
S. Wood, D. Metcalf, D. Devine and
C. Robinson,
“Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms,”
J. Antimicrob. Chemother., 57
(4), 680
–684
(2006). https://doi.org/10.1093/jac/dkl021 Google Scholar
T. P. Paulino, K. F. Ribeiro, A. C. Tedesco, and
P. Ciancaglini,
“Use of hand held photopolymerizer to photoinactivate Streptococcus mutans,”
Arch. Oral Biol., 50
(3), 353
–359
(2005). https://doi.org/10.1016/j.archoralbio.2004.09.002 Google Scholar
I. M. Bevilacqua, R. A. Nicolau, S. Khouri, G. R. Teodoro, R. A. Zangaro, and
M. T. Pacheco,
“The impact of photodynamic therapy on the viability of Streptococcus mutans in a planktonic culture,”
Photomed. Laser Surg., 25
(6), 513
–518
(2007). https://doi.org/10.1089/pho.2007.2109 Google Scholar
K. Konopka and
T. Goslinski,
“Photodynamic therapy in dentistry,”
J. Dent. Res., 86
(8), 694
–707
(2007). https://doi.org/10.1177/154405910708600803 Google Scholar
M. Wilson,
“Lethal photosensitisation of oral bacteria and its potential application in the photodynamic therapy of oral infections,”
Photochem. Photobiol. Sci., 3
(5), 412
–418
(2004). https://doi.org/10.1039/b211266c Google Scholar
S. George and
A. Kishen,
“Photophysical, photochemical, and photobiological characterization of methylene blue formulations for light-activated root canal disinfection,”
J. Biomed. Opt., 12
(3), 034029
(2007). https://doi.org/10.1117/1.2745982 Google Scholar
M. R. Hamblin and
T. Hasan,
“Photodynamic therapy: a new antimicrobial approach to infectious disease?,”
Photochem. Photobiol. Sci., 3
(5), 436
–450
(2004). https://doi.org/10.1039/b311900a Google Scholar
K. L. Chhour, M. A. Nadkarni, R. Byun, F. E. Martin, N. A. Jacques, and
N. Hunter,
“Molecular analysis of microbial diversity in advanced caries,”
J. Clin. Microbiol., 43
(2), 843
–849
(2005). https://doi.org/10.1128/JCM.43.2.843-849.2005 Google Scholar
M. A. Munson, A. Banerjee, T. F. Watson, and
W. G. Wade,
“Molecular analysis of the microflora associated with dental caries,”
J. Clin. Microbiol., 42
(7), 3023
–3029
(2004). https://doi.org/10.1128/JCM.42.7.3023-3029.2004 Google Scholar
J. A. Aas, A. L. Griffen, S. R. Dardis, A. M. Lee, I. Olsen, F. E. Dewhirst, E. J. Leys, and
B. J. Paster,
“Bacteria of dental caries in primary and permanent teeth in children and young adults,”
J. Clin. Microbiol., 46
(4), 1407
–1417
(2008). https://doi.org/10.1128/JCM.01410-07 Google Scholar
A. S. Garcez, S. C. Nunez, M. R. Hamblin, and
M. S. Ribeiro,
“Antimicrobial effects of photodynamic therapy on patients with necrotic pulps and periapical lesion,”
J. Endod., 34
(2), 138
–142
(2008). https://doi.org/10.1016/j.joen.2007.10.020 Google Scholar
D. S. Wambier, F. A. dos Santos, A. C. Guedes-Pinto, R. G. Jaeger, and
M. R. Simionato,
“Ultrastructural and microbiological analysis of the dentin layers affected by caries lesions in primary molars treated by minimal intervention,”
Pediatr. Dent., 29
(3), 228
–234
(2007). Google Scholar
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||