|
|
1.IntroductionThe patterning of the embryonic heart is a complex process involving both genetic and environmental factors. Many of the genetic factors have been extensively studied and yet the role of environmental signals, including the flow of blood resulting from the heart’s own pumping action, remains poorly understood. To examine cardiac hemodynamics in vivo, particle tracking and particle image velocimetry approaches have been developed that utilize a range of imaging modalities including confocal laser scanning microscopy,1–3 ultrasound,4 and optical coherence tomography.5 The two major clinical techniques used for in vivo velocity measurements are magnetic resonance imaging (MRI) and ultrasound. Unfortunately, the near-wall resolutions of these modalities, 1000 to 1200 and 250 to 300 μm, respectively,6 are insufficient for high-resolution measurements in small vessels. Small animal versions of these systems are available for research purposes, with ultrasound systems being able to increase the spatial resolution of measurements at the expense of penetration7,8 and MRI systems attaining higher spatial resolution at the expense of temporal resolution.9,10 Laser Doppler is widely used for blood flow velocity measurements and relies on the optical Doppler effect of a frequency shift due to the movement of a scattering particle.11 Additionally, laser speckle techniques have been used for flow measurements,12 utilizing the alteration of speckle contrast due to particle movement to deduce that movement. A recent study has performed 3-D flow reconstruction using ultrasound particle image velocimetry;13 however, the spatial resolution was an order of magnitude larger than that of the current study. More detailed reviews of all of these techniques can be found in Vennemann et al.14 and Fouras et al.15 Early work investigating the role of blood flow in the development of the heart has largely been carried out in the chick,8,16–18 utilizing the advantages of the external development of this system to allow physical manipulation. Work investigating blood flow has also been carried out in other species, such as the mouse19,20 and rat.21,22 A variety of surgical manipulations have been carried out on the developing chicken heart based on tying off arteries or veins, thereby altering the patterns of blood flow and increasing the pressure load on the heart. These studies have identified effects on the proliferation of myocytes,8 ventricular septal defects and semilunar valve malformations,16 as well as atrioventricular valve defects;17 together with a wide range of effects on chamber shape, stroke volume, heart rate, and cardiac output.18 Blood flow was assessed in these studies by the injection of dye into the bloodstream and video recording16 or by ultrasound echocardiography.8 In other cases, blood flow itself was not observed, but the resulting changes in morphology were examined. These studies clearly defined a role for blood flow in the patterning of the heart but were hampered by an inability to differentiate the effect of changes in blood flow from the longer term effects of increased cardiac pressure, together with difficulties in the detailed measurement of intracardiac blood flow caused by the high spatial and temporal resolutions required. Research in the zebrafish model system has begun to remove these limitations and allow the detailed examination of intracardiac blood flow. Zebrafish offer many advantages for the study of intracardiac flow, including the optical transparency of the early embryo, which allows the application of bright field imaging techniques for improved spatial and temporal resolution. Particle image velocimetry (PIV), an optical imaging method for measuring direction and velocity of flow patterns,23 is ideal for in vivo whole-field blood velocity measurements. PIV requires a pair of images to be acquired at a specified time interval. Images are divided into interrogation windows and cross correlation used to determine the modal displacement of the window; this, combined with the known time interval, gives the instantaneous velocity. Microparticle image velocimetry (μPIV), the application of PIV on a microscopic level, is a common variation of standard PIV, with its own advantages and disadvantages. For further details of the specifics of μPIV, the reader is referred to Santiago et al.24 Hove et al. were the first to exploit the transparency of zebrafish to conduct in vivo μPIV and assessed the effect of intracardiac fluid forces on cardiogenesis.2 Because of the opacity of living tissue, in vivo PIV has only been conducted on a limited basis in small transparent vessels in rodents,25,26 chicken embryos,27–29 and zebrafish embryos.2,30 These studies have either injected tracer particles25,28–30 or used the red blood cells themselves as the tracer particles.2,26,27 Hove et al. applied PIV analysis to the heart using bright field imaging, using the red blood cells as tracers.2 This method produces images in which the signal from the blood cells is contained within the same gray scale as other objects that are not of interest, for instance the heart wall. Because of the nature of PIV, both stationary and moving objects will contribute to the cross correlation and thus will impact the final velocity measurement. Hove et al. identified the presence of the heart wall and superficial tissue in their images as interfering with accurate velocity calculation, suggesting their velocity measurements represented an underestimation of the true value.2 Lu et al. removed the impact of the wall by utilizing defocusing particle tracking velocimetry (PTV) on fluorescent tracer particles injected into the bloodstream, measuring velocity in three dimensions.30 The use of fluorescent particles allows for the optical filtering of the contribution of the heart wall from the velocity calculation improving the accuracy of the flow measurements. However, as PTV requires the distance between tracer particles to be larger than their displacement, there is a severe limitation in the spatial resolution of the technique, which provides only sparse instantaneous vector fields. Other studies in the zebrafish have utilized high-speed confocal or selective plane illumination microscopy of fluorescently labeled samples, together with the synchronization of confocal slices, to form dynamic 3-D models31 for examining the pumping mechanism of the embryonic heart,1 and investigating valve formation.3,32 Despite the significant findings of these studies, the requirement for fluorescent imaging reduces the temporal resolution because of the longer integration times required. The removal of stationary structures is common in methods such as in vitro X-ray velocimetry by removal of an average image composed by combining every image over the entire acquisition.33 However, the dynamic nature of the heart makes subtracting an average image inappropriate. Cardiac-phase averaging, taking images at the same point in the cardiac cycle from successive heartbeats, has previously been utilized to improve signal-to-noise ratio in cardiac imaging by optical coherence tomography.5 Here we describe a cardiac-phase averaging method to remove the contribution of the heart wall and other interfering structures from bright field μPIV analysis to improve the accuracy of blood flow measurements and allow the use of high-speed bright field imaging, providing improved temporal and spatial resolution. This technique has eliminated the severe underestimation of velocity measurements caused by the presence of stationary structures in μPIV images, enabling accurate and quantitative detection of hemodynamic changes within the heart. 2.Results and Discussion2.1.Image Acquisition and Phase Average FilteringHigh-speed bright field imaging (2000 frames per second) was used to capture cardiac contraction over a 2-s window (approximately four cardiac cycles) in zebrafish ranging in age from 3 days post fertilization (dpf) to 6-dpf ( at each stage). Acquisition rates at this speed ensure that the desired pixel displacement is met for PIV analysis ( of the interrogation window) at the high spatial resolution used in this study (0.28 μm pixel size). At this rate of acquisition, we obtained instantaneous velocity measurement time points per cycle (depending on fish age). The temporal oversampling due to the high-speed acquisition of data can be used as an opportunity to improve the velocity measurements by binning the measurements into 100 sections in each cardiac cycle and averaging them to reduce erroneous vectors. Mathematical analysis of blood flow has successfully modeled cardiac output with a finite Fourier model having seven harmonics;34 as a result, a minimum of 14 bins is required to accurately model cardiac function. Measurement-based studies have used larger numbers of bins to ensure the accuracy of results, for example, 42 bins per cardiac cycle, as used by Leo et al.35 Cardiac defects may result in changes to the pattern of blood flow as well as the magnitude, resulting in a more complex model; therefore, we have utilized 100 bins in our analysis to ensure the accurate measurement of irregular flow patterns. Additionally, as each imaging sequence contains multiple cardiac cycles, it is possible to phase average the PIV measurements. These steps require first dividing each cycle into bins and then aligning the periods so that they are in phase with each other. By initially performing PIV on a small section of the overall image, the onset of contraction could be determined and used to align the cardiac cycles (Fig. 1). The binning of images from each cardiac cycle also removes the effect of variance in the length of each cardiac cycle (a variance of 10 to 15 ms being common). Each frame within a cycle is allocated a relative bin within the cardiac cycle. This provides the ability to compare frames from different periods in this quasi-periodic flow, enhancing the accuracy of the velocity measurements acquired by reducing erroneous vectors. Because of the high temporal resolution of the acquisitions ( frames for one cardiac cycle of a 4-dpf embryo), each of the 100 bins contains approximately eight averaged velocity measurements from each cycle. Fig. 1Flow data is interrogated by performing PIV on a small section of the image to identify the start of each cardiac cycle. Each cardiac cycle is then divided into 100 bins. The velocity measurements from each bin in multiple cardiac cycles are combined to create a temporally averaged velocity measurement. For reference each cardiac cycle is approximately 800 frames in length. 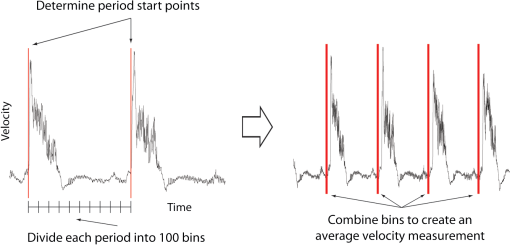 To remove the contribution of the static heart structures from the PIV analysis of blood flow, which causes underestimation of velocity, averaged images are created for each of the 100 bins within a cycle. Each average image is subtracted from the individual frames within the bin, resulting in the filtering of the static heart structures, and those moving less than (which would appear stationary at these frame rates) are also removed from the imaging sequence as illustrated in Fig. 2 (Video 1). Following filtering, PIV analysis, using the full temporal resolution available, is carried out on the unbinned filtered images. Fig. 2Bright field microscopy images of a 4-dpf zebrafish heart. (a) Raw image of the zebrafish heart, (b) average image formed by the binning process described in Fig. 1, and (c) final image produced by subtracting the average image (b) from the raw image (a) (Video 1, QuickTime, 9.5 MB) [URL: http://dx.doi.org/10.1117/1.JBO.17.3.036007.1]. 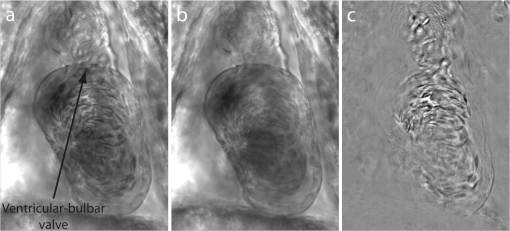 2.2.Particle Image Velocimetry AnalysisThe computational expense of PIV can be reduced by defining the region of interest within the images for analysis. We carried out imaging on zebrafish expressing green fluorescent protein under the control of the cardiac myosin light chain 2 promoter. For each bright field acquisition, we captured a corresponding sequence of fluorescent images at a rate of 100 frames per second immediately following bright field capture. These fluorescent images were manually phase matched to the bright field images in order to provide a synchronized data set (Fig. 3) and thresholded to create a mask for the PIV analysis. The fluorescence image clearly identifies the area of interest for velocity calculation and is ideal for masking of the bright field images. The mask images were used to restrict the area of the image analyzed for PIV, reducing the computational expense of the technique. Additionally, by utilizing a mask we immediately combine heart wall information obtained from fluorescence imaging with the velocity measurements obtained from bright field imaging. Fig. 3Synchronization of bright field and fluorescent images for PIV mask creation. (a) Bright field microscopy image, (b) fluorescence image used for mask creation, and (c) combination of the two images following temporal alignment. The fluorescence image is seen to clearly identify the area of interest for velocity calculation. 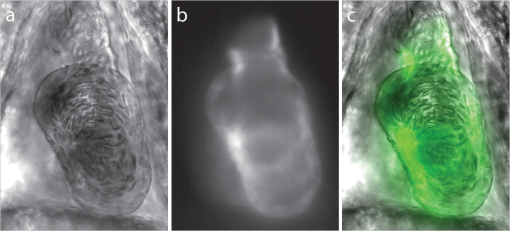 PIV analysis was carried out on each of the masked data sets to provide detailed velocity measurements over the period of zebrafish development from 3 to 6-dpf. Figure 4 (Video 2) shows the peak flow from the atrium to the ventricle and clearly illustrates the complexities of the flow being investigated. Video 2 illustrates the temporal resolution of the averaging technique, showing all 100 time-points (10 bins per second). These data show that for large parts of the cardiac cycle, the flow within the ventricle is negligible, with short periods of high inflow or outflow. Flow into the ventricle displays a vortical nature brought about by the enlargement of the chamber, while flow through the ventricular-bulbar valve is relatively parallel. Immediately after the valve the flow once again shows vortical nature owing to the expansion of the bulbus arteriosus. Fig. 4Blood flow velocity measurements in a 6-dpf zebrafish during peak flow from the atrium into the ventricle. Vectors show direction and magnitude of velocity (standard color rainbow, red is high). Vectors are evaluated at 4.5-µm spacing with an interrogation window size of . For clarity only every second vector is shown. Video 2 provides all 100 time-point measurements (QuickTime, 11.2 MB) [URL: http://dx.doi.org/10.1117/1.JBO.17.3.036007.2]. 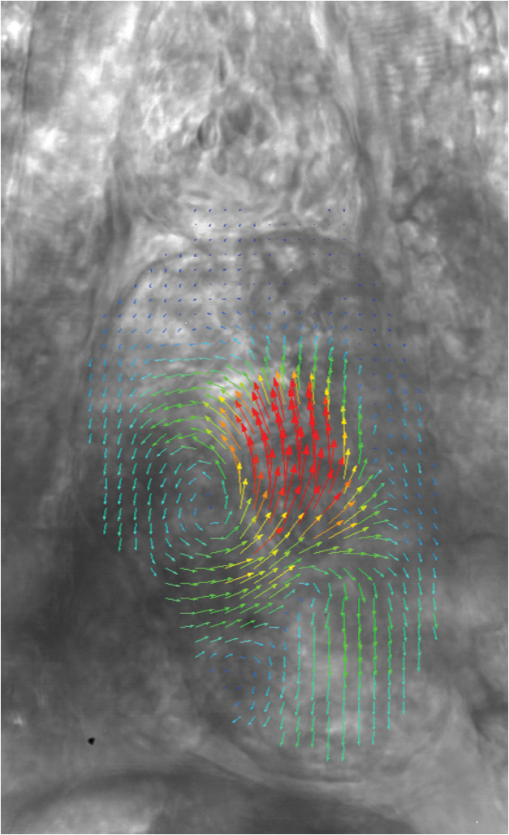 To determine the improvement in PIV analysis following image filtering, we compared the cross correlation peaks obtained with and without cardiac-phase filtering to an idealized result generated with synthetic data (Fig. 5). Stationary structures within the image pairs will be included in the cross correlation, resulting in a shift of the apparent maximum of cross correlation towards the centroid of these structures and, in this instance, reducing the measured velocity. The noise resulting from these structures and the distortion of the cross correlation peak are clearly evident in the unfiltered data [Fig. 5(b)] but removed as a result of cardiac-phase filtering [Fig. 5(c)]. To examine the effect of cardiac-phase filtering on the velocity measurements, we compared the unfiltered data to the filtered data [Fig. 6(a)]. This comparison identified more than a 3-fold difference in average velocity during peak flow ( in filtered data compared to in the unfiltered data sets). Fig. 5Cross correlation images used in PIV analysis. (a) Cross correlation peak for synthetic, ideal data. (b) Cross correlation from 6-dpf unfiltered images. A clear ridge is evident distorting the peak, indicative of low-frequency noise such as stationary structures. (c) Filtering of the data analyzed in (b) demonstrating successful removal of noise from the analysis and improvement in the cross correlation, as evidenced by the similar appearance to the ideal case in (a). 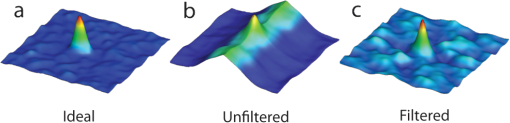 Fig. 6Average velocity measurements through the ventricular-bulbar valve. (a) Comparison of velocity measurements before (blue squares) and after (red circles) cardiac-phase average filtering in a 6-dpf zebrafish. (b) Symbols provide velocity measurements for individual fish (red circles, green squares, blue triangles, and gray diamonds) at 5-dpf, and the solid line shows the average of these. Video 3 provides the bright field imaging of the fish displaying retrograde flow through the ventricular-bulbar valve (green squares, QuickTime, 10.2 MB). (c) Average velocity measurements for each age group: 3-dpf (gray diamonds), 4-dpf (blue triangles), 5-dpf (green squares) and 6-dpf (red circles). Retrograde flow is evident in 3-dpf fish prior to systole between 0.8 and 1 period. (Video 3, MOV, 9.7 MB) [URL: http://dx.doi.org/10.1117/1.JBO.17.3.036007.3] 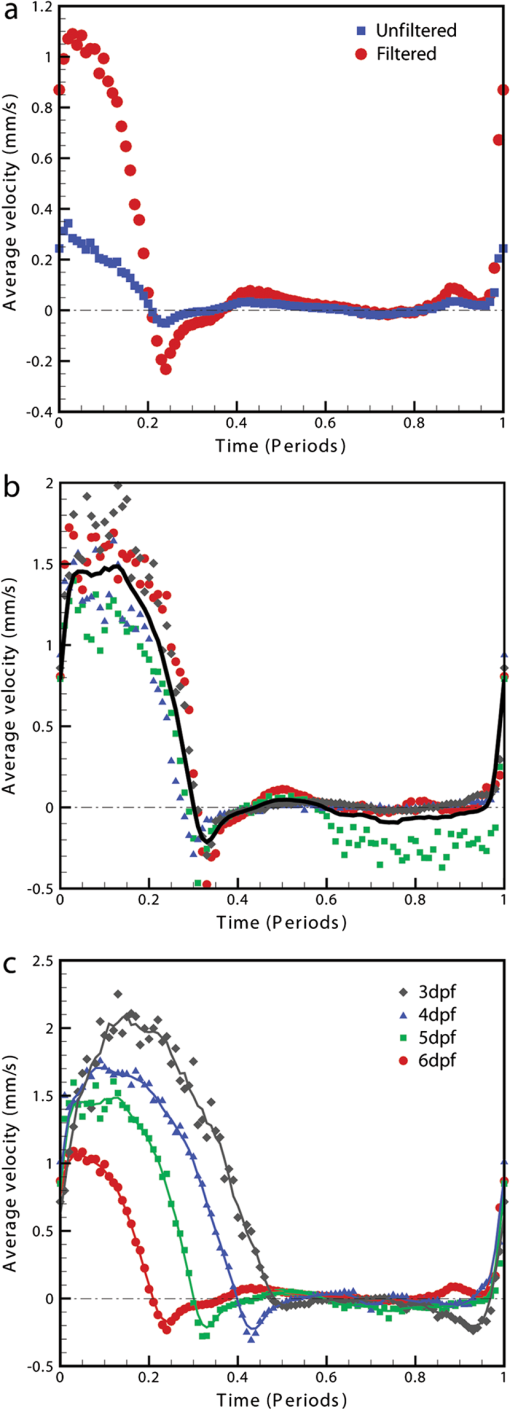 Within each age group filtered velocity measurements were highly reproducible and were used to form an average for each time point [Fig. 6(b)]. Figure 6(b) also shows the sensitivity of our approach. Note that Fig. 6(b) shows one fish exhibiting retrograde motion through the ventricular-bulbar valve in the latter part of the cycle. Remarkably, this retrograde flow, clearly evident in the quantitative analysis of this fish, can be seen to correspond to the leaking of one red blood cell (RBC) at a time back through the valve (Video 3). Analysis of blood flow between 3 and 6-dpf identified a reduction in both peak velocity and the contractile period of the cardiac cycle as the fish increase in age [Fig. 6(c)]. The contractile period is seen to reduce approximately 10% each day between 3 and 6-dpf. Interestingly, this reduction in contractile period is not accounted for by a substantial change in heart rate (average 158 beats per minute). A steady decrease in the velocity is also witnessed during this age range [Fig. 7(a)]. Figure 7(b) provides the maximum velocity measurement recorded through the valve, rather than the spatial average used in Fig. 6, which nevertheless shows a similar trend to the spatially averaged results. This gives an indication of the maximum velocities measured in the center of the valve. We observe a high variance in maximum velocity between individuals, particularly in the younger age groups. Even within a single batch of fertilized embryos raised in the same conditions, there is subtle variation in the rate of development between individuals. We believe the high variance is due to these subtle variations in developmental rate as well as variation between individuals. The difference in development become less significant with time, and therefore there is decreasing variation with increasing age. Fig. 7Maximum average velocity (a) and maximum velocity (b) measurements acquired through the ventricular-bulbar valve. Maximum velocity and maximum average velocities are measured before and after spatial averaging is performed, respectively. Linear regression lines are provided for clarity. 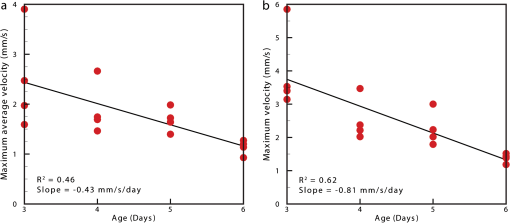 The measurements provided in Figs. 6 and 7 should not be confused with flow measurements or with oxygen transportation capacity. They represent the velocity at which the present RBCs are traveling. Two possible explanations for this reduction in both contractile time and velocity are the increase in size of the ventricular-bulbar valve and the increased hematocrit brought about by the ongoing production of RBCs during the early development of the embryonic zebrafish. These factors, individually or combined, may account for the reduction in velocity, while maintaining or increasing the fish’s ability to circulate oxygen. It is important that automated analyses of flow are able to detect subtle changes in heart function. One area of interest is the reverse flow through the valve. The motion witnessed in Fig. 6(c) immediately after systole in the 4-, 5-, and 6-dpf fish could easily be mistaken for retrograde flow through the valve; however, this motion is due to the closure of the valve pushing several RBCs back into the ventricle. While this trait is not present in the 3-dpf fish, reverse velocity measurements are evident immediately before systole. This motion is true retrograde flow caused by the force applied to the ventricular-bulbar valve as the ventricle expands. This force opens the underdeveloped valve, causing RBCs to move back into the ventricle through the ventricular-bulbar valve from the difference in pressure. These results demonstrate the capability of this approach to detect subtle developmental changes in the ventricular-bulbar valve. 3.ConclusionsA technique is presented for cardiac-phase filtering, demonstrating the adverse effect of the heart wall on velocity measurements obtained from bright field imaging. Removal of the heart wall was seen to result in the correction of a severe (3-fold) underestimation in velocity measurements. Additionally, the high spatial and temporal resolution used in this study was utilized to detect developmental changes within the embryonic heart. The contractile period of the heart was seen to significantly reduce as the fish increase in age (51% of the cardiac cycle for 3-dpf and 23% for 6-dpf), as measured by flow through the ventricular bulbar valve. Furthermore, the velocity through the valve was seen to also significantly decrease with age. Overall, this study demonstrates the ability of cardiac-phase filtering PIV to perform in vivo quantitative studies of developmental changes in the zebrafish heart. 4.Methods4.1.Microparticle Image VelocimetryAn in-house code was used for performing the image processing and velocity measurements in this study.36 This code has been developed over a number of years and rigorously validated by Fouras et al.37 and Nesbitt et al.38 Whole field velocity measurements were performed on the ventricle of each subject, which were masked by fluorescence image data. An interrogation window size of was used with a spacing between measurements of 4.5 µm. Velocity measurements though the ventricular-bulbar valve were calculated on a subregion of the full images in size ( pixels). An initial large subregion of ( pixels) was chosen so that the ventricular-bulbar valve was centered within the region. An interrogation window size of was used with a spacing between measurements of 2.25 μm. The larger initial subregion ensures that RBCs in the desired subregion do not leave the image in the subsequent frame. The measurements outside the desired subregion are later discarded, and a spatial average of the remaining vectors is used to obtain the average velocity through the valve. Because of the small region size, no masking was used for these measurements. 4.2.Preparation of Zebrafish SamplesHomozygous transgenic cardiac myosin light chain2-GFP zebrafish embryos39 were collected and raised in E3 embryo medium (50 mm NaCl, 1.67 Mm KCl, 3.3 mM CaCl, 3.3 mM , in deionized water) at 28 °C. Embryos were anesthetized with 0.016% tricaine (3-amino benzoic acid ethyl ester) and mounted in 0.7% low-melting-point agarose dissolved in E3 embryo medium together with 0.016% tricaine, prior to imaging. The embryos were mounted in Fluorodish imaging chambers (World precision instruments). 4.3.Imaging SetupAn inverted microscope (Leica DM ILM) with a objective lens (Nikon bright field CFI Plan Apo ) was coupled via an optical spacer (Leica ) to a high-speed camera (IDT Y4) to obtain an effective pixel size of 0.28 µm. A three-axis robotic sample stage (Aerotech) was used for high-resolution (, resolution, 1 μm; resolution, 0.1 μm) specimen positioning. Zebrafish with fluorescently labeled cardiac muscle cells were mounted such that viewing the heart was possible from below, enabling the main chambers of the heart to be in focus. Specimens were aligned to minimize flow in the out-of-plane direction through the ventricular-bulbar valve. Consistent orientation of the embryos was achieved by using the anatomical features surrounding the heart. Bright field imaging was used for PIV measurements, while fluorescence imaging was used to acquire heart wall information. The fluorescence images were acquired immediately after the bright field acquisition, with the only change to the optical path between the specimen and the camera being the inclusion of a dichroic filter. Temperature during image acquisition was . AcknowledgmentsWe are grateful to Nick Lam for assistance with the clmc2GFP fish and to Monash Micro Imaging for the loan of filters for fluorescent imaging. ReferencesA. S. Forouharet al.,
“The embryonic vertebrate heart tube is a dynamic suction pump,”
Science, 312
(5774), 751
–753
(2006). http://dx.doi.org/10.1126/science.1123775 SCIEAS 0036-8075 Google Scholar
J. R. Hoveet al.,
“Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis,”
Nature, 421
(6919), 172
–177
(2003). http://dx.doi.org/10.1038/nature01282 NATUAS 0028-0836 Google Scholar
J. Vermotet al.,
“Reversing blood flows act through klf2a to ensure normal valvulogenesis in the developing heart,”
PLoS Biol., 7
(11), e1000246
(2009). http://dx.doi.org/10.1371/journal.pbio.1000246 1544-9173 Google Scholar
B. C. W. Groenendijket al.,
“The endothelin-1 pathway and the development of cardiovascular defects in the haemodynamically challenged chicken embryo,”
J. Vasc. Res., 45
(1), 54
–68
(2008). http://dx.doi.org/10.1159/000109077 JVREE9 1018-1172 Google Scholar
S. Bhatet al.,
“Multiple-cardiac-cycle noise reduction in dynamic optical coherence tomography of the embryonic heart and vasculature,”
Opt. Lett., 34
(23), 3704
–3706
(2009). http://dx.doi.org/10.1364/OL.34.003704 OPLEDP 0146-9592 Google Scholar
R. S. RenemanT. ArtsA. P. G. Hoeks,
“Wall shear stress—an important determinant of endothelial cell function and structure—in the arterial system in vivo,”
J. Vasc. Res., 43
(3), 251
–269
(2006). http://dx.doi.org/10.1159/000091648 JVREE9 1018-1172 Google Scholar
L. SunC. LienK. K. Shung,
“In vivo cardiac imaging of adult zebrafish using high frequency ultrasound (45–75 MHz),”
Ultrasound Med. Biol., 34
(1), 31
–39
(2008). http://dx.doi.org/10.1016/j.ultrasmedbio.2007.07.002 USMBA3 0301-5629 Google Scholar
A. T. deAlmeidaT. McQuinnD. Sedmera,
“Increased ventricular preload is compensated by myocyte proliferation in normal and hypoplastic fetal chick left ventricle,”
Circ. Res., 100
(9), 1363
–1370
(2007). http://dx.doi.org/10.1161/01.RES.0000266606.88463.cb CIRUAL 0009-7330 Google Scholar
H. BenvenisteS. Blackband,
“MR microscopy and high resolution small animal MRI: applications in neuroscience research,”
Prog. Neurobiol., 67
(5), 393
–420
(2002). http://dx.doi.org/10.1016/S0301-0082(02)00020-5 PGNBA5 0301-0082 Google Scholar
R. G. Wiseet al.,
“Simultaneous measurement of blood and myocardial velocity in the rat heart by phase contrast MRI using sparse q-space sampling,”
J. Magn. Reson. Imaging, 22
(5), 614
–627
(2005). http://dx.doi.org/10.1002/(ISSN)1522-2586 1053-1807 Google Scholar
A. DavisJ. IzattF. Rothenberg,
“Quantitative measurement of blood flow dynamics in embryonic vasculature using spectral Doppler velocimetry,”
Anat. Rec., 292
(3), 311
–319
(2009). http://dx.doi.org/10.1002/ar.v292:3 ANREAK 0003-276X Google Scholar
S. Yuanet al.,
“Determination of optimal exposure time for imaging of blood flow changes with laser speckle contrast imaging,”
Appl. Opt., 44
(10), 1823
–1830
(2005). http://dx.doi.org/10.1364/AO.44.001823 APOPAI 0003-6935 Google Scholar
C. Poelmaet al.,
“3D Flow reconstruction using ultrasound PIV,”
Exp. Fluids, 50
(4), 777
–785
(2011). http://dx.doi.org/10.1007/s00348-009-0781-8 EXFLDU 0723-4864 Google Scholar
P. VennemannR. LindkenJ. Westerweel,
“In vivo whole-field blood velocity measurement techniques,”
Exp. Fluids, 42
(4), 495
–511
(2007). http://dx.doi.org/10.1007/s00348-007-0276-4 EXFLDU 0723-4864 Google Scholar
A. Fouraset al.,
“The past, present and future of -ray technology for in vivo imaging of function and form,”
Appl J. Phys., 105
(10), 102009
(2009). http://dx.doi.org/10.1063/1.3115643 JAPIAU 0021-8979 Google Scholar
B. Hogerset al.,
“Extraembryonic venous obstructions lead to cardiovascular malformations and can be embryolethal,”
Cardiovasc. Res., 41
(1), 87
–99
(1999). http://dx.doi.org/10.1016/S0008-6363(98)00218-1 CVREAU 0008-6363 Google Scholar
D. Sedmeraet al.,
“Remodeling of chick embryonic ventricular myoarchitecture under experimentally changed loading conditions,”
Anat. Rec., 254
(2), 238
–252
(1999). http://dx.doi.org/10.1002/(ISSN)1097-0185 ANREAK 0003-276X Google Scholar
B. C. W. Groenendijket al.,
“The role of shear stress on ET-1, KLF2, and NOS-3 expression in the developing cardiovascular system of chicken embryos in a venous ligation model,”
Physiology, 22
(6), 380
–389
(2007). http://dx.doi.org/10.1152/physiol.00023.2007 Google Scholar
M. J. MaclennanB. B. Keller,
“Umbilical arterial blood flow in the mouse embryo during development and following acutely increased heart rate,”
Ultrasound Med. Biol., 25
(3), 361
–370
(1999). http://dx.doi.org/10.1016/S0301-5629(98)00147-1 USMBA3 0301-5629 Google Scholar
I. V. Larinaet al.,
“Live imaging of blood flow in mammalian embryos using Doppler swept-source optical coherence tomography,”
J. Biomed. Opt., 13
(6), 060506
(2008). http://dx.doi.org/10.1117/1.3046716 JBOPFO 1083-3668 Google Scholar
L. Niuet al.,
“Ultrasonic particle image velocimetry for improved flow gradient imaging: algorithms, methodology and validation,”
Phys. Med. Biol., 55
(7), 2103
–2120
(2010). http://dx.doi.org/10.1088/0031-9155/55/7/020 PHMBA7 0031-9155 Google Scholar
J. JeongY. SugiiM. Minamiyama,
“Measurement of RBC deformation and velocity in capillaries in vivo,”
Microvasc. Res., 71
(3), 212
–217
(2006). http://dx.doi.org/10.1016/j.mvr.2006.02.006 MIVRA6 0026-2862 Google Scholar
R. J. Adrian,
“Particle-imaging techniques for experimental fluid-mechanics,”
Annu. Rev. Fluid Mech., 23 261
–304
(1991). http://dx.doi.org/10.1146/annurev.fl.23.010191.001401 ARVFA3 0066-4189 Google Scholar
J. G. Santiagoet al.,
“A particle image velocimetry system for microfluidics,”
Exp. Fluids, 25
(4), 316
–319
(1998). http://dx.doi.org/10.1007/s003480050235 EXFLDU 0723-4864 Google Scholar
M. L. Smithet al.,
“Near-wall μPIV reveals a hydrodynamically relevant endothelial surface layer in venules in vivo,”
Biophys. J., 85
(1), 637
–645
(2003). http://dx.doi.org/10.1016/S0006-3495(03)74507-X BIOJAU 0006-3495 Google Scholar
Y. SugiiS. NishioK. Okamoto,
“In vivo PIV measurement of red blood cell velocity field in microvessels considering mesentery motion,”
Physiol. Meas., 23
(2), 403
–416
(2002). http://dx.doi.org/10.1088/0967-3334/23/2/315 PMEAE3 0967-3334 Google Scholar
J. Y. LeeH. S. JiS. J. Lee,
“Micro-PIV measurements of blood flow in extraembryonic blood vessels of chicken embryos,”
Physiol. Meas., 28
(10)), 1149
–1162
(2007). http://dx.doi.org/10.1088/0967-3334/28/10/002 PMEAE3 0967-3334 Google Scholar
C. Poelmaet al.,
“In vivo blood flow and wall shear stress measurements in the vitelline network,”
Exp. Fluids, 45
(4), 703
–713
(2008). http://dx.doi.org/10.1007/s00348-008-0476-6 EXFLDU 0723-4864 Google Scholar
P. Vennemannet al.,
“In vivo micro particle image velocimetry measurements of blood-plasma in the embryonic avian heart,”
J. Biomech., 39
(7), 1191
–1200
(2006). http://dx.doi.org/10.1016/j.jbiomech.2005.03.015 JBMCB5 0021-9290 Google Scholar
J. Luet al.,
“Three-dimensional real-time imaging of cardiac cell motions in living embryos,”
J. Biomed. Opt., 13
(1), 014006
(2008). http://dx.doi.org/10.1117/1.2830824 JBOPFO 1083-3668 Google Scholar
M. Lieblinget al.,
“Four-dimensional cardiac imaging in living embryos via postacquisition synchronization of nongated slice sequences,”
J. Biomed. Opt., 10
(5), 054001
(2005). http://dx.doi.org/10.1117/1.2061567 JBOPFO 1083-3668 Google Scholar
P. J. Scherzet al.,
“High-speed Imaging of developing heart valves reveals interplay of morphogenesis and function,”
Development, 135
(6), 1179
–87
(2008). http://dx.doi.org/10.1242/dev.010694 DEVPED 0950-1991 Google Scholar
R. A. Jamisonet al.,
“-ray velocimetry and haemodynamic forces within a stenosed femoral model at physiological flow rates,”
Ann. Biomed. Eng., 39
(6), 1643
–53
(2011). http://dx.doi.org/10.1007/s10439-011-0260-2 ABMECF 0090-6964 Google Scholar
S. A. StevensW. D. LakinW. Goetz,
“A differentiable, periodic function for pulsatile cardiac output based on heart rate and stroke volume,”
Math. Biosci., 182 201
–211
(2003). http://dx.doi.org/10.1016/S0025-5564(02)00200-6 MABIAR 0025-5564 Google Scholar
H. L. Leoet al.,
“Fluid dynamic assessment of three polymeric heart valves using particle image velocimetry,”
Ann. Biomed. Eng., 34
(6), 936
–952
(2006). http://dx.doi.org/10.1007/s10439-006-9117-5 ABMECF 0090-6964 Google Scholar
A. FourasD. Lo JaconoK. Hourigan,
“Target-free stereo PIV: a novel technique with inherent error estimation and improved accuracy,”
Exp.Fluids, 44
(2), 317
–329
(2008). http://dx.doi.org/10.1007/s00348-007-0404-1 EXFLDU 0723-4864 Google Scholar
A. Fouraset al.,
“Volumetric correlation PIV: a new technique for 3D velocity vector field measurement,”
Exp. Fluids, 47
(4), 569
–577
(2009). http://dx.doi.org/10.1007/s00348-009-0616-7 EXFLDU 0723-4864 Google Scholar
W. S. Nesbittet al.,
“A shear gradient-dependent platelet aggregation mechanism drives thrombus formation,”
Nat. Med., 15
(6), 665
–673
(2009). http://dx.doi.org/10.1038/nm.1955 1078-8956 Google Scholar
C. J. Huanget al.,
“Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish,”
Dev. Dynam., 228
(1), 30
–40
(2003). http://dx.doi.org/10.1002/dvdy.10356 DEDYEI 1097-0177 Google Scholar
|

