|
|
|
Raman microspectroscopy combines Raman spectroscopy with optical microscopy1 and has been widely applied in biomedicine, materials, and environmental science.1, 2 It uses a focused laser beam as a single-point illumination on the sample by an objective, and the Raman-scattered signal from a tiny volume (<1 μm3) is collected in a confocal configuration to obtain high sensitivity and resolution.1, 2 Raman microspectroscopy has been employed as a single-particle analyzer for noninvasive and label-free chemical analyses of single bacteria, viruses, mammalian cells, organelles, and nanoparticles.1, 2 With the help of surface-enhancement, it allows ultrasensitive chemical analysis and single molecule detection such as crystal violet.3 The combination with optical tweezers allows real-time analysis of single living cells suspended in an aqueous medium.4, 5 Recently, Raman microspectroscopy has been used to analyze airborne particles collected with the impactors,6, 7 which is important in analyses related to human health, pollution monitoring, and global climate change. However, the weak nature of Raman scattering generally limits its use since it typically takes a few or tens of seconds to obtain a Raman spectrum of an unknown particle, especially biological particles, and single-focus excitation can only analyze one particle within a Raman acquisition time. Consequently, this technique becomes time-consuming when the analysis of large numbers of single particles is desired, and the laser power shining on each particle is constrained, such as when monitoring physiological dynamics of multiple individual microbial cells in a population4 and when attempting to quantify individual airborne particles impacted at random positions on a cover slip.6, 7 In this paper, we report the development of a multifocus confocal Raman microspectroscopy system that allows parallel analysis of multiple individual particles in random positions on a cover slip based on a precise image-guided technique. The multifocus excitation technique has been used in laser scanning fluorescence microscopy,8 coherent anti-stokes Raman scattering microscopy,9 Raman imaging,10 and recently in multiple-trap laser tweezers Raman spectroscopy.4 Here, we show that this technique can be used to rapidly measure relative concentration of microparticles, to rapidly identify individual aerosol particles collected in indoor air and monitor biological dynamics of single bacterial spores during their germination. As a result, it allows parallel monitoring of dynamic process of 80 individual cells in 1 h, which otherwise would take at least 80 h to obtain the statistical data by using single-focus Raman microspectroscopy. The experimental schematic is shown in Fig. 1a. A laser beam at 780 nm was introduced into an inverted microscope (Nikon TiS) equipped with an objective (Plan Apo 60×, NA = 1.4) and a green-filtered halogen lamp was used for illumination.4, 5 An imaging camera was used to record a bright-field or phase-contrast image which was analyzed by a MATLAB program to locate the centroid positions (x i, y i) of 80 particles in a field of view. These coordinates were used to drive a pair of galvo-mirrors GM1 and GM2 (Cambridge Technology, 6220H) to rapidly steer a single laser beam with step-function waveforms to form multifocus excitation on 80 individual particles. Another galvo-mirror GM3 (Thorlabs, GVS001) in front of the spectrograph (Princeton Instruments, LS785) synchronously steered the backward Raman scattering from each focus onto different vertical positions of a multichannel CCD chip (Princeton Instruments, PIXIS 400BR) so that full Raman spectra from each focus can be collected simultaneously.10 The time that the laser focus illuminated each particle was equal within a cycle and much longer than the transition time for the laser beam to jump from one particle to another. The scanning frequency at which the laser focus was steered across each particle was high (∼10 Hz) such that an effective time-averaged pattern illumination was created during the CCD acquisition time (1 to 10 s). A 50-μm confocal pinhole was used such that the Raman spectral image of each focus on the CCD chip occupied 2 to 3 pixels (20 μm/pixel). As a result, the CCD chip with 400 vertical pixels can be used to simultaneously collect 80 Raman spectra (5 pixels/spectrum). The measured lateral and axial resolutions of the confocal system are about 0.5 and 1.2 μm, respectively. Fig. 1Experimental setup. (a) Schematic of multifocus confocal Raman microscopy. GM: galvo-mirrors; DM: dichroic mirrors; L: lens. (b) Phase-contrast image of a mixture of polystyrene beads and B. megaterium spores. Beads (numbered in green) and spores (numbered in red) have been identified by multifocus confocal Raman microscopy as shown in (c). (c) Individual Raman spectral images of 80 particles in (b) measured by the spectrograph's CCD chip with one exposure time. Typical Raman spectra of a polystyrene bead and a B. megaterium spore are shown at the top and bottom, respectively. 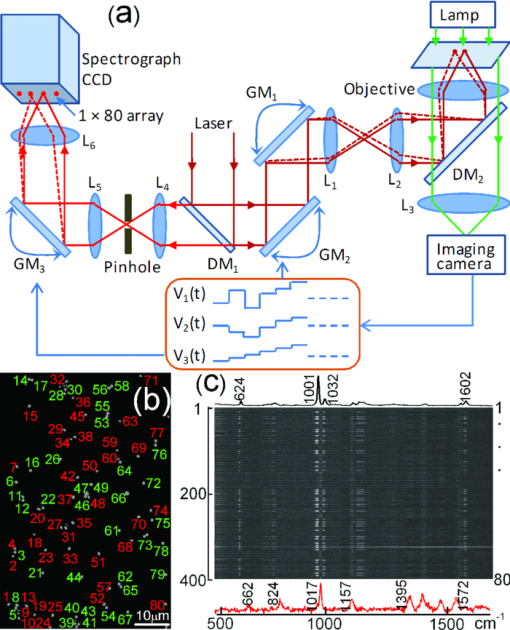 The advantages of using synchronous galvo-mirrors include: 1. the multifocus excitation pattern under the microscope is programmable and image-guided; and 2. the separation of Raman scattering from each focus on the CCD chip can be independently changed by the third galvo-mirror so that cross talk between different spectral channels can effectively be avoided.4 The difference between current work and multifoci-scan Raman imaging10 is that the 80 laser foci were only switched to each individual particle's position here, while in Raman imaging the laser foci are scanned across the entire area of the sample. This switching scheme effectively avoids useless Raman acquisition from the positions without particle occupation and thus increases the speed of multiple particle analysis. Figure 1b shows a phase-contrast image of 80 particles of an unknown mixture of 1-μm polystyrene beads and dormant Bacillus megaterium spores. Apparently, these particles cannot be distinguished by their image sizes and shapes. Figure 1c shows the Raman spectral image of the 80 particles on the CCD chip with typical Raman spectra of a polystyrene bead (top spectrum) and a B. megaterium spore (bottom spectrum) recorded with an incident power of 1.0 mW per focus (with total power of 80 mW for the 80 foci) and a 5 s exposure time. From the single-particle Raman spectra, these particles can be easily distinguished, based on characteristic bands at 1001 cm−1 for polystyrene beads and 1017 cm−1 for B. megaterium spores. The latter band is due to the presence of (∼10% of dry wt) 1:1 chelate of Ca2+ with pyridine-2,6-dicarboxylic acid (dipicolinic acid) (CaDPA) in spores.4 Due to the rapid identification capability noted above, it is possible to rapidly measure the relative percentage of specific microparticles in a mixture. As a demonstration, we mixed B. megaterium and B. subtilis spores with relative ratios of 1:3, 1:2, 1:1, 2:1, and 3:1, and ∼10 μl of each mixture was dried on a coverslip. For each mixture, we randomly measured 20 fields of view that each contained ∼40 spores, as shown in Fig. 2c, and ∼800 particles were analyzed for each mixture. Figures 2a, 2b show Raman spectra of B. megaterium and B. subtilis spores, respectively. They can be easily distinguished because of the two carotenoid-specific bands at 1157 and 1520 cm−1 uniquely from B. megaterium spores.10 Therefore, the ratio of the numbers of B. megaterium and B. subtilis spores in a mixture can be determined for each condition simply by measuring the carotenoid-specific bands. Figure 2d shows the ratios as the function of the relative concentrations of the sample mixtures and a linear relationship was observed. Fig. 2Relative concentration measurements of mixed B. megaterium and B. subtilis spores. Raman spectra of (a) B. megaterium and (b) B. subtilis spores. (c) Phase-contrast image of the mixed spores of B. megaterium (numbered in red) and B. subtilis (numbered in black). (d) Measured ratios of B. megaterium and B. subtilis spores versus the relative concentration of B. megaterium spores in the mixture. The red curve is the fitted line. 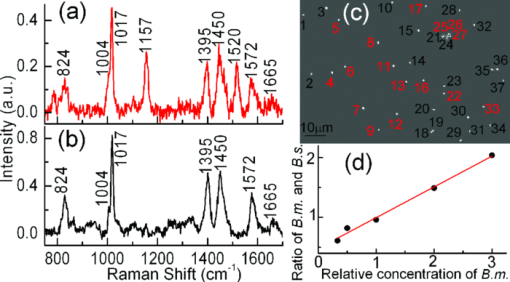 Multifocus confocal Raman microspectroscopy can also be used to rapidly identify airborne particles. Aerosol particles in a laboratory room were collected into deionized water (10 ml) with an AGI-30 impinger at a flow rate of 15 l/min for 30 min, as shown in Fig. 3b. A small drop of the collected particles were deposited and dried on a quartz coverslip. Figure 3c shows a bright field image of 40 individual unknown particles. Raman spectra of these particles were simultaneously collected with a laser power of 2.5 mW per particle and an exposure time of 5 s. These spectra can be classified into five different types, as shown in Fig. 3a. These particles were identified as i. carbon (with 1300 and 1600 cm−1 bands); ii. glassy silicates; iii. the mixed compounds of carbonates and silicates; iv. and v. silicate particles with and without fluorescence background.6, 7 Fig. 3Identification of airborne particles in the atmosphere. (a) Raman spectra of five typical aerosol particles with particle numbers as labeled in (c). (b) Air sampler that collected the aerosol particles into water for spectroscopic analysis. (c) Selected aerosol particles that were recognized with a MATLAB program. 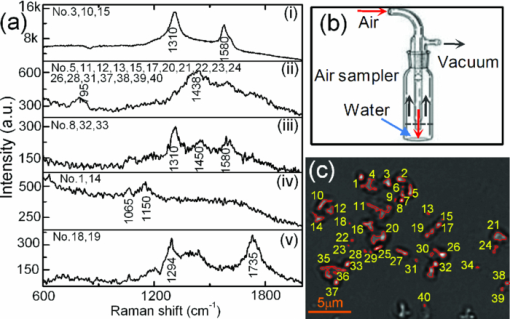 The most effective application of multifocus confocal Raman microspectroscopy could be simultaneous monitoring of dynamic physiological processes of multiple living cells adhered at random positions on a coverslip. As an example, we monitored the germination of 20 B. megaterium spores using Raman microspectroscopy and phase-contrast microscopy. The spores were first heat-activated at 60 °C for 15 min in a water bath and were then adhered firmly on the surface of a quartz coverslip. The germination solution of 0.5 mm D-glucose with 25 mm KPO4 buffer (pH 7.4) was added at t = 0 min to initiate germination at 37 °C.4, 5 The phase-contrast images were captured at a rate of 10 s per frame and the first frame was used to locate the positions of the 20 spores, as shown in Fig. 4a. During the germination process of 60 min, the microscope stage may have slight movements in the horizontal direction, causing the displacement of spores from the laser foci. Therefore, a new frame of phase-contrast images was used to recalculate the coordinates of the 20 spores every 2 min to precisely guide the laser foci to the spores. The Raman spectra of these spores were acquired with an exposure time of 20 s and a laser power of 5 mW per focus. Figures 4b, 4c show the time-lapse changes in the CaDPA level (intensity of the 1017 cm−1 band) and refractility (intensity of the phase-contrast image) of seven germinating spores, respectively. The data showed that for each germinating spore, the completion of CaDPA release precisely corresponded to the end of the rapid fall in phase-contrast image intensity and the rapid CaDPA release process normally took 1 to 2 min.5 Up to 80 spores can be monitored in parallel by using this multifocus Raman microspectroscopy. Fig. 4Simultaneous monitoring of the germination of multiple individual B. megaterium spores. (a) Phase-contrast image of 20 B. megaterium spores before the addition of germinant. (b) Intensities of the CaDPA-specific band at 1017 cm−1 and (c) intensities of phase-contrast images of seven germinating spores as a function of the germination time. 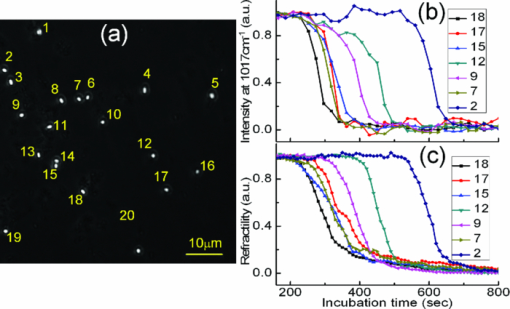 In summary, we have developed a multifocus confocal Raman microspectroscopy system based on an image-guided synchronous galvo-mirror scheme, allowing ∼80 individual particles in random positions to be analyzed simultaneously. We further demonstrated the utility of this analyzer for rapid identification of single bacteria and airborne particles collected on a cover slip, accurate concentration measurements, and the monitoring of physiological dynamics of multiple individual bacterial spores during their germination. This technique can further be integrated with differential inference-contrast and fluorescence microscopy,4 and may find broad applications in microbiology and environmental science. AcknowledgmentsThis work was supported by a Multi-University Research Initiative (MURI) award through the U.S. Army Research Laboratory and the U.S. Army Research Office (PS/YQL) via contract Grant No. W911NF-09-1-0286 and by a grant from the Army Research Office (YQL/PS) via contract Grant No. W911NF-08-1-0431. ReferencesG. J. Puppels,
F. F. M. de Mul,
C. Otto,
J. Greve,
M. Robert-Nicoud,
D. J. Arndt-Jovin, and
T. M. Jovin,
“Studying single living cells and chromosomes by confocal Raman microspectroscopy,”
Nature, 347
(6290), 301
–303
(1990). https://doi.org/10.1038/347301a0 Google Scholar
G. Turrell and
J. Corset, Raman Microscopy: Developments and Applications, Academic Press, London
(1996). Google Scholar
K. Kneipp,
Y. Wang,
H. Kneipp,
L. T. Perelman,
I. Itzkan,
R. Dasari, and
M. S. Feld,
“Single molecule detection using surface-enhanced Raman scattering (SERS),”
Phys. Rev. Lett., 78
(9), 1667
–1670
(1997). https://doi.org/10.1103/PhysRevLett.78.1667 Google Scholar
L. B. Kong,
P. F. Zhang,
G. W. Wang,
P. Setlow, and
Y. Q. Li,
“Characterization of bacterial spore germination using phase contrast microscopy, fluorescence microscopy, Raman spectroscopy and optical tweezers,”
Nat. Protoc., 6
(5), 625
–639
(2011). https://doi.org/10.1038/nprot.2011.307 Google Scholar
L. B. Kong,
P. F. Zhang,
P. Setlow, and
Y. Q. Li,
“Characterization of bacterial spore germination using integrated phase contrast microscopy, Raman spectroscopy and optical tweezers,”
Anal. Chem., 82
(9), 3840
–3847
(2010). https://doi.org/10.1021/ac1003322 Google Scholar
N. P. Ivleva,
U. McKeon,
R. Niessner, and
U. Pöschl,
“Raman microspectroscopic analysis of size-resolved atmospheric aerosol particle samples collected with an ELPI: soot, humic-like substances, and inorganic compounds,”
Aerosol Sci. Technol., 41
(7), 655
–671
(2007). https://doi.org/10.1080/02786820701376391 Google Scholar
S. Mertes,
B. Dippel, and
A. Schwarzenbock,
“Quantification of graphitic carbon in atmospheric aerosol particles by Raman spectroscopy and first application for the determination of mass absorption efficiencies,”
Aerosol Sci., 35
(3), 347
–361
(2004). https://doi.org/10.1016/j.jaerosci.2003.10.002 Google Scholar
J. Bewersdorf,
R. Pick, and
S. W. Hell,
“Multifocal multiphoton microscopy,”
Opt. Lett., 23
(9), 655
–657
(1998). https://doi.org/10.1364/OL.23.000655 Google Scholar
T. Minamikawa,
M. Hashimoto,
K. Fujita,
S. Kawata, and
T. Araki,
“Multi-focus excitation coherent anti-Stokes Raman scattering (CARS) microscopy and its applications for real-time imaging,”
Opt. Express, 17
(12), 9526
–9536
(2009). https://doi.org/10.1364/OE.17.009526 Google Scholar
L. B. Kong,
P. F. Zhang,
J. Yu,
P. Setlow, and
Y. Q. Li,
“Rapid confocal Raman imaging using a synchro multifoci-scan scheme for dynamic monitoring of single living cells,”
Appl. Phys. Lett., 98
(21), 213703
(2011). https://doi.org/10.1063/1.3595482 Google Scholar
|

