|
|
|
Colorectal cancer (CRC) is a major cause of cancer-related deaths in the world.1 It is commonly believed that adenomatous polyps, or adenomas, appear to be major precursors to CRC, and the morbidity rate from CRC could be significantly reduced if these dysplastic lesions were detected.2, 3 However, it is challenging that the current endoscopic screening methods with white light detect these dysplastic lesions.4, 5 Thus, the development of new approaches to identify such lesions is of great medical significance. Recently, the remodeling of the stroma has been implicated in colonic dysplasia,6, 7 and these alterations may provide a better biomarker of these lesions than currently available screening methods. The stroma is composed primarily of type-I collagen, which is known to induce a second harmonic generation (SHG) signal.8, 9 SHG microscopy, in recent years, has emerged to be a promising technique for the qualitative and quantitative characterization of stromal biology with advantages of being label-free, inherent three-dimension resolution, near-IR excitation for superior optical penetration, lower photodamage, and capable of providing quantitative information.8, 9, 10, 11 Therefore, it has been recognized as promising approaches for cancer diagnostics.9, 12, 13, 14 To date, we have found no previous studies that quantitatively investigated the remodeling of the stroma in dysplastic colonic mucosa, and this is what motivated us to do this work. In this study, we used SHG microscopy to analyze the collagen change of normal and dysplastic colonic mucosa. SHG microscopy was achieved using a nonlinear optical system which has been described previously.14 In brief, SHG images were acquired using a commercial laser scanning microscopic imaging system (Zeiss LSM 510 META, Jena, Germany) coupled to a femtosecond Ti:sapphire laser (Coherent Mira 900-F) operating at 800 nm. The polarization direction of the laser light is the horizontal polarization. An oil immersion objective (×63 and NA = 1.4) was employed for focusing the excitation beam into tissue samples (average power less than 15 mW) and was also used to collect the backscattered intrinsic SHG signals. The images were obtained at 2.56 μs per pixel. A fine focusing stage (HRZ 200 stage, Carl Zeiss) was used to change the focus position for recording various optical sections. A total of nine fresh ex vivo colon tissue samples were obtained immediately after resection from patients undergoing a colectomy for familial adenomatous polyposis. Prior to study participation, all patients signed an informed consent, and this study was approved by the Institutional Review Board of Fujian Medical University. Colonic adenomas are dysplastic lesions. Typical adenomas are polypoid structures and the adenomas are surrounded by normal tissue. In this study, the surrounding normal regions and the polyp regions were imaged. After SHG imaging, the tissue specimens were fixed in 10% formalin and prepared for pathologic examination using standard protocols. In this work, all data were presented as a mean value plus-or-minus its standard deviation, and analyzed using Student's t-test. Differences were considered to be statistically significant when the P-values were less than 0.05. Shown in Fig. 1 are the representative en face SHG images from normal and dysplastic colonic mucosa. As can be seen from Fig. 1, the stroma can be identified because the epithelium consists mainly of columnar epithelial cells and goblet cells that are not effective in generating SHG signals and the stroma is composed primarily of type-I collagen that is capable of emitting strong SHG signals. Morphologically, normal and dysplastic colonic mucosa can be easily discriminated. In a normal case, the collagen displays a denser matrix, and the collagen fibers are almost parallel to the interface of epithelium and stroma. In dysplasia, a looser collagen matrix is observed, and the collagen fibers are located at an angle to the interface of epithelium and stroma. It is worth pointing out that these features are not visible in standard hematoxylin and eosin-stained sections, as presented in Fig. 2. Fig. 1Representative SHG images from (a) normal and (b) dysplastic colonic tissues. The size of the images is 146 × 146 μm2. 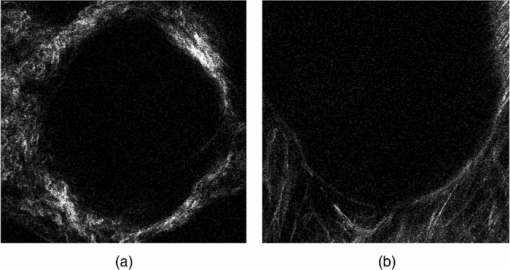 Fig. 2Typical histological images (magnification, 40×), from (a) normal and (b) dysplastic colonic tissues. 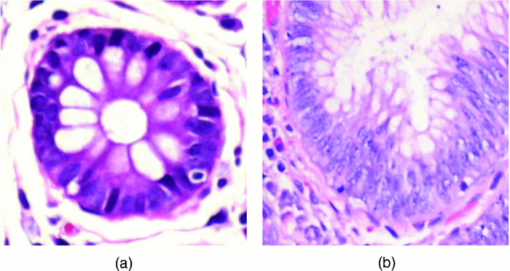 In the following, to further quantify these features, two quantitative analyses were performed. First, the depth-dependent decay (DDD) of the SHG signal was analyzed to determine the collagen density. In short, a series of SHG images were obtained at intervals of 1 μm up to a final depth of 30 μm; the average SHG intensity of selected regions at each depth was then calculated and plotted as a function of depth. Decrease in SHG signal intensity with increasing depth can be approximated using a first-order model: I = Ae −kx + C, where I is the SHG intensity, x is the imaging depth, A is a pre-exponential scaling factor, C is an arbitrary constant that adjusts the lowest signal intensity to zero, and k is the DDD constant.15 Figure 3 shows a typical plot of the exponential fits from normal and dysplastic colonic tissues. Recently, Dunn found that although scattering plays a role in DDD, absorption is the main factor responsible for DDD in turbid media at least to a depth of focus of 412 μm. Thus, although other factors, such as direction, distribution, and size of collagen fibers, may contribute to changes in the DDD value, it is a reasonable measure of collagen density.16 Moreover, the collagen fiber angle relative to the tangent of the interface of epithelium and stroma was measured every 5 μm using IMAGEJ software (National Institutes of Health), as reported previously.17 As shown in Table 1, the dysplastic colonic mucosa has a lower DDD constant and a bigger collagen fiber angle (p < 0.05), indicating loss of the collagen density and the collagen fibers located at an angle to the interface of epithelium and stroma in dysplasia, consistent with the above-mentioned observations. Therefore, these differences in the collagen density and the collagen fiber direction may be used to quantitatively discriminate between normal and dysplastic colonic mucosa, and serve as quantitative intrinsic biomarkers for quantifying the diagnosis of colonic dysplasia. Fig. 3Typical plot of the exponential fits from normal (circles), and dysplastic (triangles) colonic tissues; intensity at depth 0 μm has been normalized to unity in each case. 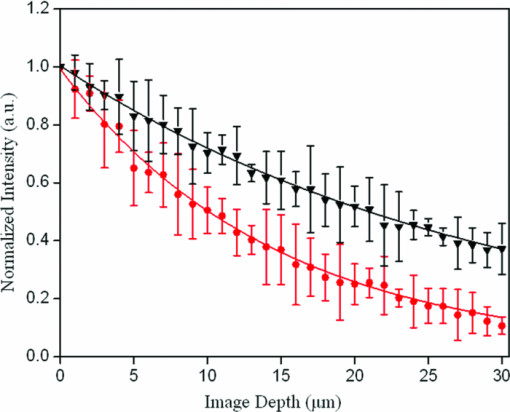 Table 1Quantitative variables with dysplasia.
In conclusion, this study demonstrates the potential of intrinsic SHG imaging to provide biochemical and morphological biomarkers, including the collagen density and the collagen fiber direction, which can be used to discriminate between normal and dysplastic colonic mucosa. With the advent of the SHG-based endoscopy,18 we expect that the analysis of collagen change using intrinsic SHG imaging may become an important noninvasive methodology that provides quantitative information about the collagen change for diagnosing colonic dysplasia. AcknowledgmentsThe project was supported by the National Natural Science Foundation of China (Grant Nos. 60908043 and 30970783), and the Natural Science Foundation of Fujian Province (Grant No. 2011J01341). ReferencesA. Jemal,
R. Siegel,
E. Ward,
Y. Hao,
J. Xu, and
M. J. Thun,
“Cancer statistics, 2009,”
Ca-Cancer J. Clin., 59 225
–249
(2009). https://doi.org/10.3322/caac.20006 Google Scholar
S. Srivastava,
D. E. Henson, and
A. Gazdar, Molecular Pathology of Early Cancer, IOS Press, Amsterdam
(1998). Google Scholar
S. J. Miller,
B. P. Joshi,
Y. Feng,
A. Gaustad,
E. R. Fearon, and
T. D. Wang,
“In vivo fluorescence-based endoscopic detection of colon dysplasia in the mouse using a novel peptide probe,”
PLoS ONE, 6 e17384
(2011). https://doi.org/10.1371/journal.pone.0017384 Google Scholar
J. C. van Rijn,
J. B. Reitsma,
J. Stoker,
P. M. Bossuyt,
S. J. van Deventer, and
E. Dekker,
“Polyp miss rate determined by tandem colonoscopy: a systematic review,”
Am. J. Gastroenterol., 101 343
–350
(2006). https://doi.org/10.1111/j.1572-0241.2006.00390.x Google Scholar
G. Gualco,
N. Reissenweber,
I. Cliche, and
C. E. Bacchi,
“Flat elevated lesions of the colon and rectum: A spectrum of neoplastic and nonneoplastic entities,”
Ann. Diagn. Pathol., 10 333
–338
(2006). https://doi.org/10.1016/j.anndiagpath.2006.03.003 Google Scholar
R. Kalluri and
M. Zeisberg,
“Fibroblasts in cancer,”
Nat. Rev. Cancer, 6 392
–401
(2006). https://doi.org/10.1038/nrc1877 Google Scholar
T. J. Romer,
M. Fitzmaurice,
R. M. Cothren,
R. Richards-Kortum,
R. Petras,
M. V. Sivak Jr., and
J. R. Kramer Jr,
“Laser-induced fluorescence microscopy of normal colon and dysplasia in colonic adenomas: implications for spectroscopic diagnosis,”
Am. J. Gastroenterol., 90 81
–87
(1995). Google Scholar
W. R. Zipfel,
R. M. Williams,
R. Christie,
A. Y. Nikitin,
B. T. Hyman, and
W. W. Webb,
“Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation,”
Proc. Natl. Acad. Sci. U.S.A., 100 7075
–7080
(2003). https://doi.org/10.1073/pnas.0832308100 Google Scholar
P. J. Campagnola1 and
C. Y. Dong,
“Second harmonic generation microscopy: principles and applications to disease diagnosis,”
Laser Photonics Rev., 5 13
–26
(2011). https://doi.org/10.1002/lpor.200910024 Google Scholar
S. M. Zhuo,
J. X. Chen,
T. S. Luo,
D. S. Zou, and
J. J. Zhao,
“Multimode nonlinear optical imaging of the dermis in ex vivo human skin based on the combination of multichannel mode and Lambda mode,”
Opt. Express, 14 7810
–7820
(2006). https://doi.org/10.1364/OE.14.007810 Google Scholar
W. R. Zipfel,
R. M. Williams, and
W. W. Webb,
“Nonlinear magic: multiphoton microscopy in the biosciences,”
Nat. Biotechnol., 21 1369
–1377
(2003). https://doi.org/10.1038/nbt899 Google Scholar
S. M. Zhuo,
J. X. Chen,
S. S. Xie,
Z. B. Hong, and
X. S. Jiang,
“Extracting diagnostic stromal organization features based on intrinsic two-photon excited fluorescence and second-harmonic generation signals,”
J. Biomed. Opt., 14 020503
(2009). https://doi.org/10.1117/1.3088029 Google Scholar
O. Nadiarnykh,
R. B. LaComb,
M. A. Brewer, and
P. J. Campagnola,
“Alterations of the extracellular matrix in ovarian cancer studied by second harmonic generation imaging microscopy,”
BMC Cancer, 10 94
(2010). https://doi.org/10.1186/1471-2407-10-94 Google Scholar
S. M. Zhuo,
J. X. Chen,
G. Z. Wu,
S. S. Xie,
L. Q. Zheng,
X. S. Jiang, and
X. Q. Zhu,
“Quantitatively linking collagen alteration and epithelial tumor progression by second harmonic generation microscopy,”
Appl. Phys. Lett., 96 213704
(2010). https://doi.org/10.1063/1.3441337 Google Scholar
B. A. Torkian,
A. T. Yeh,
R. Engel,
C. H. Sun,
B. J. Tromberg, and
B. J. Wong,
“Modeling aberrant wound healing using tissue-engineered skin constructs and multiphoton microscopy,”
Facial. Plast. Surg., 6 180
–187
(2004). https://doi.org/10.1001/archfaci.6.3.180 Google Scholar
A. K. Dunn,
V. P. Wallace,
M. Coleno,
M. W. Berns, and
B. J. Tromberg,
“Influence of optical properties on two-photon fluorescence imaging in turbid samples,”
Appl. Opt., 39 1194
–1201
(2000). https://doi.org/10.1364/AO.39.001194 Google Scholar
P. P. Provenzano,
K. W. Eliceiri,
J. M. Campbell,
D. R. Inman,
J. G. White, and
P. J. Keely,
“Collagen reorganization at the tumor-stromal interface facilitates local invasion,”
BMC Medicine, 4 38
(2006). https://doi.org/10.1186/1741-7015-4-38 Google Scholar
H. C. Bao,
A. Boussioutas,
R. Jeremy,
S. Russell, and
M. Gu,
“Second harmonic generation imaging via nonlinear endomicroscopy,”
Opt. Express, 18 1255
–1260
(2010). https://doi.org/10.1364/OE.18.001255 Google Scholar
|

