|
|
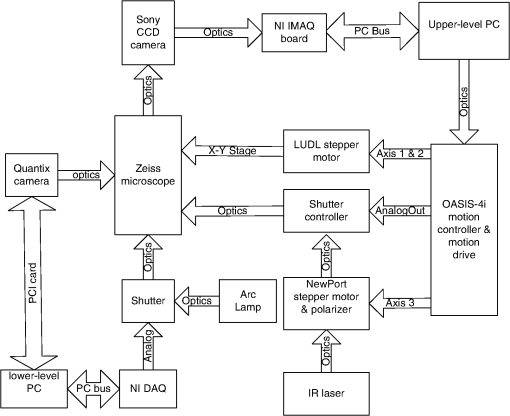
1.IntroductionSperm motility, the ability of a sperm to move efficiently towards the egg, is a valuable parameter in the assessment of sperm quality and fertilization capacity.1 The ability to evaluate sperm motility is important in areas such as sperm cryopreservation, in-vitro fertilization (IVF), and artificial insemination (AI). Factors in the vaginal canal, such as pH, antibody response, and the overall structural and functional capacity of the sperm, can affect sperm motility.2 Particularly, the viscous environment encountered by the sperm can have a major impact on its motility and its subsequent ability to reach and fertilize the egg. The objective of this study is to address the relationship between viscosity and sperm motility using optical trapping. There are various methods to score and quantify sperm motility. Computer aided analysis quantifies the overall motility of a sperm population in a short amount of time.3,4 Commercial computer assisted sperm analysis (CASA) systems have been developed to track sperm within a field of view and measure motility parameters such as curvilinear velocity (VCL), amplitude of lateral head movement, and percent of motile sperm.3,4 Examples of these commercial systems include Hamilton Thorne IVSO-CASA, SM-CMA system (Stromberg-Mika, Bad Feilnbach, Germany), and the Hobson Sperm Tracker (Hobson Sperm Tracking Ltd., Sheffield, United Kingdom). Other optically based computer aided tracking systems such as the real-time automated tracking and trapping system (RATTS) have been developed to automatically track and optically trap individual sperm.5 RATTS can measure VCL and has the added ability to measure sperm swimming force using optical tweezers to hold and release individual sperm.6 Optical tweezers (traps) can confine and manipulate microscopic particles due to the momentum of the photons in a tightly focused laser beam.7,8 RATTS utilizes optical tweezers to quantify sperm swimming forces by measuring the minimum amount of laser power need to hold the sperm in the trap. The laser escape power (Pesc) is directly proportional to the sperm’s swimming force and can be calculated using the equation, , where is the swimming force, is the laser power, is the speed of light in the medium, and is the geometrically determined trapping efficiency parameter.9 Previous studies have used optical tweezers to noninvasively study sperm motility by measuring swimming forces.6,9 The development of RATTS permits simultaneous measurement of force and VCL thus providing a multiparametric analysis of sperm motility. The goal of this study is to analyze the effect of viscosity on sperm motility by measuring both VCL and sperm swimming force using RATTS. A membrane potential-sensitive fluorescent probe is also used to examine changes in sperm energetics as a function of changes in viscosity of the swimming medium. Fluorescent probes have been used to relate mitochondrial membrane potential to sperm motility and have demonstrated that high mitochondrial membrane potentials in the sperm midpiece correlate with an increase in ATP synthesis and increased motility.10 Cyanine dyes such as 3,-dihexyloxacarbocyanine iodide [] integrate into the mitochondria and increase in fluorescent intensity as the magnitude of the membrane potential increases. There are numerous chemical probes to measure mitochondrial activity, such as carbocyanines [, ], rhodamines (TMRE, JC-1), and rosamines (CMX-Ros). is used in this study because it has been demonstrated to be an effective dye for measuring membrane potentials in sperm.11 2.Materials and Methods2.1.Optical SystemThe optical system is similar to that described previously.12 Briefly, it uses a continuous wave 1064-nm-wavelength laser (Spectra Physics, Model BL-106C, Mountain View, CA) which travels through a series of lenses and mirrors into a Zeiss Axiovert S100 microscope and a , phase III, NA 1.3 oil immersion objective (Zeiss, Thornwood, NY) where it is focused to a diffraction-limited spot of approximately . A motorized rotating polarizer controls the laser power by attenuating the beam during the sperm swimming force experiments. The beam-expanding lenses and focusing lens fill the objective back aperture with the collimated laser beam maximizing the amount of energy in the trapping focal spot. The optical system for acquiring phase and fluorescence images has been described previously.11 Briefly, a Zeiss fluor arc lamp provides the excitation light. A red filter above the image plane allows for separation of phase contrast (670 nm) and fluorescence (500 to 570 nm) images. The reflected phase contrast information is collected by a charged coupled device (CCD) camera (Cohu model 7800, San Diego, CA) at 30 fps. The fluorescence information passes through a HQ emission filter and is collected by a digital camera (Quantix 57, Roper Scientific Inc., Tuscon, AZ). 2.2.Hardware and Software SystemMotility parameters are measured using two computer-based analysis systems. First, CASA (IVOS Sperm Analyzer, Hamilton Thorne, Beverly, MA) is used to assess the initial quality of sperm samples and to measure VCL. Second, VCL and Pesc are measured simultaneously using customized software (RATTS) coded in the LabView language (National Instruments, Austin, TX). RATTS operates at video rate and provides remote robotic interfaces with the hardware. In addition, the system has fluorescent image processing capabilities.5 The operation of RATTS is performed in the upper-level system while fluorescent image acquisition, processing, and storage are done in the lower-level system, as shown in Fig. 1. The two computers are networked together over a gigabit TCP/IP cat5e crossover connection. Communication between the two systems is optimized with LabView’s shared variables and virtual instrument (VI) server function in which the lower-level system continuously polls the upper-level system for the next request. Using RATTS, sperm are tracked for an extended duration before and after laser trap experiments. Motility measurements including VCL are measured followed by subsequent trapping measurements. A more detailed description of the system has been presented previously.13 2.3.Sperm Collection and Preparation with DiOC6(3)Human samples were supplied by Infertility, Gynecology, and Obstetrics Medical Group (La Jolla, CA). The samples were frozen according to a normal human freezing protocol.14–16 Human sperm samples were thawed in a water bath at 37°C for 2 min, and then centrifuged for 10 min at 2000 rpm. The supernatant was removed and the pellet was resuspended in 1 mL of HEPES-buffered modified Human Tubal Fluid (mHTF) with filtered 5% serum substitute supplement (SSS) (Irvine Scientific, Irvine, CA). The sample was again centrifuged for 10 min at 2000 rpm and resuspended. This two-wash technique was used for all experiments. The final sperm dilutions of 30,000 sperm per mL were loaded into cover slide dishes (about 0.5 μL of sperm in 2.5 mL ) and mounted on the microscope stage.11 For fluorescent studies 3,-dihexyloxacarbocyanine iodide [], a nonratiometric carbocyanine dye supplied by Invitrogen (Carlsbad, CA), was added to the prepared sperm: 40-nM stock dye was added into 250 μL of prepared human sperm and was incubated at 37°C for 20 min.11 The sperm sample was loaded into the optical system to measure midpiece mitochondrial fluorescence. Stock solutions of dye were prepared with dimethyl sulfoxide (DMSO). 2.4.Viscous Media PreparationMedia with different viscosities were created by varying the concentration of methylcellulose (Sigma Aldrich M 7140, St. Louis, MO) in sperm suspension media. Specifically, media with viscosities of 3, 6, 9, and 15 cP were made by adding 0.5%, 1%, 1.5%, and 2% (w/w) methylcellulose (Sigma Aldrich M 7140) to the media, respectively. Initially, one-third of the mHTF was heated to 80°C. The methylcellulose powder was added to the heated mHTF media, and the mixture was agitated until the particles were evenly dispersed. For complete solubilization another one-third of the mHTF media was added as cold media to lower the temperature of dispersion. The final one-third of the mHTF media was added along with SSS to yield a final SSS solution with the desired methylcellulose concentration. The solution was agitated and subsequently cooled to 0°C to 5°C for 20 to 40 min to lower the temperature of dispersion and further hydrate the methylcellulose. The solution was then continuously agitated for an additional 30 min after the proper temperature was reached. 2.5.Refractive Index AnalysisThe refractive indices of the viscosity solutions were measured using a digital refractometer (Sper Scientific model 300034, Scottsdale, AZ) with instrument range of 1.330 to 1.5318, resolution of 0.0001, and accuracy of . Measurements were taken at 22.3°C using 1 mL of solution. The refractive index of a sperm cell was approximated to be 1.53 (Ref. 17). The effect of refractive index on optical trap stiffness was considered by comparing the differences between the refractive index of a sperm cell and the indices of the media.18 2.6.Statistical AnalysisComparisons between experimental data sets were performed in MATLAB (Mathworks, Natick, MA) using the nonparametric Wilcoxon rank sum test (based on 5% significance). The experimental results are presented in Figs. 2Fig. 3Fig. 4–5 as box plots. Each box plot displays the following data: median (center line of box), upper and lower quartile values (top and bottom of box, respectively), range (upper and lower bars), and data points lying outside 3-times the interquartile range. The notches of the box plots represent the uncertainly of the median value. If the notches do not overlap, then the medians are different at the 95% confidence level. Fig. 2CASA VCL vs. Viscosity. MATLAB was used to fit a power model [] to the medians of the data sets. The coefficients with their 95% confidence limits were found to be (72.3, 85.35) and (,), yielding the equation . The correlation coefficient was determined to be . The -values are: at 1 cP (), at 3 cP (), at 6 cP (), at 9 cP (), and at 15 cP (). 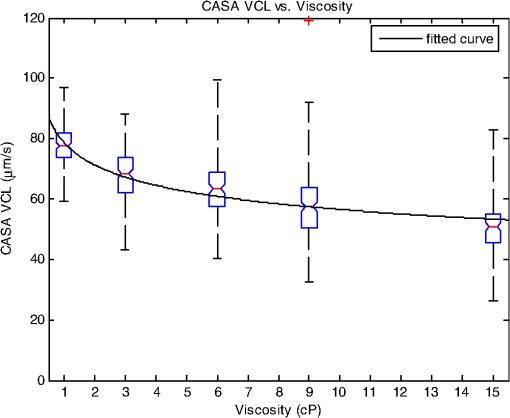 3.ResultsFor each sperm sample, motility parameters were first analyzed at 1 cP (mHTF media with 5% SSS and no methylcellulose) using the CASA system where the VCL could be closely monitored in multiple fields of view. This measurement served as an initial base-line for sperm quality/motility before subsequent experiments were performed. CASA was then used to measure VCL at 3, 6, 9, and 15 cP. The CASA VCL parameters were aggregated and graphed using MATLAB as shown in Fig. 2. The CASA VCL results demonstrate a decrease in VCL with increasing viscosity. A power model was fitted to the medians of the datasets yielding the equation with a correlation coefficient of . The same sperm samples were then measured for VCL using RATTS. The aggregated data was graphed and fitted to a power model yielding the equation with a coefficient of . Although the VCL measurements from RATTS were slightly lower than those from CASA, a similar relationship between VCL and viscosity was observed with a confidence level greater than 95%, as shown in Fig. 3. Fig. 3RATTS VCL vs. Viscosity. MATLAB was used to fit a power model () to the medians of the data sets. The coefficients with their 95% confidence limits were found to be (58.88, 71.1) and (,), yielding the equation . The correlation coefficient was determined to be . The -values are: at 1 cP (), at 3 cP (), at 6 cP (), at 9 cP (), and at 15 cP (). 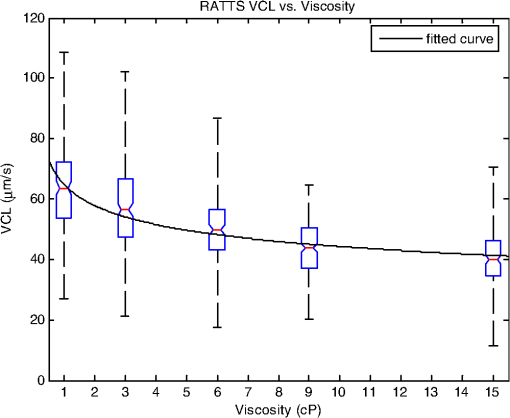 Measurements of Pesc were then performed using RATTS in 1, 3, 6, 9, and 15 cP media Fig. 4. A power model was fitted to the medians of the data sets yielding with a correlation coefficient of . The results reveal that at higher viscosities the sperm escape from the laser trap at higher laser powers. This indicates that the sperm swim with greater force as viscosity increases. Statistical analysis of the data sets reveal that the Pesc at 6, 9, and 15 cP are statistically different () from the Pesc at 1 cP, with , , and , respectively. However, the Pesc at 3 cP was found to be statistically the same () as that at 1 cP, with a -value of 0.6887. The statistical analysis indicates that viscosity affects sperm swimming force in viscosities higher than 3 cP. Fig. 4Pesc vs. Viscosity. MATLAB was used to fit a power model to the medians of the data sets. The coefficients with their 95% confidence limits were found to be (0.334, 5.573) and (1.486, 2.163), yielding the equation . The correlation coefficient was determined to be . The -values are: at 1 cP (), at 3 cP (), at 6 cP (), at 9 cP (), and at 15 cP (). The asterisks () indicate that there was no significant difference between 1 and 3 cP at 5% confidence level. 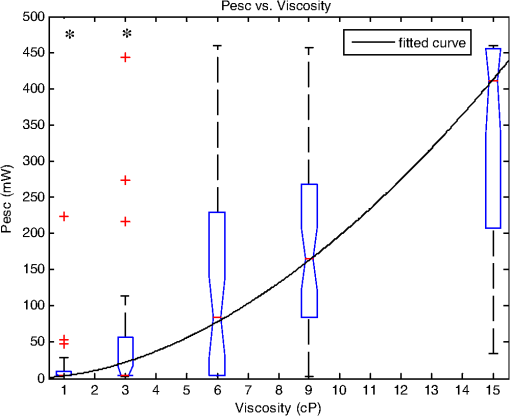 To examine if aerobic energetics plays a role in this phenomenon, sperm were incubated in media containing the membrane potential-sensitive dye following the protocol previously described.11 The -treated sperm were tested in viscosities of 1, 6, and 9 cP. The fluorescence data were aggregated and graphed in Fig. 5. The data sets for 6 and 9 cP were statistically compared to that for 1 cP using the Wilcoxon rank sum test, and yielded and , respectively. These results indicate that there is no significant change with a confidence of 95%. Therefore, it does not appear that there is an increase in mitochondrial ATP generation as the sperm encounter higher viscosities even though, as shown in Fig. 5, they are escaping from the trap with greater force. Fig. 5Fluorescence versus viscosity. Midpiece fluorescence intensity of treated sperm was measured. Asterisks () indicates that there was no significant difference in fluorescence intensity between 1, 6, and 9 cP at the 5% level. The -values are: at 1 cp (), at 6 cP (), andat 9 cP (). Fluorescence intensity was measured by subtracting the background intensity (sperm samples without fluorescence probe) from the fluorescence measurements using . 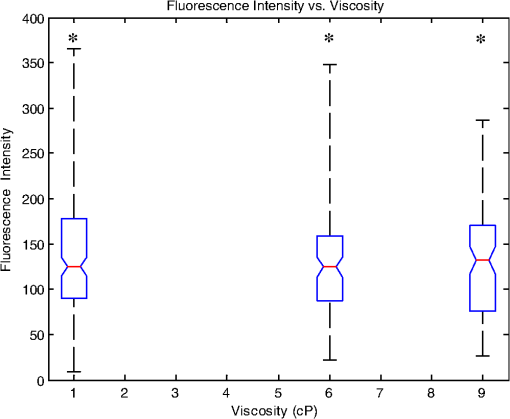 The refractive indices of the solutions were measured using a digital refractometer with a resolution of 0.0001 and an accuracy of (Table 1). The index of refraction ranged from at 1 cP to at 15 cP. As the concentration increased, the index of refraction proportionally increased at a rate of approximately 0.0013 per 1% methylcellulose. Table 1Refractive indices of the viscosity solutions
4.DiscussionVCL measured on both the CASA and RATTS systems show that as the viscosity increases, VCL decreases. This result is not surprising considering basic principles of fluid mechanics: an object moving through a fluid is subjugated to frictional forces created by the viscosity of the medium. Increasing the viscosity of the mHTF medium augments the fluid’s ability to resist shear stress. Thus as viscosity increases, sperm velocity should decrease. Although sperm swim-trajectory is complicated because of its three dimensional waveform, its speed is correlated to viscosity as fluid flow laws dictate for a linear, nonrandom, traveling object.19 This trend is modeled using MATLAB to fit the power regression as in Figs. 2 and 3. The equation relates viscosity to motility parameters as follows: CASA and RATTS . The power parameter represents the rate at which VCL is decreasing with increasing viscosity. Although the VCL measurements are slightly different, the parameters and fall within the 95% parameter confidence limits for CASA and RATTS, respectively (Figs. 2 and 3), and validate the accuracy of each system. A possible explanation for the slight difference in VCL measurements may be due the temperature of the sample; CASA heats the sperm sample to 37°C which can affect viscosity of the solution, and RATTS measurements are made at room temperature, 20°C. Although the viscosity media made for this study were calibrated at room temperature, the viscosity may have decreased due to the increased temperature, accounting for the increase in sperm VCL. The Pesc motility parameter (sperm swimming force) measured using the optical tweezers and RATTS, responded differently to increasing viscosity. Interestingly, as the viscosity increases above 3 cP, the Pesc increases. Thus, at higher viscosities the swimming force of the sperm increases. To model this behavior MATLAB was used to fit a power regression producing the equation, . By comparing the VCL and Pesc parameters, it is concluded that viscosity has a significant effect on the swimming force of the sperm. The effect of the methylcellulose on the refractive index and trap stiffness was considered.18 It was determined that from 1 to 15 cP, the index of refraction increased by 0.0026. This small increase in refractive index was calculated to contribute to a less than 1.5% change in trap stiffness from 1 to 15 cP. This small change does not explain the large nonlinear increase in laser power needed to trap sperm at increasing viscosities. A possible explanation for the increase in sperm swimming force was that sperm energetics (ATP generation) increased in response to the higher viscous environment. The sperm, in an effort to maintain its VCL would increase ATP production in the mitochondrial-rich midpiece. To investigate this possibility the mitochondrial membrane potential-sensitive dye was used to monitor the energetics of the sperm while in the trap using the methods reported previously.11,12 However, the experimental results (Fig. 5) did not reveal any difference in mitochondrial midpiece fluorescence when the sperm were swimming in media of different viscosities. Though these data suggest that the increase in swimming force is not related to an increase in mitochondrial ATP generation (aerobic respiration), future experiments should be conducted with other membrane-potential dyes such as JC1 and .12,20 In addition, the role of ATP from anaerobic respiration (glycolysis) cannot be ruled out as it has been shown to play a significant role in sperm energetics.12 Notwithstanding, the data presented in this paper demonstrate that as viscosity increases, sperm swim with greater force, but aerobic energetics remains relatively constant. This leads to the speculation that the increase in swimming force may be due to the biomechanical properties of the sperm flagellum. Future studies using high speed imaging will permit detailed analysis of flagellum waveform, and should help elucidate the biomechanical effect of viscosity on sperm motility.21 This study has focused on low viscosities which encompass the 4 to 10 cP range found in the human male reproductive tract.22–24 Future studies examining higher viscosity environments will provide insight into a sperm’s journey through the human female reproductive tract consisting of viscosities upwards of 1700 cP.24,25 In order to do these experiments, higher power laser traps will be needed, and consequent thermal effects of the trap will have to be mitigated.26 AcknowledgmentsThis work was supported by funds from the Beckman Laser Institute Inc. Foundation awarded to MWB. C. Chandsawangbhuwana would like to acknowledge support from a National Defense Science and Engineering Graduate Fellowship and a NSF Graduate Research Fellowship. ReferencesD. P. WolfP. E. Patton,
“Sperm cryopreservation: state of the art,”
J. Assist. Reprod. Genet., 6
(6), 325
–327
(1989). JARGE4 1058-0468 Google Scholar
J. Rutllantet al.,
“Rheological and ultrastructural properties of bovine vaginal fluid obtained at oestrus,”
J. Anat., 201
(1), 53
–60
(2002). http://dx.doi.org/10.1046/j.1469-7580.2002.00069.x JOANAY 0021-8782 Google Scholar
R. P. AmannD. F. Katz,
“Reflections on CASA after 25 years,”
J. Androl., 25
(3), 317
–325
(2004). JOAND3 0196-3635 Google Scholar
D. Mortimer, Practical Laboratory Andrology, Oxford University Press, USA
(1994). Google Scholar
L. Z. Shiet al.,
“Real-time automated tracking and trapping system for sperm,”
Microsc. Res. Tech., 69
(11), 894
–902
(2006). http://dx.doi.org/10.1002/jemt.20359 MRTEEO 1059-910X Google Scholar
E. Araujo Jr.et al.,
“Relative force of human epididymal sperm,”
Fertil. Steril., 62
(3), 585
–590
(1994). FESTAS 0015-0282 Google Scholar
A. Ashkin,
“The study of cells by optical trapping and manipulation of living cells using infrared laser beams,”
ASGSB Bull.: Publication of the American Society for Gravitational and Space Biology, 4
(2), 133
–136
(1991). Google Scholar
A. Ashkin,
“Forces of a single-beam gradient laser trap on a dielectric sphere in the ray optics regime,”
Biophys. J., 61
(2), 569
–582
(1992). http://dx.doi.org/10.1016/S0006-3495(92)81860-X BIOJAU 0006-3495 Google Scholar
K. Koniget al.,
“Determination of motility forces of human spermatozoa using an 800 nm optical trap,”
Cell. Mol. Biol. (Noisy-le-grand), 42
(4), 501
–509
(1996). Google Scholar
C. Marchettiet al.,
“Comparison of four fluorochromes for the detection of the inner mitochondrial membrane potential in human spermatozoa and their correlation with sperm motility,”
Hum. Reprod., 19
(10), 2267
(2004). http://dx.doi.org/10.1093/humrep/deh416 HUREEE 0268-1161 Google Scholar
T. Chenet al.,
“Optical tweezers and non-ratiometric fluorescent-dye-based studies of respiration in sperm mitochondria,”
J. Opt., 13
(4), 044010
(2011). JOOPDB 0150-536X Google Scholar
J. M. Nascimentoet al.,
“Comparison of glycolysis and oxidative phosphorylation as energy sources for mammalian sperm motility, using the combination of fluorescence imaging, laser tweezers, and real time automated tracking and trapping,”
J. Cell. Physiol., 217
(3), 745
–751
(2008). http://dx.doi.org/10.1002/jcp.v217:3 JCLLAX 0021-9541 Google Scholar
J. M. Nascimentoet al.,
“Analysis of sperm motility using optical tweezers,”
J Biomed. Opt., 11
(4), 044001
(2006). http://dx.doi.org/10.1117/1.2337559 JBOPFO 1083-3668 Google Scholar
Lori B. Andrewset al.,
“New guidelines for the use of semen donor insemination: 1986. The American Fertility Society,”
Fertil. Steril., 46
(4 Suppl. 2), 93S
–110S
(1986). FESTAS 0015-0282 Google Scholar
S. J. DiMarzoet al.,
“Pregnancy rates with fresh versus computer-controlled cryopreserved semen for artificial insemination by donor in a private practice setting,”
Am. J. Obstet. Gynecol., 162
(6), 1483
(1990). AJOGAH 0002-9378 Google Scholar
P. SerafiniR. P. Marrs,
“Computerized staged-freezing technique improves sperm survival and preserves penetration of zona-free hamster ova,”
Fertil. Steril., 45
(6), 854
–858
(1986). FESTAS 0015-0282 Google Scholar
C. Van DuijnC. Van VoorstG. Hellinga,
“Precision measurements of dimensions, shape and mass density of spermatozoan heads in normal and subfertile human males,”
Eur. J. Obstet. Gynecol., 2
(2), 37
–54
(1972). Google Scholar
R. R. Brauet al.,
“Passive and active microrheology with optical tweezers,”
J. Opt. A, 9
(S), 103
–112
(2007). http://dx.doi.org/10.1088/1464-4258/9/8/S01 JOAOF8 1464-4258 Google Scholar
D. M. Woolley,
“Motility of spermatozoa at surfaces,”
Reproduction, 126
(2), 259
–270
(2003). http://dx.doi.org/10.1530/rep.0.1260259 1470-1626 Google Scholar
A. MeiE. BotvinickM. Berns,
“Monitoring sperm mitochondrial respiration response in a laser trap using ratiometric fluorescence,”
Proc. SPIE, 5930 59302F
(2005). http://dx.doi.org/10.1117/12.618126 Google Scholar
D. J. Smithet al.,
“Bend propagation in the flagella of migrating human sperm, and its modulation by viscosity,”
Cell Motil. Cytoskeleton, 66
(4), 220
–236
(2009). http://dx.doi.org/10.1002/cm.v66:4 CMCYEO 0886-1544 Google Scholar
C. H. Leeet al.,
“Effects of chelating agents on the rheological property of cervical mucus,”
Contraception, 65
(6), 435
–440
(2002). http://dx.doi.org/10.1016/S0010-7824(02)00300-1 CCPTAY 0010-7824 Google Scholar
M. C. LinT. C. TsaiY. S. Yang,
“Measurement of viscosity of human semen with a rotational viscometer,”
J. Formos. Med. Assoc., 91
(4), 419
–423
(1992). JFASEO 0929-6646 Google Scholar
D. B. Troy, Remington: The Science and Practice of Pharmacy, Philadelphia College of Pharmacy and Science, New York
(2005). Google Scholar
P. Y. TamD. F. KatzS. A. Berger,
“Non-linear viscoelastic properties of cervical mucus,”
Biorheology, 17
(5–6), 465
–478
(1980). BRHLAU 0006-355X Google Scholar
Y. Liuet al.,
“Physiological monitoring of optically trapped cells: assessing the effects of confinement by 1064-nm laser tweezers using microfluorometry,”
Biophys. J., 71
(4), 2158
–2167
(1996). http://dx.doi.org/10.1016/S0006-3495(96)79417-1 BIOJAU 0006-3495 Google Scholar
|