|
|
1.IntroductionThe interaction between light and biological tissue is determined by the intensity and wavelength of the light and by the optical properties (absorption and scattering) of the tissue. Biological tissues can be penetrated relatively deeply by near-infrared (NIR) light, and thus the brain can be studied. In the present work NIR-LEDs (light-emitting diodes) are used to couple light into the head tissue. The light is strongly scattered, because the brain is a strong scatterer in the NIR.1, 2 The intensity of the re-emerging light is measured by a photodiode detector, and thus the optical properties of the brain can be obtained. Every tissue also has its own absorption spectrum. Biological tissues contain three main absorbers: water, hemoglobin, and lipid. Below a wavelength of 650 nm, strong absorption occurs for oxyhemoglobin (O2Hb) and deoxyhemoglobin (HHb), whereas water shows strong absorption above 950 nm. As a consequence of this, light does not penetrate deeply into the tissue in these regions. To be able to interrogate deep layers of tissue, near-infrared spectroscopy (NIRS) uses NIR wavelengths between 650 and 950 nm. NIRS is primarily employed to measure tissue oxygenation with parameters such as O2Hb, HHb, total hemoglobin (tHb) concentration, tissue oxygen saturation (StO2), and cerebral blood flow.3 NIRS has the advantage to enable bedside continuous and noninvasive monitoring of these parameters. Furthermore, it can be combined with other types of cerebral monitoring such as EEG or aEEG, and it is cost effective.2 For preterm infants undergoing intensive-care, cerebral oxygenation and hemodynamics measurements are important, since ischemic or hypoxic events in the brain frequently occur and may lead to death or serious lesions in the developing brain. For reliable and quantitative monitoring in a clinical environment, a high reproducibility of the measurement is important. The higher the reproducibility, the higher the clinical sensitivity and specificity. Menke stated that between O2Hb, HHb, tHb and StO2, StO2 is likely to be the most reproducible parameter. In previous studies, the reproducibility of StO2 was examined for different NIRS devices. Sorensen and Greisen4 reported an accuracy of 5.2% for StO2 for the NIRO 300 (Hamamatsu Photonics, Hamamatsu, Japan). This was considered as insufficient for clinical purposes.4 Arri 5 obtained a reproducibility of 4.6% for the OxiplexTS (ISS, Champaign, Illinois) and determined that low reproducibility is associated with inhomogeneous tissue. The aim of this study was to test the reproducibility of a novel sensor geometry which reduces the influence of superficial inhomogeneities. 2.Patients and Methods2.1.PatientsAfter parental informed consent was obtained, 12 female and 18 male neonates admitted to the intermediate care ward who were in stable clinical condition without need for supplemental oxygen, were measured in a supine or prone position. The demographic data of these infants are displayed in Table 1. This study has been approved by the local Ethical Committee. Table 1Demographic data of the measured infants.
2.2.ProtocolSince inhomogeneities such as nonscattering cerebral spinal fluid (e.g., in the fissura longitudinalis cerebralis) affect the accuracy of NIRS measurements, the NIRS sensor was positioned laterally between the frontal and temporal cerebral region. The child slept during the measurement time and was monitored during the whole investigation. The sensor was fixed with a gaze tube and the sensor was covered with a towel to reduce ambient light. If the newborn infant was calm, the readjustment of the instrument and the measurement were started immediately. The instrument does not need time to settle, due to its self-calibrating nature (see Sec. 2.3). Each measurement took approximately 1 min. The sensor was repositioned after each measurement (break time). To determine reproducibility, it is important not to include true physiological variation in the parameters. For this reason measurements were carried out in stable infants within a short period of time. To avoid previously observed 5a spatial variation, the sensor's position remained on the same side of the head and was changed only by a few millimeters. After each repositioning, the power of the light sources was automatically readjusted by the instrument and the detected light levels were visually inspected to avoid saturation of the detectors, before a new measurement was started. This procedure was repeated until 4 measurements were completed. The break time phases and the measurement phases were marked using a switch box; the emitted analog signal was simultaneously recorded and coded the break time and the measurement phases for the analysis. 2.3.NIRS MeasurementThe MCP II is a NIRS device developed in the Division of Neonatology, at the University Hospital Zurich and has been described in detail elsewhere.6 The sensor (Fig. 1) consists of four light source positions and four light detectors. The sources and detectors have an orthogonal configuration. Each light source position includes LEDs of three different wavelengths (750, 800, 875 nm). The LEDs are time-multiplexed. The light is detected by photodiodes. In principle, each LED detector combination can be measured. The channels can be sampled at a rate of 100 Hz. A laptop using Linux recorded the raw data and visualized them. Fig. 1The sensor configuration including two self-calibrating probes: The inner probe is visualized by the thick line, which connects the inner light sources and detectors (S2, S3, D2, D3) and includes four light bundles, two with a distance of 1.25 cm and two with a distance of 2.5 cm. The outer probe is visualized by the thick dotted line, which connects S1, S4, D1, and D4. This outer probe was not used in the analysis. 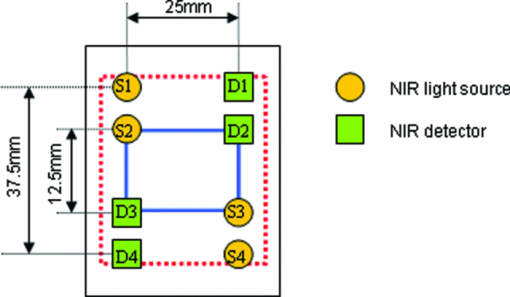 The sensor incorporates two completely symmetric configurations, depicted as inner and outer probes in Fig. 1, each constituting a self-calibrating probe geometry according to Ref. 7. The advantage of this geometry is that it is insensitive to superficial inhomogeneities and does not require a calibration to measure StO2. The self-calibrating principle requires two sources and two detectors (like the inner probe in Fig. 1), which are symmetrically arranged to include four light bundles with two source detector distances (in our case light bundles S2-D3 and S3-D2 with a distance of r 1 = 1.25 cm and light bundles S2-D2 and S3-D3 with a distance of r 2 = 2.5 cm). The self-calibrating principle is implemented by calculating the linear slope (S), i.e., the decrease in intensity (I) over distance (r), here for the example of the inner probe: Eq. 1[TeX:] \documentclass[12pt]{minimal}\begin{document}\begin{equation} \hspace*{-8pt}S = \ln [ {r^{2*} I(r)} ] = \frac{{0.5 ^* \ln \left({\displaystyle\frac{{I_{{\rm S2D2}} ^{\,\,\,\quad *} I_{{\rm S3D3}} }}{{I_{{\rm S2D3}} ^{\,\,\,\quad *} I_{{\rm S3D2}} }}} \right) + 2^*\ln \left({\displaystyle\frac{{r_2 }}{{r_1 }}} \right)}}{{r_2 - r_1 }}. \end{equation}\end{document}
[TeX:]
\documentclass[12pt]{minimal}\begin{document}\begin{equation*}
\mu _a \cdot \mu '_s = \frac{{S^2 }}{3}.\end{equation*}\end{document}
From
[TeX:]
$\mu _a \cdot \mu ^\prime _s$
at three wavelengths we can calculate
[TeX:]
${\rm O}_2 {\rm Hb} \cdot \mu ^\prime _s$
and
[TeX:]
${\rm HHb} \cdot \mu ^\prime _s$
. Note that the
[TeX:]
$\mu ^\prime _s$
is unknown, and thus the precise concentration of O2Hb and HHb are not known. However, when StO2 is calculated,
[TeX:]
$\mu ^\prime _s$
cancels out:
[TeX:]
\documentclass[12pt]{minimal}\begin{document}\begin{equation*}
{\rm StO}_2 = \frac{{{\rm O}_2 {\rm Hb} \cdot \mu '_s }}{{{\rm O}_2 {\rm Hb} \cdot \mu '_s + {\rm HHb} \cdot \mu '_s }} = \frac{{{\rm O}_2 {\rm Hb}}}{{{\rm O}_2 {\rm Hb} + {\rm Hhb}}}.
\end{equation*}\end{document}
A small error in the assumption is that [TeX:] $\mu ^\prime _s$ changes with wavelength and we adjusted for this by assuming the following [TeX:] $\mu ^\prime _s$ values: 3.81 cm−1 for 750 nm, 3.49 cm−1 for 800 nm, and 3.01 cm−1 for 875 nm. These values were linearly extrapolated from previous measurements.5 We also corrected for the absorption of water by assuming that the concentration of water is 90%. The algorithm was implemented in a MATLAB ® script and used offline to calculate StO2. Due to the low intensity of the emitted light, these measurements are harmless for the eyes and skin of the newborn children. Moreover, heating effects on the tissue are negligible. The sensor is embedded in a soft, flexible, and dark medical grade silicone. For this reason, it can be adapted to the curvature of the head and has a high wearing comfort. Despite the high flexibility of the sensor, the tiny heads of preterm infants had such a strong cranial curvature that only the inner probe of the sensor (Fig. 1) firmly touched the head, while the outer probe of the sensor was visibly not touching the skin and the respective measurements were consequently discarded. For this reason we only present data of the inner probe. 2.4.StatisticsWe used the open source statistics package R version 2.6.1 (2007-11-26) and in particular the function LME (linear mixed effects) with the StO2 values as random variable and the factor subject. The results were evaluated using the summary function. In addition, the residuals were checked for normality by the Kolmogorov–Smirnov and Shapiro Wilk test, which were both not significant, and also by inspecting the Q-Q plot, which showed a normal distribution, with the exception of one outlier. Hence we report the results with and without the outlier. A correlation analysis was carried out to test for correlations between the StO2 and the postnatal age, gestational age, and birthweight. 3.ResultsFor the complete data set, the mean StO2 was 79.99 ± 4.47%, the between-infant variability 4.20%, and the within-infant variability 2.76%. The error of measurement only accounts to 30.1% of the variability. When removing the one measurement, which was an outlier, the between-infant variability was 4.31%, the within-infant variability 2.46%, which in turn accounted for 24.5% of the variability. When the postnatal age was logarithmic transformed, there was a significant (p = 0.019, r = −0.424) linear correlation with StO2 (Fig. 2). The gestational age and birthweight had no significant effect on StO2 4.Discussion and ConclusionNIRS could be a valuable device for noninvasive monitoring of cerebral oxygen supply at the bedside, in particular the continuous monitoring of regional changes of StO2. Continuous cerebral monitoring of newborns undergoing intensive care is a demand in modern neonatology in the quest to early detect or prevent abnormalities and thus reduce neonatal morbidity and mortality. Monitoring the cerebral perfusion, oxygenation and function could be a significant step. Up to now no method has been established to monitor and prevent cerebral pathologies in a clinical routine. So far NIRS has rarely been used in clinical settings due to the partially challenging application and the lack of reproducibility. Another limitation is that several NIRS devices are commercially available and each has its own technology with individual strengths and limitations. Recent reproducibility studies were performed, which show little convincing results. Menke 3 were the first to test the reproducibility of the Critikon 2020 (Johnson and Johnson Medical, Newport, United Kingdom). They measured the reproducibility of StO2 (grand mean 69.7%), O2Hb, HHb and tHb. StO2 showed the best reproducibility with a low within-infant and between-infant variation of 1.7%, respectively, 4.1% in neonates. However, this device has been shown to underestimate changes of StO2 in vitro 9, 10 and in vivo, 11 and thus the reproducibility is likely to be larger in reality. The reason for this underestimation is based on the algorithm of the Critikon 2020 (Ref. 12) which uses the diffusion approximation with the infinite boundary condition, i.e., the tissue is assumed to surround the sensor in all directions and the tissue thickness is infinite. In today's instruments, such as our algorithm and the one of the Hamamatsu NIRO 300, the diffusion approximation for the semi-infinite boundary condition is mostly used, which assumes the tissue to be a flat surface extending to infinity. This corresponds reasonably to the situation of the NIRS measurement over the head. Our mean StO2 value of 79.99 ± 4.47% was considerably higher than the one of Menke 3 (67.9 %) measured by a Critikon 2020 and Lemmers 13 (∼70%), measured by the INVOS 4100 (Somanetics Corp., Troy, Michigan), and a little higher than the one of Sorensen and Greisen4 (74.6 ± 8.5%) measured by a NIRO 300. The algorithm of the Critikon 2020 used by Menke 3 is based on quite different assumptions, i.e., it assumes an infinite boundary condition of the diffusion approximation, which is a likely explanation for the difference in StO2. The algorithm of the INVOS 4100 is, to the best of our knowledge, not published, and thus it is impossible to discuss instrumental reasons for the different StO2 values. The NIRO 300 used by Sorensen and Greisen4 showed a small difference in mean StO2. Although the NIRO 300 assumes a semi-infinite boundary condition15 like our algorithm, it does not feature self-calibration of the sensor, it employs longer source detector distances, and it does not account for the water absorption, all instrumental factors which are different from our approach. In addition, the populations of Sorensen and Greisen4 and Lemmers 13 were younger by 6.3 or 4.6 weeks, respectively. All these reasons may be possible explanations for the difference of 5.4% or 10% between the studies. The significant decrease in StO2 with postnatal age is in line with previous findings.16 Although during the first three days of life StO2 increases,17 after passing this period, it generally decreases.16 Sorensen and Greisen carried out 3 to 8 measurements per neonate using the NIRO 300 (Hamamatsu, Hamamatsu, Japan) on 37 preterm infants. For each measurement, the sensor was replaced on any position on the head assuming that no regional differences in cerebral oxygenation exist. The mean StO2 was 74.6% and the reproducibility of the measurement was 5.2%. Since the between infant variability was similar to the reproducibility, Sorensen and Greisen concluded that StO2 measurements are clinically not feasible due to a lack of reproducibility.4 However, the study was carried out in clinically stable infants and it may still be possible to detect infants at risk, if their StO2 is low (<65%), and thus StO2 still may be clinically useful. Also, Dullenkopf 18 showed that StO2 measurements using the NIRO 300 over the lateral forehead of neonates and infants were not well reproduced under clinical conditions during sensor exchange experiments. However, the protocol of the study is not directly comparable to Sorensen and Greisen and our study. In real life applications the light coupling between the tissue, the sources, and the detectors, as well the sensitivity of the detectors, might be unknown and time dependent (e.g., sweat, pressure on sensor affect coupling). In addition, there may be superficial conditions that affect the light coupling, such as hair and birth marks. Such inhomogeneities violate the assumption of the algorithm, which assumes complete homogeneity. The MCPII uses a novel geometry with two sources and two detectors which is shown in Fig. 1. This special geometry removes the effect of inhomogeneities and varying coupling.7 It was the aim of this study to test whether this geometry leads to a higher reproducibility of StO2 values in neonates. Indeed this novel sensor geometry achieved a higher reproducibility compared to the Hamamatsu NIRO 300, which also employs a semi-infinite boundary condition. The higher reproducibility of the Critikon 2020 can probably be explained by two reasons: 1. An algorithm assumes an infinite boundary condition and thus any changes in StO2 are underestimated, which leads to a higher reproducibility. 2. The Critikon 2020 is the only other instrument that has an incorporated coupling compensation system, which removes coupling errors and thus may lead to a higher reproducibility. These results may indicate that the errors in light coupling are a key factor affecting the reproducibility of StO2 measurements. There are other reasons for a higher reproducibility in our study compared to Sorensen and Greisen,4 i.e., the higher postnatal and gestational age is expected to lead to a higher reproducibility. The reason for this could be due to basic anatomical and physiological conditions. Older infants have a larger head circumference and therefore a larger brain size, which makes it easier to find a potential measuring area which fulfils the assumptions of semi-infinite boundary condition. Furthermore, it is less difficult to ensure a close-fitting sensor on the head, especially for the MCPII and its neonatal sensor. In addition, the head of a preterm infant is more transparent and thus inhomogeneities may have a larger effect. Considering that particularly preterm infants often require cerebral monitoring, in future studies the reproducibility in infants with a low postnatal and gestational age will be addressed. In conclusion, these results confirm our hypothesis that the novel sensor geometry using a coupling compensation yields higher reproducibility. AcknowledgmentsWe gratefully acknowledge the support of the clinical staff, the participation of the infants, and the funding from the Swiss National Science Foundation. ReferencesV. Tuchin, Tissue Optics: Light Scattering Methods and Instruments for Medical Diagnosis (2000), The International Society of Optical Engineering(2000). Google Scholar
M. Wolf and
G. Greisen,
“Advances in near-infrared spectroscopy to study the brain of the preterm and term neonate,”
Clin. Perinatol., 36
(4), 807
–834
(2009). https://doi.org/10.1016/j.clp.2009.07.007 Google Scholar
J. Menke, U. Voss, G. Moller, and
G. Jorch,
“Reproducibility of cerebral near infrared spectroscopy in neonates,”
Biol. Neonate, 83
(1), 6
–11
(2003). https://doi.org/10.1159/000067006 Google Scholar
L. C. Sorensen and
G. Greisen,
“Precision of measurement of cerebral tissue oxygenation index using near-infrared spectroscopy in preterm neonates,”
J. Biomed. Opt., 11
(5), 054005
(2006). https://doi.org/10.1117/1.2357730 Google Scholar
S. J. Arri, T. Muehlemann, M. Biallas, H. U. Bucher, and
M. Wolf,
“Precision of cerebral oxygenation and hemoglobin concentration measurements in neonates measured by near-infrared spectroscopy,”
J. Biomed. Opt., 16
(4), 047005
(2011). https://doi.org/10.1117/1.3570303 Google Scholar
M. Keel, M. Wolf, O. Baenziger, V. Dietz, K. von Siebenthal, and
H. U. Bucher,
“Regional differences of cerebral hemoglobin concentration in preterm infants measured by near infrared spectrophotometry,”
Technol. Health Care, 7
(1), 63
–73
(1999). Google Scholar
D. Haensse, P. Szabo, D. Brown, J. C. Fauchere, P. Niederer, H. U. Bucher, and
M. Wolf,
“A new multichannel near infrared spectrophotometry system for functional studies of the brain in adults and neonates,”
Opt. Express, 13
(12), 4525
–4538
(2005). https://doi.org/10.1364/OPEX.13.004525 Google Scholar
D. M. Hueber, S. Fantini, A. E. Cerussi, and
B. Barbieri,
“New optical probe designs for absolute (self-calibrating) NIR tissue hemoglobin measurements,”
Proc. SPIE, 3597 618
–631
(1999). https://doi.org/10.1117/12.356784 Google Scholar
S. Fantini, M. A. Franceschini, and
E. Gratton,
“Semi-infinite-geometry boundary-problem for light migration in highly scattering media - a frequency-domain study in the diffusion-approximation,”
J. Opt. Soc. Am. B, 11
(10), 2128
–2138
(1994). https://doi.org/10.1364/JOSAB.11.002128 Google Scholar
M. Wolf, O. Baenziger, M. Keel, V. Dietz, K. von Siebenthal, and
H. U. Bucher,
“Testing near-infrared spectrophotometry using a liquid neonatal head phantom,”
Proc. SPIE, 3566 79
–86
(1998). https://doi.org/10.1117/12.334354 Google Scholar
M. Wolf, M. Keel, V. Dietz, K. von Siebenthal, H. U. Bucher, and
O. Baenziger,
“The influence of a clear layer on near-infrared spectrophotometry measurements using a liquid neonatal head phantom,”
Phys. Med. Biol., 44
(7), 1743
–1753
(1999). https://doi.org/10.1088/0031-9155/44/7/313 Google Scholar
M. Wolf, K. von Siebenthal, M. Keel, V. Dietz, O. Baenziger, and
H. U. Bucher,
“Tissue oxygen saturation measured by near infrared spectrophotometry correlates with arterial oxygen saturation during induced oxygenation changes in neonates,”
Physiol. Meas., 21
(4), 481
–491
(2000). https://doi.org/10.1088/0967-3334/21/4/305 Google Scholar
M. Wolf, P. Evans, H. U. Bucher, V. Dietz, M. Keel, R. Strebel, and
K. von Siebenthal,
“Measurement of absolute cerebral haemoglobin concentration in adults and neonates,”
Adv. Exp. Med. Biol., 428 219
–227
(1997). Google Scholar
P. M. Lemmers, M. Toet, L. J. van Schelven, and
F. van Bel,
“Cerebral oxygenation and cerebral oxygen extraction in the preterm infant: the impact of respiratory distress syndrome,”
Exp. Brain Res., 173
(3), 458
–467
(2006). https://doi.org/10.1007/s00221-006-0388-8 Google Scholar
S. J. Matcher, P. Kirkpatrick, K. Nahid, M. Cope, and
D. T. Delpy,
“Absolute quantification methods in tissue near infrared spectroscopy,”
Proc. SPIE, 2389 486
–495
(1995). https://doi.org/10.1117/12.209997 Google Scholar
N. Roche-Labarbe, S. A. Carp, A. Surova, M. Patel, D. A. Boas, P. E. Grant, and
M. A. Franceschini,
“Noninvasive optical measures of CBV, StO2, CBF index, and rCMRO(2) in human premature neonates’ brains in the first six weeks of life,”
Hum. Brain Mapp, 31
(3), 341
–352
(2010). https://doi.org/10.1002/hbm.20868 Google Scholar
G. Naulaers, G. Morren, S. Van Huffel, P. Casaer, and
H. Devlieger,
“Measurement of tissue oxygenation index during the first three days in premature born infants,”
Adv. Exp. Med. Biol., 510 379
–383
(2003). Google Scholar
A. Dullenkopf, A. Kolarova, G. Schulz, B. Frey, O. Baenziger, and
M. Weiss,
“Reproducibility of cerebral oxygenation measurement in neonates and infants in the clinical setting using the NIRO 300 oximeter,”
Pediatric Critical Care Medicine, 6
(3), 344
–347
(2005). https://doi.org/10.1097/01.PCC.0000161282.69283.75 Google Scholar
|


