|
|
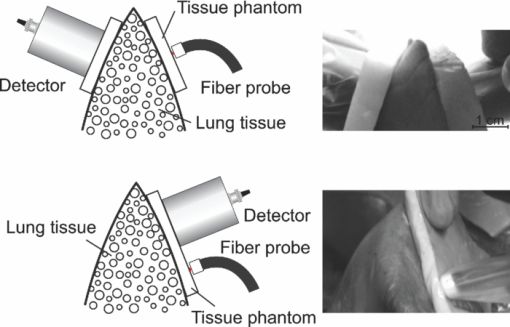
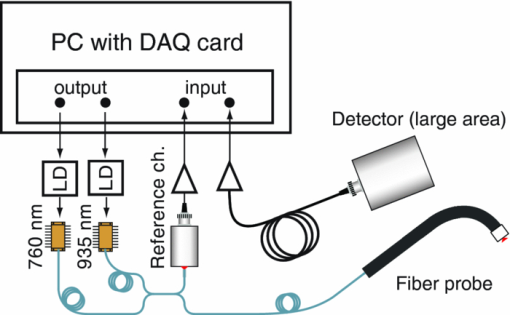
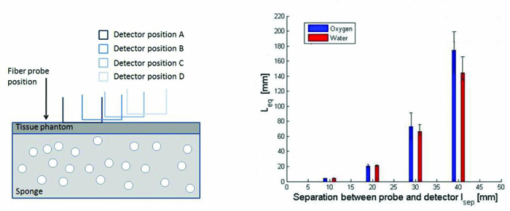
1.IntroductionThe fetal lungs are among the last vital organs to mature during pregnancy, and the functionality of this organ is therefore highly affected by a preterm birth. In Europe and the US between 6% and 15% of the deliveries are preterm1, 2 and result in large intensive-care costs.3 A major mortality factor of these infants is the respiratory distress syndrome (RDS).4 It is caused by a combination of an insufficient production of surfactant and immaturity of the lungs. Surfactant is a compound that distends the alveoli so that they do not collapse. RDS is treated with exogenous surfactant administration and respiratory support, such as nasal continuous positive airways pressure or mechanical ventilation with additional oxygen supply. Treatment in neonatal intensive-care units includes several sophisticated monitoring and regulating systems, controlling and measuring the oxygenation, temperature, etc. The diagnostic method to study the lung functionality is mainly x-ray scanning. Recently, electrical impedance tomography has been applied to estimate the air volume on the research level.5 This technique involves multiple electrical electrodes which can cause irritation. Nonoptimal handling of respiration assistance increases morbidity and may result in brain damage and chronic lung disease. It would be very valuable with a minimally invasive technique to continuously monitor the lung function, including the oxygen contents and the air-filled volumes and their dynamics in these preterm neonates. With increasing survival rate of the most immature infants, the need for assessing lung function increases. We present a feasibility study on a new technique allowing for gas monitoring inside the lungs of preterm infants. The technique is based on diode laser absorption spectroscopy and is noninvasive and nondamaging, making the monitoring repeatable. The gas is sensed by sending in low-intensity laser light and detecting the absorption imprints of lung gases in the diffusely emerging light. This is accomplished by tuning the lasers across sharp absorption lines due to energy-level transitions in molecular oxygen and water vapor, occurring around 760 and 935 nm, respectively. Measurements presented here are restricted to studies on realistic phantoms, with ventilated animal lung tissue, surrounded by gelatin layers of relevant thickness and with scattering and absorption properties to simulate measurements through the thoracic wall of the baby. We show that the technique yields relevant and understandable signals, prompting the planning of a clinical trial on premature babies. The now employed method of noninvasive sensing of free gas located inside the human body using laser spectroscopy was first demonstrated by our group in 2005, and has been investigated as a tool to diagnose paranasal sinus problems.6, 7, 8, 9, 10, 11 The possibility to obtain information on the gas filled volume through water vapor monitoring, and the ventilation of the sinus through a combination of oxygen and water vapor sensing was demonstrated in a clinical trial.12 Ventilation could also be assessed by observing the temporal response of the oxygen signal on flushing the nasal cavity with nitrogen gas.8, 10 Furthermore, nonintrusive monitoring of gas located in the mastoid bone, a porous gas-filled bone structure behind the ear, has been demonstrated and also shows clinical correlation.13 The fact that light can penetrate into tissue in the region 600 to 1400 nm, the tissue optical window, makes it possible to probe the gas in a cavity or distributed in pores, although surrounded by tissue. Within this optical window, where the tissue appears translucent and light can be faintly transmitted up to a couple of centimeters, molecular oxygen and water vapor exhibit weak absorption lines. The scattering and absorption of the tissue, responsible for the limited penetration depth, require a proper understanding of diffuse light propagation. This special regime, with absorption of gas inside highly scattering media, is referred to as gas in scattering media absorption spectroscopy, GASMAS, and was first demonstrated in 2001.14 The technique has been used in several nonmedical applications including the study of wood drying,15 for a pharmaceutical tablet,16 as well as food monitoring.17, 18 Analytical laser spectroscopy for concentration assessment relies on the use of the Beer–Lambert law for light attenuation through the sample. Basically, the product of concentration and path length is measured. With a well-known path length through a nonscattering sample in a cuvette, the concentration can be obtained directly after calibration. In scattering media, the path length is not well-defined—a distribution of path lengths is obtained, and the concentration determination is difficult. By normalizing the oxygen signal on the water vapor signal, the absolute oxygen concentration can be calculated, since the concentration of water vapor is only determined by the accurately known temperature. The procedure assumes that the bulk material absorption and scattering properties are the same for the two wavelengths used. This is not quite the case, and we will discuss implications below. Measurements through gas-containing strongly scattering media can be performed in two basic geometrical arrangements—in transmission or in reflectance. For thick samples, no light would propagate to a detector on the back side of the sample—then the use of reflectance geometry is only possible. Clearly, a larger source-detector separation then leads to a deeper sampling volume at the same time as the diffuse light level is decreased. We have explored both geometries in our phantom studies simulating neonatal lung monitoring. The general conclusion was found to be that transmission measurements are likely to be possible only for the most premature (smallest) babies, while reflectance measurements can be pursued on larger children, limited to the case when the thickness of the thoracic wall impairs the possibility of the photons to interrogate the underlying lung parenchyma. First the tissue phantoms and the experimental setup are described. Then measurements are reported with a presentation of the main results. Finally, conclusions are drawn, and suggestions for future work are made. 2.Materials and MethodsSince the objective of the study was to investigate the possibility of optical assessment of gas in neonatal lungs in phantom studies, it was of crucial importance to construct models with relevant size and optical properties similar to those of human tissue. Based on x-ray images from the Pediatrics Clinic, Lund University, the chest diameter of a 27 week preterm infant, weighing about 1 kg is around 6 cm, with a typical chest-wall thickness of 0.6 cm, while the corresponding numbers for a full-born infant at birth (3 kg) is about 10 and 1.2 cm, respectively. In contrast, the most immature infants weighing 0.4 kg has a chest diameter of 4.5 cm. Model phantoms were constructed correspondingly. For the optical measurements we used our dual wavelength diode laser spectroscopic system, developed primarily for our sinus cavity studies.10, 11 Phantoms and the measurement system will now be described briefly. A more detailed account can be found in Ref. 19. 2.1.Tissue PhantomsThe measurements were performed on animal lung tissue, obtained from a local slaughter house specializing in boar game. Porcine lungs have similarities to those of humans. For each measurement series, fresh samples were collected and transported chilled and with a time interval not exceeding approximately 10 h between slaughter and measurements. By using intact sections of the lungs with appropriate thickness, conditions for small children could be selected. The lung tissue was ventilated with compressed air gently supplied through a bronchus and with a lung perforation incised for pressure relief. Alternatively, a stream of pure nitrogen could be supplied. For simulating thoracic walls of appropriate thickness, slabs of gelatin were prepared from a solution containing scattering titanium oxide particles and absorbing ink. The optical parameters for scattering μs and absorption μa achieved in this way approached those expected for the relevant tissue as suggested by literature.20 Layers of appropriate thickness were placed around the section of ventilated boar lung to build up a realistic phantom for a premature baby or small child. Clearly, data for neonatals are scarce and the presence of ribs and connective tissue induce uncertainties. We have used data pertinent to adults while corresponding parameters for neonatals are known to be less demanding for optical techniques. The phantoms and the placement of the laser probe and the detector are illustrated for both measurement geometries in Fig. 1. 2.2.Dual Wavelength Diode Laser SpectrometerA laser spectroscopic instrument initially developed for human sinus cavity measurements was employed as illustrated in Fig. 2. The instrument and data processing are described in detail in Refs. 10 and 11; here only a short account will be given. Free oxygen and water vapor gas are detected using wavelength modulated diode lasers operating in the spectral range around 760 and 935 nm, respectively. The characteristic absorptive imprints from the small amount of gas are very faint, but can be picked up by the detection system which operates using lock-in techniques, where the second derivative (2f) of the intensity dips are recorded. Gas detection in the presence of scattering liquid or solid materials relies on the fact that free gases have about 10,000 times sharper absorption lines in comparison with the latter materials. The light from the two diode lasers are merged into a single fiber, and the main part of the light is brought to the sample, whereas a fraction of the light goes to a separate detector for reference measurements. Using this procedure, artifacts due to interference fringes, etc., can be largely eliminated. The two gases can be measured individually because the corresponding lasers are modulated with separate frequencies allowing the two signal contributions recorded with the same detector to be separated. Due to the scattering of light in the tissue a large-area detector (18 × 18 mm2) is utilized. 3.Measurements and Results3.1.Measurements on a Simple PhantomIn order to get a feeling of tissue interrogation depth as a function of source-detector separation, preliminary experiments were first performed with a wetted sponge placed under a 6-mm thick gelatin tissue phantom, an arrangement which has resemblance to the premature 1 kg child case. Figure 3 illustrates the results showing an increasing gas signal for both oxygen as well as water vapor when the injection-detection separation is increased. 3.2.Measurements on Lung TissueMeasurements were performed on fresh boar lung tissue, which was ventilated through a stream of compressed air supplied by a plastic hose connected to a bronchus segment. Measurements in transmission as well as reflection geometry were performed and with different arrangements of tissue-like gelatin slabs of different thickness and composition. Oxygen as well as water vapor signals were recorded and compared. The purpose was to investigate the possibilities and limitations of the technique and the geometrical sizes possible to interrogate. Measurement geometries similar to those shown in Fig. 1 were used. Recorded signals for the case of transmission measurements through 1-cm thick tissue-like gelatin placed on each side of a 2- to 3-cm thick ventilated boar lung section are shown in Fig. 4, while corresponding data in reflectance geometry are shown in the right hand part. Second derivative signals for oxygen and water, respectively, are shown, recorded when repeatedly ramping the diode laser driving current, thus passing the absorptive feature. As can be seen, quite useful signal-to-noise ratios are obtained for both gases. Experimental low-noise line shapes, recorded for free air samples, following a procedure described, e.g., in Ref. 11, are fitted to the experimental data and yield quantities denoted by L eq, the distance passed by the laser beam in normal 21% oxygen air of 100% water vapor saturation at 14ºC (the actual lung-tissue temperature), to yield the same signals as the ones obtained when the multiply scattered light passes through the tissue. Fig. 4Experimental gas absorption recordings in transmission and reflectance measurements through a ventilated boar lung tissue phantom, with 1-cm thick thoracic-wall–like gelatin slabs covering the tissue. The optrode spacing is about 5 and 2 cm for the transmission and reflectance cases, respectively. 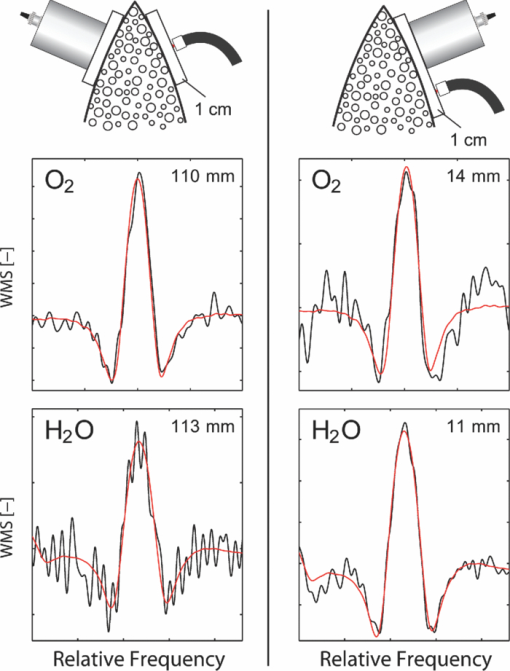 We note that the signals for the case of transmission correspond to much longer effective light-passing distances than the physical geometrical ones, due to the multiple scattering of light. The signals are much lower in reflectance geometry, since a substantial part of the light only passes through the gas-free gelatin layer. By normalizing the oxygen signal on the water vapor signal, the effect of the unknown path length can be eliminated, under the assumption that the material interrogated has similar optical properties with regard to absorption and scattering for the two wavelengths used, 760 nm for oxygen and 935 nm for water vapor. Actually, as can be derived from the L eq values indicated in connection with the signals, the oxygen/water vapor ratio approaches 1, corresponding to a 21% concentration of oxygen in the boar lung in the transmission case, whereas it is slightly higher than 1 for the reflectance case, which is also evident in Fig. 3. We will discuss this observation later. The gas absorption from the lung phantom was further analyzed with respect to the origin of the signal. The oxygen and water vapor gas signals obtained from a tissue phantom consisting of ventilated boar tissue, placed below a 0.7-cm thick slab of thoracic-wall–like gelatin are shown in Fig. 5. The injection fiber/detector center separation in reflectance geometry was 1.5 cm. The oxygen and the water vapor signals for an air-ventilated lung show a ratio higher than 1. It can be seen that upon exchanging air for a stream of pure nitrogen, little influence of the water vapor signal is observed. Ideally, for full moisturing of the dry nitrogen gas on entering the lung tissue, identical signals would have been expected. Regarding the oxygen signal, the exchange of air for pure nitrogen as expected results in a disappearance of the signal. It was noticed that this result was only obtained after the lung had been mechanically compressed to simulate breath. If the boar lung segment was flushed with nitrogen without first mechanically compressing the lung tissue, the oxygen signal remained largely unaffected, since gas exchange in the air-filled alveoles was too slow without active “breathing.” Fig. 5Reflection measurement of oxygen and water vapor in a tissue phantom (0.7 cm) with boar lung covered with a gelatin slab. It is observed how the oxygen signal is depressed when the lung is flushed with nitrogen. It is also observed that the water vapor signal is largely independent of whether the lung is air- or nitrogen filled. 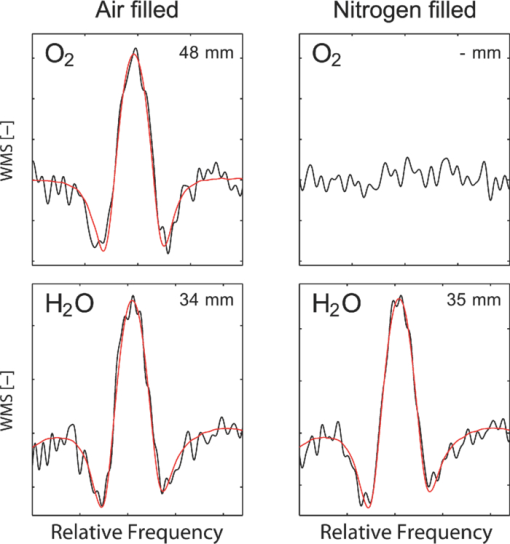 Further measurements were performed in reflection geometry, using thoracic-wall phantom slabs of different thicknesses. Figure 6 shows data obtained. As expected, the signals become weaker when the thoracic-wall layer becomes thicker, and the gas signals increase for larger light-injection–detector separations (accompanied with a strong reduction in the total light intensities, not indicated). The ratio between the oxygen and water vapor signals are again observed to become larger than 1, reflecting the fact that the water vapor monitoring light gets more absorbed and effectively samples a more shallow region. Preliminary measurements using the water vapor absorption band around 820 nm show that a deeper penetration due to less tissue absorption can be obtained, while still maintaining adequate signal levels. Fig. 6Reflection measurement of lung with gelatin tissue phantom of different thickness. Data for oxygen are shown in the left hand panels, while corresponding data for the ratio of oxygen and water vapor signals are shown in the right hand panels. 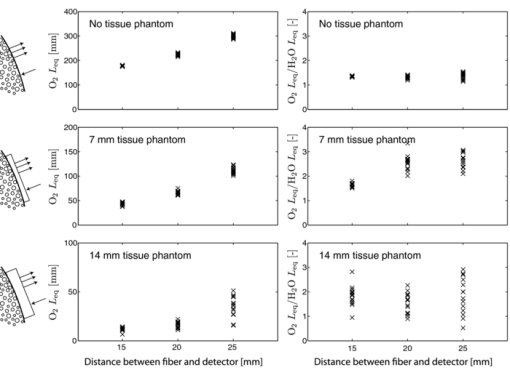 While discussing optimal interrogation wavelengths we note that the very interesting respiratory gas carbon dioxide cannot be measured nonintrusively by the present technique, since the relevant absorption wavelengths for this molecule fall in a spectral region of heavy absorption by the tissue surrounding the gas volume. 4.DiscussionWe have shown in tissue phantom measurements that it should be possible to noninvasively monitor the lungs of preterm newborn infants using the techniques which we have developed. We anticipate that the method will be able to noninvasively assess differences in aveolar oxygenation in different areas of the lungs, i.e., that it could be usable for assessing resuscitation effects, assessing inhomogeneity of lung aerisation; such as pneumothorax, atelectasis, and emphysema. Further research will show the clinical applicability. The oxygen concentration is derived from normalization of oxygen signal to water vapor signal, while the water vapor signal gives information on the gas-filled volume, and changes in that volume, e.g., when breathing. Thus, the present measurements yield different and complementary information to assessments of blood oxygenation, e.g., by near-IR spectroscopy. An accuracy of 1% to 2% units (5% to 10% accuracy in the measured value) for oxygen for corrected values, and a higher sensitivity to relative change could be expected based on our experience from human sinus cavity monitoring with similar recording conditions. Since pulmonary problems constitute a main concern for these infants, the new possibility to monitor lung function, including oxygenation in real time, is an attractive finding. While transmission geometry seems to be possible for very immature infants, a more realistic geometry for larger infants would be the reflectance measurements as the amount of light penetrating thick tissue layers becomes small. Oxygenation in different lung lobes might be measurable, and changes in lung function due to different medications and interventions can be monitored instantaneously. The use of the weaker water vapor absorption band around 820 nm exhibits diagnostic advantages compared to the 935 nm band. Oxygenation would then be measurable with a smaller empirical correction due to different optical properties of the tissue for the wavelengths interrogating the two gases. The correction factor to obtain absolute oxygen concentrations could be determined experimentally or through simulations, e.g., of the Monte Carlo type. In simulations, the detailed influence of the fact that tissue absorption is larger at 935 or 820 nm than at 760 nm, and that scattering has the opposite tendency can be accounted for. By knowing the weight of the infant, and thus its approximate dimensions, a factor supported by such simulations and also initial experience can be applied. By a very recent development, we have also been able to assess the path length with the frequency-modulated continuous-wave technique quasisimultaneously with the gas imprint in an only slightly modified GASMAS setup.21 By incorporating this realistic technology in clinical equipment, we expect very accurate oxygen monitoring to be achievable, since differences in optical propagation could be measured and compensated for. For practical monitoring of preterm infants, the light injection and detection might be performed using soft, realistic pads which are attached to the body, or by using optrodes incorporated in a small vest for real-time, continuous monitoring of lung function. Less discomfort than encountered when applying electrical impedance tomography, or electric plethysmography employing electrodes, can be expected. Rather than estimating only lung volume, as is the case for the electrical methods, the spectroscopic method presented here also provides the oxygen contents, and in a more nonintrusive way. Our encouraging results suggest that a clinical trial to evaluate the benefits and limitations of the new method should be pursued, particularly in view of the neonatal optical properties being less demanding than those assumed (for adults) in our phantoms measurements. Actually, a very recent first case study, to be reported,22 demonstrated the possibility to attain strong water vapor signals from a newborn baby of full size (4 kg), clearly showing that also the weaker oxygen signals will be readily attainable for the intended patient group, which much smaller weight and dimensions. AcknowledgmentsThis work was supported by the Swedish Research Council through a direct grant and through a Linnaeus grant to the Lund Laser Centre, and through the Lund University Medical Faculty. The authors are grateful to Stefan Andersson-Engels, Erik Alerstam, and Can T. Xu for supporting measurements of tissue phantom optical parameters, to Lars Björklund for valuable discussions, and to Skanska Vilt AB for supplying fresh boar lung specimen. ReferencesM. M. Slattery and
J. J. Morrison,
“Preterm delivery,”
Lancet, 360 1489
–1497
(2002). https://doi.org/10.1016/S0140-6736(02)11476-0 Google Scholar
R. L. Goldenberg,
J. F. Culhane,
J. D. Iams, and
R. Romero,
“Epidemiology and causes of preterm birth,”
Lancet, 371 75
–84
(2008). https://doi.org/10.1016/S0140-6736(08)60074-4 Google Scholar
W. M. Gilbert,
T. S. Nesbitt, and
B. Danielsen,
“The cost of prematurity: quantification by gestational age and birth weight,”
Obstet. Gynecol., 102 488
–492
(2003). https://doi.org/10.1016/S0029-7844(03)00617-3 Google Scholar
L. B. Ware and
M. A. Matthay,
“Medical progress - The acute respiratory distress syndrome,”
New Engl. J. Med., 342 1334
–1349
(2000). https://doi.org/10.1056/NEJM200005043421806 Google Scholar
M. B. van Veenedaal,
M. Miedema,
F. H. C. de Jongh,
J. H. van der Lee,
I. Frerichs, and
A. H. van Kaam,
“Effect of closed endotracheal suction in high-frequency ventilated premature infants measured with electrical impedance tomography,”
Intensive Care Med., 35 2130
–2134
(2009). https://doi.org/10.1007/s00134-009-1663-5 Google Scholar
L. Persson,
K. Svanberg, and
S. Svanberg,
“On the potential of human sinus cavity diagnostics using diode laser gas spectroscopy,”
Appl. Phys. B, 82 313
–317
(2006). https://doi.org/10.1007/s00340-005-1968-1 Google Scholar
L. Persson,
E. Kristensson,
L. Simunsson, and
S. Svanberg,
“Monte Carlo simulations related to gas-based optical diagnosis of human sinusitis,”
J. Biomed. Opt., 12 054002
(2007). https://doi.org/10.1117/1.2777179 Google Scholar
L. Persson,
M. Andersson,
M. Cassel-Engquist,
K. Svanberg, and
S. Svanberg,
“Gas monitoring in human sinuses using tunable diode laser spectroscopy,”
J. Biomed. Opt., 12 054001
(2007). https://doi.org/10.1117/1.2777189 Google Scholar
L. Persson,
F. Andersson,
M. Andersson, and
S. Svanberg,
“Approach to optical interference fringes reduction in diode laser absorption spectroscopy,”
Appl. Phys. B, 87 523
–530
(2007). https://doi.org/10.1007/s00340-007-2593-y Google Scholar
L. Persson,
M. Lewander,
M. Andersson,
K. Svanberg, and
S. Svanberg,
“Simultaneous detection of molecular oxygen and water vapor in the tissue optical window using tunable diode laser spectroscopy,”
Appl. Opt., 47 2028
–2034
(2008). https://doi.org/10.1364/AO.47.002028 Google Scholar
M. Lewander,
Z. Guan,
K. Svanberg,
S. Svanberg, and
T. Svensson,
“Clinical system for non-invasive in situ monitoring of gases in the human paranasal sinuses,”
Opt. Express, 17 10849
–10863
(2009). https://doi.org/10.1364/OE.17.010849 Google Scholar
M. Lewander,
S. Lindberg,
T. Svensson,
R. Siemund,
K. Svanberg, and
S. Svanberg,
“Non-invasive diagnostics of the maxillary and frontal sinuses based on diode laser gas spectroscopy,”
Rhinology, Google Scholar
S. Lindberg,
M. Lewander,
T. Svensson,
R. Siemund,
K. Svanberg, and
S. Svanberg,
“Method for studying gas composition in the human mastoid using laser spectroscopy,”
Ann. Oto. Rhinol. Larynol., Google Scholar
M. Sjöholm,
G. Somesfalean,
J. Alnis,
S. Andersson-Engels, and
S. Svanberg,
“Analysis of gas dispersed in scattering media,”
Opt. Lett., 26 16
–18
(2001). https://doi.org/10.1364/OL.26.000016 Google Scholar
M. Andersson,
L. Persson,
M. Sjöholm, and
S. Svanberg,
“Spectroscopic studies of wood-drying processes,”
Opt. Express, 14 3641
–3653
(2006). https://doi.org/10.1364/OE.14.003641 Google Scholar
T. Svensson,
M. Andersson,
L. Rippe,
S. Svanberg,
S. Andersson-Engels,
J. Johansson, and
S. Folestad,
“VCSEL-based oxygen spectroscopy for structural analysis of pharmaceutical solids,”
Appl. Phys. B, 90 345
–354
(2008). https://doi.org/10.1007/s00340-007-2901-6 Google Scholar
M. Lewander,
Z. G. Guan,
L. Persson,
A. Olsson, and
S. Svanberg,
“Food monitoring based on diode laser gas spectroscopy,”
Appl. Phys. B, 93 619
–625
(2008). https://doi.org/10.1007/s00340-008-3192-2 Google Scholar
M. Lewander,
T. Svensson,
S. Svanberg, and
A. Olsson,
“Non-intrusive measurements of headspace gas composition in liquid food packages made of translucent materials,”
Packag. Technol. Sci., 24 271
–280
(2011). https://doi.org/10.1002/pts.934 Google Scholar
A. Bruzelius,
“Sensing of free gas in human tissue with diode laser spectroscopy: Application to lung monitoring in premature neonates,”
(2010) Google Scholar
C. R. Simpson,
M. Kohl,
M. Essenpreis, and
M. Cope,
“Near-infrared optical properties of ex vivo human skin and subcutaneous tissues measured using the Monte Carlo inversion technique,”
Phys. Med. Biol., 43 2465
–2478
(1998). https://doi.org/10.1088/0031-9155/43/9/003 Google Scholar
L. Mei,
H. Jayaweera,
P. Lundin,
S. Svanberg, and
G. Somesfalean,
“Gas spectroscopy and optical path-length assessment in scattering media using a frequency-modulated continuous-wave diode laser,”
Opt. Lett., 36 3036
–3038
(2011). https://doi.org/10.1364/OL.36.003036 Google Scholar
P. Lundin,
E. Krite Svanberg,
M. Lewander,
L. Cocola,
S. Andersson-Engels,
J. Jahr,
V. Fellman,
K. Svanberg, and
S. Svanberg,
“Non-invasive gas monitoring in newborn infants using diode laser absorption spectroscopy – a case study,”
Google Scholar
|