|
|
1.IntroductionThere is no healthcare specialty that is free from the morbidity and costs of wound development in a patient. In 2009, U.S. hospitals discharged over 1,300,000 patients with chronic wounds and more than 547,000 with traumatic wounds (classified as body surface area burn or open wound).1 U.S. healthcare costs related to wound treatment are well over $20 billion yearly, and the impact of wound healing on these expenditures is extensive.2 In addition, if every surgical procedure is considered a case of an acute wound, the significance of wound healing is simply tremendous. Although the wound-healing process of acute wounds such as surgical incisions is fairly well understood, the modified wound-healing process encountered in patients with chronic wounds and some traumatic acute wounds still requires elucidation. Normal healing of an acute wound is directed by a cascade of growth factors and cell signaling that allows the wounds to repair quickly. Chronic wounds and some traumatic acute wounds are much slower to heal and behave differently for several underlying reasons. There may be a pathologic process such as infection that prevents the wound from healing normally. Additionally, wound healing may be complicated by a prolonged inflammatory phase that inhibits normal levels of chemical mediators and cell recruitment. Finally, the patient’s general condition contributes to the rate of wound healing; malnutrition and comorbidities such as diabetes are associated with impaired wound healing.3 Improved objective assessment of wounds would be conducive to better treatment of them, which might result in faster healing times, decreased infection rates, and decreased local and systemic complications of injury. For instance, if visits to the operating room were reduced by one instance per patient for 140 patients at one hospital, the cost savings would be over $2 million. The eventual timing of wound closure is often subjective, and there exists a need for an objective evaluation of the molecular environment of wounds throughout the wound-healing process. The use of vibrational spectroscopy and imaging for increased diagnostic accuracy and better wound treatment can produce improved clinical outcomes and decreased patient morbidity, resulting in an earlier return to an improved quality of life. 2.Wound Pathophysiology and the Process of Wound HealingSeveral parameters are used to classify wounds: the layers of tissue involved, the origin and duration of the wound, and the type of wound closure used (i.e., surgical closure with sutures or formation of scar tissue). Origin and duration dictate whether a wound is classified as chronic or acute. Wounds resulting from trauma or surgery are acute wounds and generally proceed normally through the wound-healing process. An incision site in the abdomen, a third-degree burn, or a crushed limb is termed an “acute wound.” Wounds arising from chronic inflammation, repetitive insult, or vascular compromise that fail to heal normally or in a timely manner are called “chronic wounds.” Pressure ulcers and diabetic foot ulcers are examples of chronic wounds. Acute wounds generally begin with a single, abrupt insult and progress through the healing process in an orderly manner. Conversely, chronic wounds are usually caused by a pathologic process such as infection or poor circulation. In general, the wound-healing process proceeds through regeneration and/or repair. “Wound regeneration” is the renewal of the damaged tissue with healthy tissue that is the same, whereas “wound repair” is the replacement of the damaged tissue by scar tissue. Wounds that are confined to the superficial layers of skin heal by regeneration, but wounds that penetrate deep into the subcutaneous layers are not able to regenerate and heal by scar formation. The overall sequence of events that precedes injury is thought to be similar for chronic and acute wounds whereby chronic wounds simply stall at one or more stages during the wound-healing process.4 The first step in wound healing is hemostasis, the vascular response that triggers platelet activation and aggregation, clot formation, and vasoconstriction. The second step in wound healing is inflammation—capillaries vasodilate, and neutrophils and macrophages migrate to the wound bed to debride the wound and secrete growth factors to promote angiogenesis and connective tissue synthesis (tissue inhibitors of matrix metalloproteases, matrix metalloproteases, transforming growth factor- and transforming growth factor-, interleukin-1, interleukin-6, interleukin-8, epidermal growth factor, and keratinocyte growth factor). The third step in wound healing is proliferation, a multistep process involving epithelialization (early formation of the new wound bed from fibroblasts), neoangiogenesis (induction of new vasculature), and matrix and/or collagen deposition. The final step in wound healing is wound contraction and maturation and/or remodeling—the wound edges close, and a stronger, more orderly matrix forms scar tissue.4 Numerous factors that can affect the wound-healing process make an already complicated process even more difficult to accurately assess. These factors include age, stress, nutrition, tissue perfusion and oxygenation, infection, and other comorbidities, such as obesity, diabetes mellitus, immunosuppression, pulmonary disease, renal disease, and vascular disease. Unfortunately, in some cases, wound healing is complicated by dehiscence, in which “closed” wounds fall apart and reopen. The events leading up to wound dehiscence are not well understood but are suspected to result from an intensely exaggerated inflammatory response.4 Currently, wounds are evaluated on the basis of parameters such as location of injury, adequacy of perfusion, gross appearance of the wound, wound tensile strength, and the patient’s general condition. Although parameters such as the location of injury, the gross appearance of the wound, and the patient’s general condition are fairly obvious and can be reasonably assessed, parameters such as the adequacy of perfusion and tensile strength are not readily quantifiable during surgery. It has previously been demonstrated that there is a greater incidence of associated vascular injury in slowly healing wounds than in normally healing wounds.5 It is also well established that the tensile strength of the wound is dependent on collagen deposition.6 There exists a need for technologies that can be used to noninvasively and objectively assess these challenging parameters. 3.Raman and Fourier Transform Infrared SpectroscopyRaman and Fourier transform infrared (FTIR) spectroscopy are types of vibrational spectroscopy that measure the vibrational frequencies of molecules as the molecules are excited by incident photons. Every molecule has a unique fingerprint of vibrational frequencies, which makes Raman and FTIR spectroscopy highly specific techniques for molecular identification. Both techniques can be employed noninvasively, making them ideal for biomedical applications. Raman spectroscopy and FTIR spectroscopy are sometimes referred to as “sister” techniques and provide complementary information about molecules, but they differ in several fundamental ways. Raman spectroscopy arises from the inelastic scattering of ultraviolet, visible, or near-infrared light when a photon interacts with a molecule. Raman scattering is an inherently weak process, and, as such, samples are typically illuminated by laser light. Light scattered by the sample is diffracted into individual wavelengths by a spectrograph and collected by a detector such as a CCD or CMOS sensor.7 Raman systems can be coupled to a microscope and motorized stage for high-resolution imaging8–14 or to a fiberoptic probe for bulk in vivo sampling.15–20 Raman spectroscopy’s independence from a specific sample thickness and lack of spectral interference from water make it an ideal technique for biomedical applications. One disadvantage of Raman spectroscopy in the biomedical arena, however, is its inherently weak signal, which can be overwhelmed by sample fluorescence. Often this is overcome by excitation in the near-infrared region of the spectrum where biological molecules tend not to fluoresce. There are other advanced configurations and applications of Raman spectroscopy, but they lie outside the scope of this review.21–25. FTIR spectroscopy consists of the absorbance of frequencies of light by a molecule that contains the same vibrational frequencies within its molecular bonds. A beam of infrared light is passed through or reflected by a sample. Some light is absorbed by the sample’s vibrational frequencies, and the remaining light is transmitted to an interferometer and then collected by a detector, such as a mercury cadmium telluride photoconductive detector or an indium gallium arsenide photodiode detector.26 As with Raman spectroscopic systems, FTIR systems can be coupled to a microscope27–39 or a fiberoptic probe.40 FTIR spectroscopy is sensitive to the presence of water, however, and in vivo sampling can be challenging. One disadvantage of FTIR spectroscopy is that it requires that light be able to pass through the sample and thus is confined to use with thin samples, such as tissue sections on optically transparent windows. Both Raman spectroscopy and FTIR spectroscopy offer the capability to accurately detect and identify the various molecules that compose the extracellular matrix in their native state during wound healing. They are both imaging techniques in which the precise biochemical composition of biologic samples can be obtained by noninvasive and nondestructive means.41–44 Both have been proven to be effective in studying tissues at the molecular level using diverse clinical and diagnostic applications, including the analysis of cellular structure and the determination of tumor grade and type.9,42,45–48 Pathologic alterations of wounds are accompanied by fundamental changes in the molecular environment that can be analyzed by vibrational spectroscopy.49,50 The identified changes might provide the objective markers of acute wound healing, which could then be integrated with clinical characteristics to guide the management of traumatic wounds. For instance, changes in collagen vibrational bands could be correlated with alterations in collagen deposition and reepithelialization of the wound bed. 4.Vibrational Spectroscopic Studies of Wound Healing4.1.WoundsThe application of vibrational spectroscopy, such as Raman spectroscopy and FTIR spectroscopy, to study wound healing is a developing field of interest. Both ex vivo and in vivo models of wound healing have been explored in animals and humans, but all studies published to date have focused on acute wounds versus chronic wounds. In all surgical cases, an acute wound is inflicted once a surgical incision is made. Thus, all surgical wounds are classified as acute wounds and are typically examples of the normal healing process. In early ex vivo studies by Wijelath and co-workers, FTIR attenuated total reflection (ATR) spectroscopy illustrated modified healing patterns in arterial grafts implanted into dogs. Standard histological analysis of the graft implants showed little or no activity in the first 10 days after implantation, but FTIR-ATR spectroscopy demonstrated changes within the fibrin layer of the graft that could be correlated to endothelialization of the wound.51,52 Gough et al. utilized synchrotron FTIR spectroscopic mapping to monitor peridural scarring in rats following laminectomy.53 Their results derived from untreated rats were compared to data from rats treated with L-2-oxothiazolidine-4-carboxylate (OTC). FTIR spectroscopic maps of laminectomized tissue sections indicated a decrease in lipid and phosphate bands, which are indicators of inflammatory cells. Immunohistochemistry confirmed these results and showed a diminished number of activated macrophages in OTC-treated rats. More recently, investigators successfully employed Raman spectroscopy to differentiate normal from injured tissue in rodent models of brain injury54 and spinal cord injury.55 In two rodent models of incisional wound healing, Raman spectra collected in vivo demonstrated increased protein configuration surrounding the wounds and increased cellularity56 as well as conformational changes within the proteins themselves.57 To date, published applications of vibrational spectroscopy to study wound healing in humans have been performed on ex vivo biopsies of wounds. In 2008, Mendelsohn et al. utilized both FTIR and Raman spectroscopy to correlate spectroscopic changes with the reepithelialization of the wound bed of cutaneous incisional wounds.49 Spectroscopic results were compared directly with immunohistochemical images of serial tissue sections and gene array analysis data. FTIR images collected four days after wounding precisely depicted the keratin-rich migrating epithelial tongue from the collagen-rich wound bed with focal data analysis of the to spectral region (Fig. 1). Similar spectral features are exhibited by factors 1 to 4 (f1 to f4), but the factors are spatially distinct within the sample itself. These represent keratin-rich areas confirmed by immunohistochemistry. Factors 5 and 6 are spectrally distinct from factors 1 to 4 and represent collagen-rich areas of the sample. Confocal Raman microspectroscopic images of tissue sections demonstrate the time dependence of elastin distribution in the wound up to six days after wounding (Fig. 2).49 By day 2, the elastin distribution (f1) and the distribution of a collagen factor (f3) were significantly decreased, whereas the distribution of a second collagen factor (f2) decreased. Their study clearly demonstrates the utility of vibrational spectroscopy and imaging to monitor component-specific changes in skin in an acute wound-healing model. Fig. 1Infrared characterization (factor analysis conducted over the 1185 to region) of wounded and nonwounded areas six days after wounding is shown. (a) Optical image of an unstained section with the edge of the wounded area marked by a vertical dashed line. (b—g) The score images are shown for various components of the tissue. (b) f1 is the stratum corneum and part of the viable epidermis. (c) f2 is the suprabasal epidermis. (d) f3 is the basal epidermal layer. (e) f4 is the outer leading edge of the migrating epithelial tongue. (f and g) f5 and f6 are the collagen-rich areas, respectively. (h) The factor loadings of f1 to f4 are characteristic of keratin-rich areas. The factor loadings of f5 and f6 are characteristic of collagen-rich areas. Reprinted with permission from John Wiley and Sons [J. Cell. Mol. Med. 12(5B), 2145–2154 (2008)]. 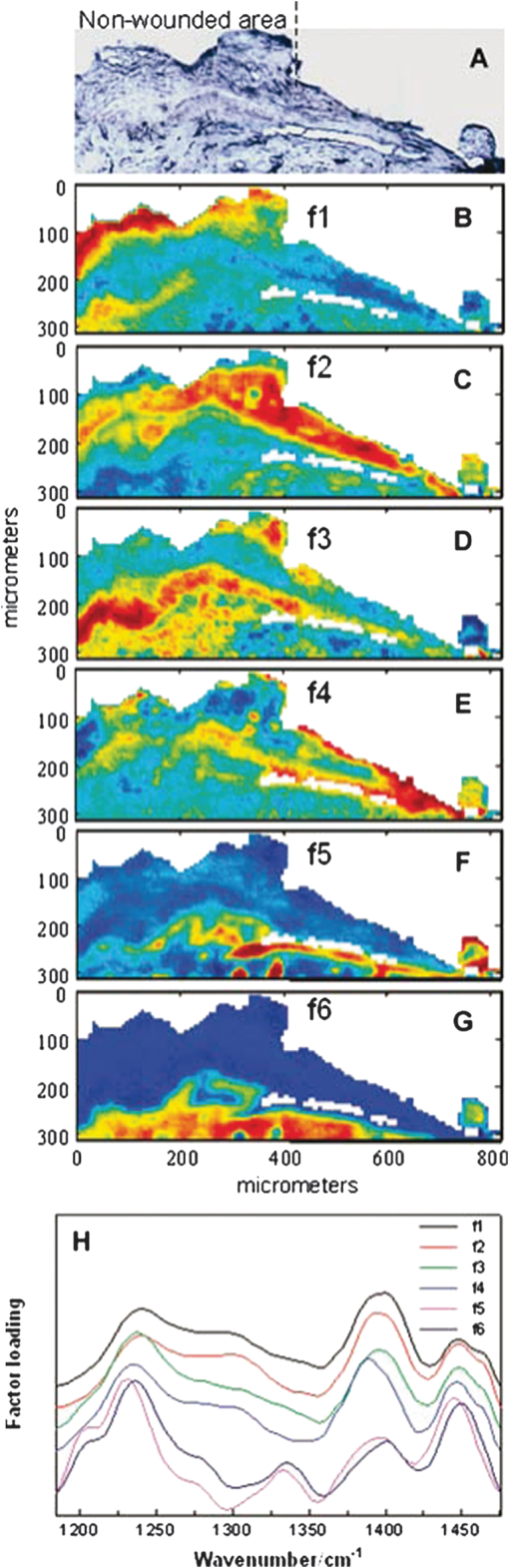 Fig. 2Factor analysis of a confocal Raman dataset delineates skin regions near a wound edge 0.5 days after wounding. Data analysis was conducted over the 800 to region, yielding four factor loading images that map to anatomically distinct regions in the skin. (a) The spatial distribution of scores for f1 highlights the stratum corneum region of the skin, which is rich in keratin-filled corneocytes and lipids. (b) f2 shows high scores in the underlying epidermal region. (c) High scores for f3 reside near the dermal-epidermal boundary region. (d) The size, location, and spatial distribution of several smaller regions with high scores for f4 are identified as cell nuclei. (e) Factor loadings reveal several spectral features specific to the microanatomy of the epidermis in human skin. Reprinted with permission from John Wiley and Sons [J. Cell. Mol. Med. 12(5B), 2145–2154 (2008)]. 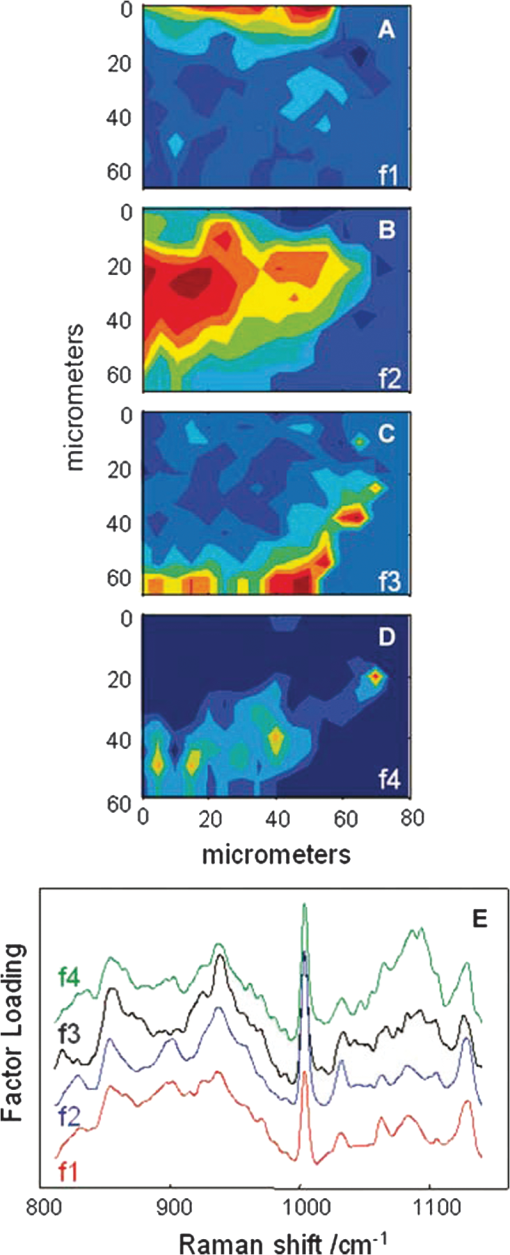 Our group has used Raman spectroscopic mapping to monitor changes within the wound bed. Tissue biopsies were collected from Operation Iraqi Freedom and Operation Enduring Freedom combat-wounded soldiers at each surgical debridement during the wound-healing process.58 Spectral maps revealed differences in the amide scissoring band area ratios that correlated with wound outcome (Fig. 3), i.e., normal healing or impaired healing. Raman spectroscopic results were corroborated with collagen gene expression profiles. In impaired healing wounds, a decrease in collagen-like bands was confirmed by decreased expression of the COL1A1 and COL3A1 genes (for type I and type III collagens, respectively).58 In addition to monitoring the wound bed itself, FTIR and Raman spectroscopy were utilized to monitor complications of wound healing, such as infection, the formation of biofilm from subsequent infection, and heterotopic ossification (HO), to which acute and chronic wounds are susceptible. Fig. 3Photographs are shown for a patient with a normal healing wound (a) and one whose wound healing was impaired (b). (c) This graph shows the percentage difference of the 1665-to- band area ratios calculated from the first and last debridement 1665-to- band area ratios for wounds classified as healing normally (black bars) and those wounds in which healing was classified as impaired (white bars). 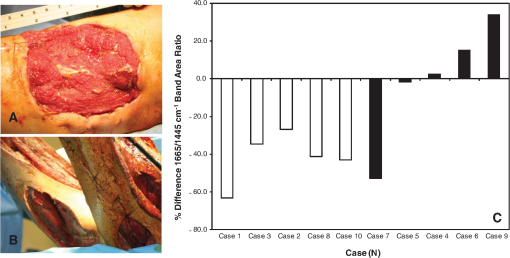 4.2.InfectionFor acute wounds such as surgical incisions, infection is the most prevalent postsurgical complication.59 Chronic wounds provide a bed of growth for pathogens—they are warm, deep, and sometimes full of necrotic tissue. Chronic wounds are more often infected than acute wounds, but acute combat wounds present a subset of acute wounds with a high infection rate.60 Identifying the pathogens responsible for wound bioburden is especially important because the prevalence of multi-drug-resistant bacteria is increasing, necessitating treatment with appropriate antimicrobial agents. Because of the specificity of Raman and FTIR spectroscopy, they can also be used to evaluate the bioburden of wounds. There have been numerous FTIR and Raman spectroscopic studies of microorganisms, many of which have been focused on rapid identification of the microorganisms.61–72 Differences in the Raman spectral profile of three bacterial species as well as three bacterial strains are evident in Fig. 4 (unpublished data). Both Klebsiella pneumoniae and Acinetobacter baumannii are Gram-positive bacteria, whereas methicillin-resistant Staphylococcus aureus is a Gram-negative bacterium. Differences in the Raman spectral profile, however, are due not strictly to peptidoglycan content but to other structural differences in the proteins as well. Inherent chemical differences in different bacterial species and strains, as demonstrated in Fig. 4, make possible the high specificity of Raman spectroscopy. When the Raman spectra of wound effluent collected from two patients colonized with different bacteria are compared (Fig. 4), the spectral profiles show differences in amino acid content and alterations in glycosidic linkages. Fig. 4Raman spectra of (a) methicillin-resistant Staphylococcus aureus (solid), Klebsiella pneumoniae (middle dashed line), and Acinetobacter baumannii (bottom dashed line). (b) Lines represent three different strains of A. baumannii. (c) Raman spectra obtained from wound effluent from a wound colonized with Escherichia coli (solid line) and from a wound colonized with A. baumannii (dashed line). Gray boxes highlight regions of the spectra where chemical differences are prevalent. 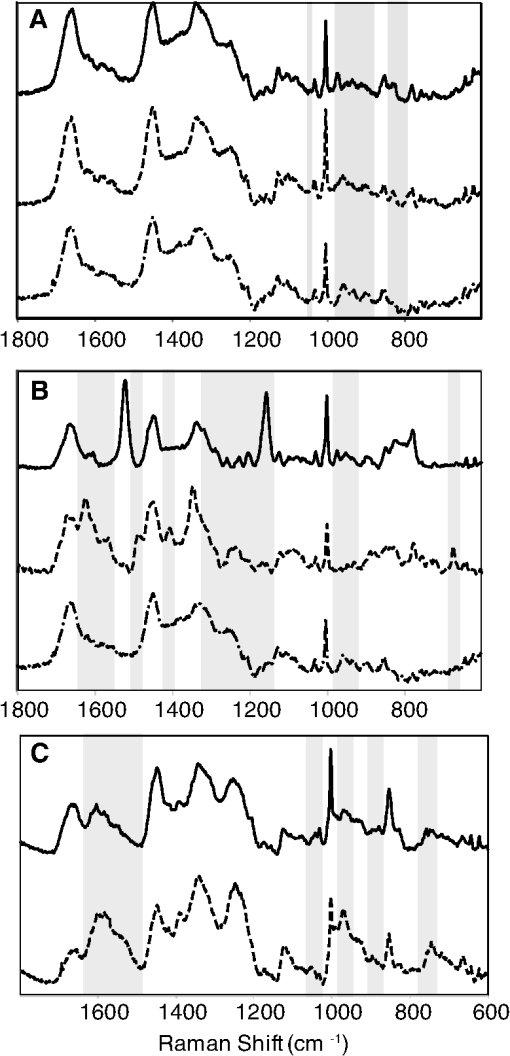 4.3.Heterotopic OssificationAnother complication of wound healing, “heterotopic ossification,” is defined as the pathological formation of bone in soft tissue. HO formation has been observed following orthopedic surgery (total hip arthroplasty as well as acetabular and elbow fracture surgery), burn injury, traumatic brain injury, and spinal cord injury.73 HO formation is not commonly observed in civilian traumatic wounds without the presence of head injury or spinal injury and develops in only 20% and 11% of these patients, respectively.74 During the current military conflicts in Iraq and Afghanistan, HO has been a frequent and common clinical problem in soldiers with traumatic combat wounds. Currently, operative excision is the only treatment for mature, symptomatic HO. Identifying tissue that will develop into HO is not trivial, however, and can only be confirmed once mineralized tissue is evidenced on a radiograph. Tissue mineralization could easily be monitored with Raman spectroscopy.12,75–88 Information could be gained that would reveal the quality of the bone being formed during HO. For example, is the bone “normal” but developing in soft tissue, or is the bone “pathological,” developing by an different mineralization mechanism altogether.80,89–94 While Raman and FTIR spectroscopy have been used extensively to study the process of biomineralization,84–95 they have not previously been used to provide insight into the pathological process of HO. We have collected Raman spectra of uninjured muscle, injured muscle, and “pre-HO” tissue (defined as palpably firm or “woody” tissue without roentgenographic evidence of HO) found within high-energy penetrating wounds (Fig. 5).95 When we compared uninjured to injured muscle, we found an apparent decrease in the 1340 and vibrational bands in the injured muscle as well as an increase in the vibrational band. This suggests collagen-specific alterations within the tissue as a result of traumatic injury. In one case, a patient exhibited “pre-HO” muscle during a debridement procedure. Upon Raman spectroscopic examination, it was clear that the tissue was indeed mineralized, even in “soft” tissue areas. Mineral vibrational bands at 1,070, 960, and , typical of a carbonated apatite, were prominent in the spectrum. These vibrational bands are attributed to the phosphate and carbonate stretching modes of bone. Thus, Raman spectroscopy can potentially be utilized to identify areas of tissue affected by early HO as well as areas of tissue that may be predisposed to HO formation. Fig. 5Raman spectra of (a) uninjured muscle and/or control tissue, (b) combat-injured muscle, and (c) preheterotopic ossification combat-injured muscle. The gray boxes highlight spectral changes in the amide III envelope (1340 to ) and the appearance of mineral vibrational bands at 1,070, 960, and . 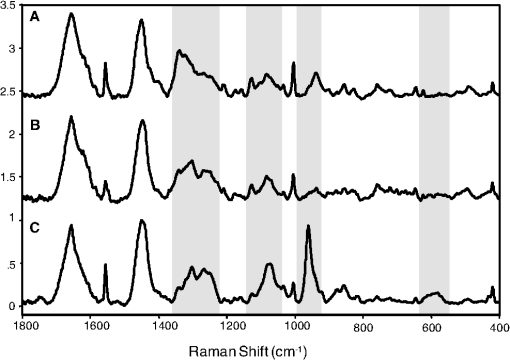 5.ConclusionsThe potential of vibrational spectroscopy to provide detailed information, noninvasively, about molecular and even structural changes within the components of the wound bed itself enable a more thorough understanding of the wound-healing process. Vibrational spectroscopic modalities such as Raman and FTIR spectroscopy can provide an objective means of evaluation by monitoring key components of wound bed reepithelialization, such as keratin, elastin, and collagen; by identifying and quantifying bacterial load; and by detecting HO. These techniques have the potential to offer improved objective assessment of combat wounds, resulting in faster healing times, decreased infection rates, and decreased local and systemic complications of injury. This, in turn, will produce improved clinical outcomes, decreased patient morbidity, and reduced medical costs. AcknowledgmentsThe views expressed in this paper are those of the authors and do not reflect the official policy of the Department of the Army, the Department of the Navy, the Department of Defense, or the U.S. government. We are military service members (or employees of the U.S. government). This work was prepared as part of our official duties. Title 17 U.S.C. § 105 states, “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. § 101 defines a U.S. government work as a work prepared by a military service member or employee of the U.S. government as part of that person’s official duties. This effort was supported in part by the U.S. Navy Bureau of Medicine and Surgery under the Medical Development Program and Office of Naval Research work unit number 602115HP.3720.001.A1015. This study was approved by the National Naval Medical Center Institutional Review Board (NNMC IRB) in compliance with all federal regulations governing the protection of human subjects. The NNMC IRB-approved protocol number is NNMC.2005.0069/NMRC.2005.0012, and the protocol title is “The Use of Vacuum Assisted Wound Closure Device in the treatment of Extremity Wounds.” We certify that all individuals who qualify as authors have been listed; that each has participated in the conception and design of this work, the analysis of data (when applicable), the writing of the document, and the approval of the submission of this version; that the document represents valid work; that if we used information derived from another source, we obtained all necessary approvals to use it and made appropriate acknowledgments thereof in the document; and that each author takes public responsibility for it. References, Agency for Healthcare Research and Quality, Healthcare Cost & Utilization Project (HCUP),
(2011) http://www.ahrq.gov/data/hcup/ , accessed December ). 2011). Google Scholar
D. J. SamsonF. LefevreN. Aronson, Wound-Healing Technologies: Low-Level Laser and Vacuum-Assisted Closure,
(2011) http://www.ahrq.gov/downloads/pub/evidence/pdf/woundtech/woundtech.pdf , accessed December ). 2011). Google Scholar
R. A. BryantD. P. Nix,
“Acute & Chronic Wounds: Current Management Concepts,”
3rd ed.Mosby, St. Louis(2007). Google Scholar
R. A. BryantD. P. Nix,
“Acute & Chronic Wounds: Current Management Concepts,”
3rd ed.Mosby, St.Louis(2012). Google Scholar
J. S. Hawksworthet al.,
“Inflammatory biomarkers in combat wound healing,”
Ann. Surg., 250
(6), 1002
–1007
(2009). http://dx.doi.org/10.1097/SLA.0b013e3181b248d9 ANSUA5 0003-4932 Google Scholar
“Schwartz’s Manual of Surgery,”
8th ed.McGraw-Hill, New York
(2006). Google Scholar
“Handbook of Raman Spectroscopy: From the Research Laboratory to the Process Line,”
Marcel Dekker, New York
(2001). Google Scholar
A. Kohleret al.,
“Multivariate image analysis of a set of FTIR microspectroscopy images of aged bovine muscle tissue combining image and design information,”
Anal. Bioanal. Chem., 389
(4), 1143
–1153
(2007). http://dx.doi.org/10.1007/s00216-007-1414-9 ABCNBP 1618-2642 Google Scholar
T. Meyeret al.,
“Nonlinear microscopy, infrared, and Raman microspectroscopy for brain tumor analysis,”
J. Biomed. Opt., 16
(2), 021113
(2011). http://dx.doi.org/10.1117/1.3533268 JBOPFO 1083-3668 Google Scholar
S. Kerenet al.,
“Noninvasive molecular imaging of small living subjects using Raman spectroscopy,”
Proc. Natl. Acad. Sci. U. S. A., 105
(15), 5844
–5849
(2008). http://dx.doi.org/10.1073/pnas.0710575105 PNASA6 0027-8424 Google Scholar
M. Kazanciet al.,
“Bone osteonal tissues by Raman spectral mapping: orientation-composition,”
J. Struct. Biol., 156
(3), 489
–496
(2006). http://dx.doi.org/10.1016/j.jsb.2006.06.011 JSBIEM 1047-8477 Google Scholar
M. D. MorrisW. F. Finney,
“Recent developments in Raman and infrared spectroscopy and imaging of bone tissue,”
Spectroscopy, 18
(2), 155
–159
(2004). 0887-6703 Google Scholar
W. Gellermannet al.,
“Raman imaging of human macular pigments,”
Opt. Lett., 27
(10), 833
–835
(2002). http://dx.doi.org/10.1364/OL.27.000833 OPLEDP 0146-9592 Google Scholar
N. J. KlineP. J. Treado,
“Raman chemical imaging of breast tissue,”
J. Raman Spectrosc., 28
(2–3), 119
–124
(1997). http://dx.doi.org/10.1002/(ISSN)1097-4555 JRSPAF 0377-0486 Google Scholar
M. V. Schulmerichet al.,
“Subsurface and transcutaneous Raman spectroscopy and mapping using concentric illumination rings and collection with a circular fiber-optic array,”
Appl. Spectrosc., 61
(7), 671
–678
(2007). http://dx.doi.org/10.1366/000370207781393307 APSPA4 0003-7028 Google Scholar
Y. Hattoriet al.,
“In vivo Raman study of the living rat esophagus and stomach using a micro-Raman probe under an endoscope,”
Appl. Spectrosc., 61
(6), 579
–584
(2007). http://dx.doi.org/10.1366/000370207781269747 APSPA4 0003-7028 Google Scholar
M. V. Schulmerichet al.,
“Transcutaneous Raman spectroscopy of bone tissue using a non-confocal fiber optic array probe,”
Proc. SPIE, 6093 60930O
(2006). http://dx.doi.org/10.1117/12.644781 PSISDG 0277-786X Google Scholar
J. G. Wuet al.,
“Distinguishing malignant from normal oral tissues using FTIR fiber-optic techniques,”
Biopolymers, 62
(4), 185
–192
(2001). http://dx.doi.org/10.1002/(ISSN)1097-0282 BIPMAA 0006-3525 Google Scholar
M. G. Shimet al.,
“Study of fiber-optic orobes for in vivo medical Raman spectroscopy,”
Appl. Spectrosc., 53
(6), 619
–627
(1999). http://dx.doi.org/10.1366/0003702991947225 APSPA4 0003-7028 Google Scholar
A. S. Hakaet al.,
“In vivo margin assessment during partial mastectomy breast surgery using raman spectroscopy,”
Cancer Res., 66
(6), 3317
–3322
(2006). http://dx.doi.org/10.1158/0008-5472.CAN-05-2815 CNREA8 0008-5472 Google Scholar
J. Grunet al.,
“Identification of bacteria from two-dimensional resonant-Raman spectra,”
Anal. Chem., 79
(14), 5489
–5493
(2007). http://dx.doi.org/10.1021/ac070681h ANCHAM 0003-2700 Google Scholar
C. A. Lieberet al.,
“In vivo nonmelanoma skin cancer diagnosis using Raman microspectroscopy,”
Lasers Surg. Med., 40
(7), 461
–467
(2008). http://dx.doi.org/10.1002/lsm.v40:7 LSMEDI 0196-8092 Google Scholar
R. Manoharanet al.,
“Ultraviolet resonance Raman spectroscopy for detection of colon cancer,”
Laser. Life Sci., 6
(4), 217
–227
(1995). http://dx.doi.org/10.1109/IEMBS.2005.1616107 0886-0467 Google Scholar
K. R. Wardet al.,
“Oxygenation monitoring of tissue vasculature by resonance Raman spectroscopy,”
Anal. Chem., 79
(4), 1514
–1518
(2007). http://dx.doi.org/10.1021/ac061072x ANCHAM 0003-2700 Google Scholar
P. S. Bernsteinet al.,
“Resonance Raman measurement of macular carotenoids in the living human eye,”
Arch. Biochem. Biophys., 430
(2), 163
–169
(2004). http://dx.doi.org/10.1016/j.abb.2004.07.004 ABBIA4 0003-9861 Google Scholar
“Handbook of Vibrational Spectroscopy,”
5 John Wiley & Sons, New York
(2002). Google Scholar
C. Pezzeiet al.,
“Characterization of normal and malignant prostate tissue by Fourier transform infrared microspectroscopy,”
Mol. Biosyst., 6
(11), 2287
–2295
(2010). http://dx.doi.org/10.1039/c0mb00041h MBOIBW 1742-2051 Google Scholar
K. Wehbeet al.,
“FT-IR spectral imaging of blood vessels reveals protein secondary structure deviations induced by tumor growth,”
Anal. Bioanal. Chem., 392
(1–2), 129
–135
(2008). http://dx.doi.org/10.1007/s00216-008-2230-6 ABCNBP 1618-2642 Google Scholar
E. Lyet al.,
“Combination of FTIR spectral imaging and chemometrics for tumour detection from paraffin-embedded biopsies,”
Analyst, 133
(2), 197
–205
(2008). http://dx.doi.org/10.1039/b715924b ANLYAG 0365-4885 Google Scholar
C. Petiboiset al.,
“Histological mapping of biochemical changes in solid tumors by FT-IR spectral imaging,”
FEBS Lett., 581
(28), 5469
–5474
(2007). http://dx.doi.org/10.1016/j.febslet.2007.10.052 FEBLAL 0014-5793 Google Scholar
R. Bhargava,
“Towards a practical Fourier transform infrared chemical imaging protocol for cancer histopathology,”
Anal. Bioanal. Chem., 389
(4), 1155
–1169
(2007). http://dx.doi.org/10.1007/s00216-007-1511-9 ABCNBP 1618-2642 Google Scholar
C. H. Petteret al.,
“Development and application of Fourier-transform infrared chemical imaging of tumour in human tissue,”
Curr. Med. Chem., 16
(3), 318
–326
(2009). CMCHE7 0929-8673 Google Scholar
B. Birdet al.,
“Infrared micro-spectral imaging: distinction of tissue types in axillary lymph node histology,”
BMC Clin. Pathol., 8
(8), 8
(2008). http://dx.doi.org/10.1186/1472-6890-8-8 BCPMB3 1472-6890 Google Scholar
R. Zoehreret al.,
“Bone quality determined by Fourier transform infrared imaging analysis in mild primary hyperparathyroidism,”
J. Clin. Endocrinol. Metab., 93
(9), 3484
–3489
(2008). http://dx.doi.org/10.1210/jc.2008-0530 JCEMAZ 0021-972X Google Scholar
S. Gourion-ArsiquaudP. A. WestA. L. Boskey,
“Fourier transform-infrared microspectroscopy and microscopic imaging,”
Methods Mol. Biol., 455 293
–303
(2008). http://dx.doi.org/10.1007/978-1-59745-104-8_20 MMBYBO 0097-0816 Google Scholar
X. Biet al.,
“Fourier transform infrared imaging spectroscopy investigations in the pathogenesis and repair of cartilage,”
Biochim. Biophys. Acta, 1758
(7), 934
–941
(2006). http://dx.doi.org/10.1016/j.bbamem.2006.05.014 BBACAQ 0006-3002 Google Scholar
E. David-Vaudeyet al.,
“Fourier Transform Infrared Imaging of focal lesions in human osteoarthritic cartilage,”
Eur. Cell Mater., 10 51
–60
(2005). 1473-2262 Google Scholar
I. W. LevinR. Bhargava,
“Fourier transform infrared vibrational spectroscopic imaging: integrating microscopy and molecular recognition,”
Annu. Rev. Phys. Chem., 56 429
–474
(2005). http://dx.doi.org/10.1146/annurev.physchem.56.092503.141205 ARPLAP 0066-426X Google Scholar
D. C. Fernandezet al.,
“Infrared spectroscopic imaging for histopathologic recognition,”
Nat. Biotechnol., 23
(4), 469
–474
(2005). http://dx.doi.org/10.1038/nbt1080 NABIF9 1087-0156 Google Scholar
V. K. Katukuriet al.,
“Detection of colonic inflammation with Fourier transform infrared spectroscopy using a flexible silver halide fiber,”
Biomed. Opt. Express., 1
(3), 1014
–1025
(2010). http://dx.doi.org/10.1364/BOE.1.001014 BOEICL 2156-7085 Google Scholar
C. Kendallet al.,
“Exploiting the diagnostic potential of biomolecular fingerprinting with vibrational spectroscopy,”
Faraday Discuss., 149 279
–290
(2011). http://dx.doi.org/10.1039/C005379A FDISE6FDISE6 0301-72491359-6640 Google Scholar
C. Kendallet al.,
“Vibrational spectroscopy: a clinical tool for cancer diagnostics,”
Analyst, 134
(6), 1029
–1045
(2009). http://dx.doi.org/10.1039/b822130h ANLYAG 0365-4885 Google Scholar
C. Krafftet al,
“Raman and FTIR microscopic imaging of colon tissue: a comparative study,”
J. Biophotonics, 1
(2), 154
–169
(2008). http://dx.doi.org/10.1002/jbio.200710005 JBOIBX 1864-063X Google Scholar
C. Krafftet al.,
“Methodology for fiber-optic Raman mapping and FTIR imaging of metastases in mouse brains,”
Anal. Bioanal. Chem., 389
(4), 1133
–1142
(2007). http://dx.doi.org/10.1007/s00216-007-1453-2 ABCNBP 1618-2642 Google Scholar
C. Krafftet al.,
“Disease recognition by infrared and Raman spectroscopy,”
J. Biophotonics, 2
(1–2), 13
–28
(2009). http://dx.doi.org/10.1002/jbio.200810024 JBOIBX 1864-063X Google Scholar
C. Murali Krishnaet al.,
“An overview on applications of optical spectroscopy in cervical cancers,”
J. Cancer Res. Ther., 4
(1), 26
–36
(2008). http://dx.doi.org/10.4103/0973-1482.39602 JCRTBK 0973-1482 Google Scholar
C. M. Krishnaet al.,
“FTIR and Raman microspectroscopy of normal, benign, and malignant formalin-fixed ovarian tissues,”
Anal. Bioanal. Chem., 387
(5), 1649
–1656
(2007). http://dx.doi.org/10.1007/s00216-006-0827-1 ABCNBP 1618-2642 Google Scholar
S. F. Wenget al.,
“FTIR fiber optics and FT-Raman spectroscopic studies for the diagnosis of cancer,”
Am. Clin. Lab., 19
(7), 20
(2000). ACLAE7 1041-3235 Google Scholar
K. L. Chan Andrewet al.,
“A coordinated approach to cutaneous wound healing: vibrational microscopy and molecular biology,”
J. Cell. Mol. Med., 12
(5B), 2145
–2154
(2008). JCMMC9 1582-4934 Google Scholar
G. Chenet al.,
“Nonlinear spectral imaging of human hypertrophic scar based on two-photon excited fluorescence and second-harmonic generation,”
Br. J. Dermatol., 161
(1), 48
–55
(2009). http://dx.doi.org/10.1111/j.1365-2133.2009.09094.x BJDEAZ 0007-0963 Google Scholar
J. Murray-WijelathD. J. LymanE. S. Wijelath,
“Vascular graft healing. III. FTIR analysis of ePTFE graft samples from implanted bigrafts,”
J. Biomed. Mater. Res. B Appl. Biomater., 70
(2), 223
–232
(2004). http://dx.doi.org/10.1002/jbm.b.30044 JBMRGL 1552-4973 Google Scholar
D. J. Lymanet al.,
“Vascular graft healing. II. FTIR analysis of polyester graft samples from implanted bi-grafts,”
J. Biomed. Mater. Res., 58
(3), 221
–237
(2001). http://dx.doi.org/10.1002/(ISSN)1097-4636 JBMRBG 0021-9304 Google Scholar
R. Wienset al.,
“Synchrotron FTIR microspectroscopic analysis of the effects of anti-inflammatory therapeutics on wound healing in laminectomized rats,”
Anal. Bioanal. Chem., 387
(5), 1679
–1689
(2007). http://dx.doi.org/10.1007/s00216-006-1095-9 ABCNBP 1618-2642 Google Scholar
L. L. Tayet al.,
“Detection of acute brain injury by Raman spectral signature,”
Analyst, 136
(8), 1620
–1626
(2011). http://dx.doi.org/10.1039/C0AN00897D ANLYAG 0365-4885 Google Scholar
T. Saxenaet al.,
“Raman spectroscopic investigation of spinal cord injury in a rat model,”
J. Biomed. Opt., 16
(2), 027003
(2011). http://dx.doi.org/10.1117/1.3549700 JBOPFO 1083-3668 Google Scholar
A. Makowskiet al.,
“Laser preconditioning for wound healing: a Raman spectroscopy analysis,”
Lasers Surg. Med., 42
(22), 10
–11
(2010). LSMEDI 0196-8092 Google Scholar
A. Alimovaet al.,
“In vivo molecular evaluation of guinea pig skin incisions healing after surgical suture and laser tissue welding using Raman spectroscopy,”
J. Photochem. Photobiol. B, 96
(3), 178
–183
(2009). http://dx.doi.org/10.1016/j.jphotobiol.2009.06.004 JPPBEG 1011-1344 Google Scholar
N. J. Craneet al.,
“Monitoring the healing of combat wounds using Raman spectroscopic mapping,”
Wound Repair Regen., 18
(4), 409
–416
(2010). 1067-1927 Google Scholar
A. J. Mangramet al.,
“Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee,”
Am. J. Infect. Control, 27
(2), 96
–134
(1999). AJICDC 0196-6553 Google Scholar
F. R. Sheppardet al.,
“The majority of US combat casualty soft-tissue wounds are not infected or colonized upon arrival or during treatment at a continental US military medical facility,”
Am. J. Surg., 200
(4), 489
–495
(2010). http://dx.doi.org/10.1016/j.amjsurg.2010.03.001 AJOOA7 0096-6347 Google Scholar
P. C. Buijtelset al.,
“Rapid identification of mycobacteria by Raman spectroscopy,”
J. Clin. Microbiol., 46
(3), 961
–965
(2008). http://dx.doi.org/10.1128JCM.01763-07 JCMIDW 1070-633X Google Scholar
M. F. Escorizaet al.,
“Raman spectroscopy and chemical imaging for quantification of filtered waterborne bacteria,”
J. Microbiol. Methods, 66
(1), 63
–72
(2006). http://dx.doi.org/10.1016/j.mimet.2005.10.013 JMIMDQ 0167-7012 Google Scholar
K. Maquelinet al.,
“Identification of medically relevant microorganisms by vibrational spectroscopy,”
J. Microbiol. Methods, 51
(3), 255
–271
(2002). http://dx.doi.org/10.1016/S0167-7012(02)00127-6 JMIMDQ 0167-7012 Google Scholar
Q. Wuet al.,
“Intensities of E. coli nucleic acid Raman spectra excited selectively from whole cells with 251-nm light,”
Anal. Chem., 72
(13), 2981
–2986
(2000). http://dx.doi.org/10.1021/ac990932p ANCHAMANCHAM 0003-27000003-2700 Google Scholar
L. Zeiriet al.,
“Surface-enhanced Raman spectroscopy as a tool for probing specific biochemical components in bacteria,”
Appl. Spectrosc., 58
(1), 33
–40
(2004). http://dx.doi.org/10.1366/000370204322729441 APSPA4 0003-7028 Google Scholar
K. Maquelinet al.,
“Rapid epidemiological analysis of Acinetobacter strains by Raman spectroscopy,”
J. Microbiol. Methods, 64
(1), 126
–131
(2006). http://dx.doi.org/10.1016/j.mimet.2005.04.028 JMIMDQ 0167-7012 Google Scholar
K. Maquelinet al.,
“Raman spectroscopic method for identification of clinically relevant microorganisms growing on solid culture medium,”
Anal. Chem., 72
(1), 12
–19
(2000). http://dx.doi.org/10.1021/ac991011h ANCHAM 0003-2700 Google Scholar
K. S. Kalasinskyet al.,
“Raman chemical imaging spectroscopy reagentless detection and identification of pathogens: signature development and evaluation,”
Anal. Chem., 79
(7), 2658
–2673
(2007). http://dx.doi.org/10.1021/ac0700575 ANCHAM 0003-2700 Google Scholar
L. Zeiriet al.,
“Silver metal induced surface enhanced Raman of bacteria,”
Colloids Surf A Physicochem. Eng. Asp, 208 357
–362
(2002). http://dx.doi.org/10.1016/S0927-7757(02)00162-0 CPEAEH 0927-7757 Google Scholar
D. I. EllisR. Goodacre,
“Metabolic fingerprinting in disease diagnosis: biomedical applications of infrared and Raman spectroscopy,”
Analyst, 131
(8), 875
–885
(2006). http://dx.doi.org/10.1039/b602376m ANLYAG 0365-4885 Google Scholar
K. Maquelinet al.,
“Prospective study of the performance of vibrational spectroscopies for rapid identification of bacterial and fungal pathogens recovered from blood cultures,”
J. Clin. Microbiol., 41
(1), 324
–329
(2003). http://dx.doi.org/10.1128/JCM.41.1.324-329.2003 JCMIDW 1070-633X Google Scholar
C. L. Winderet al.,
“The rapid identification of Acinetobacter species using Fourier transform infrared spectroscopy,”
J. Appl. Microbiol., 96
(2), 328
–339
(2004). JAMIFK 1364-5072 Google Scholar
F. S. Kaplanet al.,
“Heterotopic ossification,”
Am. J. Acad. Orthop. Surg., 12
(2), 116
–125
(2004). 1067-151X Google Scholar
D. E. Garland,
“Clinical observations on fractures and heterotopic ossification in the spinal cord and traumatic brain injured populations,”
Clin. Orthop. Relat. Res., 233 86
–101
(1988). CORTBR 0009-921X Google Scholar
A. CardenM. D. Morris,
“Application of vibrational spectroscopy to the study of mineralized tissues,”
J. Biomed. Opt., 5
(3), 259
–268
(2000). http://dx.doi.org/10.1117/1.429994 JBOPFO 1083-3668 Google Scholar
A. Cardenet al.,
“Raman imaging of bone mineral and matrix: composition and function,”
Proc. SPIE, 3608 132
–138
(1999). http://dx.doi.org/10.1117/12.345394 PSISDG 0277-786X Google Scholar
A. Cardenet al.,
“Ultrastructural changes accompanying the mechanical deformation of bone tissue: a Raman imaging study,”
Calcif. Tissue Int., 72
(2), 166
–175
(2003). http://dx.doi.org/10.1007/s00223-002-1039-0 CTINDZ 0171-967X Google Scholar
C. J. de Grauwet al.,
“Investigation of bone and calcium phosphate coatings and crystallinity determination using Raman microspectroscopy,”
Cells Mater, 6
(1–3), 57
–62
(1996). CMBLFF 1425-8153 Google Scholar
M. D. Morriset al.,
“Bone microstructure deformation observed by Raman microscopy,”
Proc. SPIE, 4254 81
–89
(2001). http://dx.doi.org/10.1117/12.427949 PSISDG 0277-786X Google Scholar
M. D. Morriset al.,
“Raman spectroscopy of early mineralization of normal and pathological calvaria,”
Proc. SPIE, 4614 28
–39
(2002). http://dx.doi.org/10.1117/12.460796 PSISDG 0277-786X Google Scholar
M. D. Morriset al.,
“Raman microscopy of de novo woven bone tissue,”
Proc. SPIE, 4254 90
–96
(2001). http://dx.doi.org/10.1117/12.427950 PSISDG 0277-786X Google Scholar
G. Penelet al.,
“Raman microspectrometry studies of brushite cement: in vivo evolution in a sheep model,”
Bone, 25
(2), 81S
–84S
(1999). 8756-3282 Google Scholar
G. PezzottiS. Sakakura,
“Study of the toughening mechanisms in bone and biomimetic hydroxyapatite materials using Raman microprobe spectroscopy,”
J. Biomed. Mater. Res., 65A
(2), 229
–236
(2003). http://dx.doi.org/10.1002/(ISSN)1097-4636 JBMRBG 0021-9304 Google Scholar
J. A. Pezzutiet al.,
“Hyperspectral Raman imaging of bone growth and regrowth chemistry,”
Proc. SPIE, 3261 270
–276
(1998). http://dx.doi.org/10.1117/12.310566 PSISDG 0277-786X Google Scholar
R. SmithI. Rehman,
“Fourier transform Raman spectroscopic studies of human bone,”
J. Mater. Sci. Mater. Med., 5 775
–778
(1995). http://dx.doi.org/10.1007/BF00120375 JSMMEL 0957-4530 Google Scholar
C. P. TarnowskiM. A. Ignelzi Jr.M. D. Morris,
“Mineralization of developing mouse calvaria as revealed by Raman microspectroscopy,”
J. Bone Miner. Res., 17
(6), 1118
–1126
(2002). http://dx.doi.org/10.1359/jbmr.2002.17.6.1118 JBMREJ 0884-0431 Google Scholar
J. Timlinet al.,
“Raman spectroscopic imaging markers for fatigue-related microdamage in bovine bone,”
Anal. Chem., 72
(10), 2229
–2236
(2000). http://dx.doi.org/10.1021/ac9913560 ANCHAM 0003-2700 Google Scholar
J. A. Timlinet al.,
“Spatial distribution of phosphate species in mature and newly generated mammalian bone by hyperspectral Raman imaging,”
J. Biomed. Opt., 4
(1), 28
–34
(1999). http://dx.doi.org/10.1117/1.429918 JBOPFO 1083-3668 Google Scholar
N. J. Craneet al.,
“Raman spectroscopic evidence for octacalcium phosphate and other transient mineral species deposited during intramembraneous mineralization,”
Bone, 39
(3), 434
–442
(2006). 8756-3282 Google Scholar
J. J. Freemanet al.,
“Raman spectroscopic detection of changes in bioapatite in mouse femora as a function of age and in vitro fluoride treatment,”
Calcif. Tissue Int., 68
(3), 156
–162
(2001). http://dx.doi.org/10.1007/s002230001206 CTINDZ 0171-967X Google Scholar
M. D. Morriset al.,
“Effects of applied load on bone tissue as observed by Raman spectroscopy,”
Proc. SPIE, 4614 47
–54
(2002). http://dx.doi.org/10.1117/12.460798 PSISDG 0277-786X Google Scholar
J. D. Pasteriset al.,
“Lack of OH in nanocrystalline apatite as a function of degree of atomic order: implications for bone and biomaterials,”
Biomaterials, 25
(2), 229
–238
(2004). http://dx.doi.org/10.1016/S0142-9612(03)00487-3 BIMADU 0142-9612 Google Scholar
G. Penelet al.,
“MicroRaman spectral study of the and vibrational modes in synthetic and biological apatites,”
Calcif. Tissue Int., 63
(6), 475
–481
(1998). http://dx.doi.org/10.1007/s002239900561 CTINDZ 0171-967X Google Scholar
P. Taddeiet al.,
“Vibrational spectroscopic characterization of new calcium phosphate bioactive coatings,”
Biospectroscopy, 57
(3), 140
–148
(2000). http://dx.doi.org/10.1002/(SICI)1097-0282(2000)57:3<140::AID-BIP2>3.0.CO;2-9 BIOSFS 1075-4261 Google Scholar
B. K. Potteret al.,
“Heterotopic ossification following combat-related trauma,”
J. Bone Joint Surg. Am., 92
(2), 74
–89
(2010). http://dx.doi.org/10.2106/JBJS.F.00412 JBJSA3 0021-9355 Google Scholar
|

