|
|
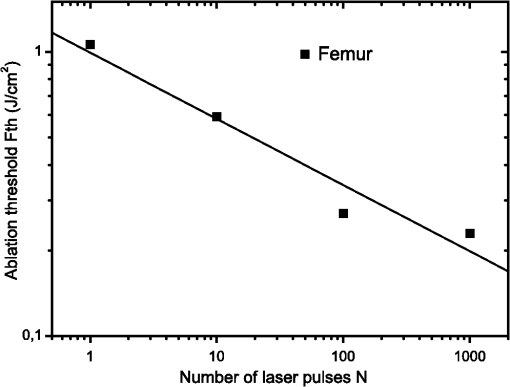
1.IntroductionFemtosecond lasers have been used widely in laser surgery for contact-free tissue removal. Targets as hard dental,1,2 restorative materials,3,4 and bone tissues5,6 have been studied with lasers in order to complement conventional drilling or cutting tools. Use of ultrafast laser pulses to modify, sculpture, and remove various materials has recently made a breakthrough.7 Different lasers have been evaluated for the removal and preparation of hard dental tissues and bones, and some of these are already in clinical use.8–10 Among the possible applications of femtosecond laser systems, their ability to cut bone in surgical applications without vibration and thermal damage presents numerous advantages over the use of other mechanical instruments. As the mechanical vibration can reduce surgical precision,6 and the rotational trajectories on the tissue can lead to collateral damage,5,11 and, in fact, as the most important feature of conservative surgery is high precision, this is something an ultrafast laser can offer. Still, it is heavily debated whether the laser can replace mechanical instruments for many other applications working with hard tissues.12 One of the biggest limitations is the slow rate of material removal, and then, in many cases, the usually unacceptable collateral damage, which is caused by overheating. In order to obtain a laser system that has an ablation efficiency comparable to mechanical instruments, the laser pulse rate must be maximal without causing thermal damage. If the repetition rate is very high, the incoming pulses may interact with the plasma, which is highly absorbing, and lead to heat generation in the sample. Additionally, the presence of plume ejected from the target may also hinder incoming pulses from reaching the target and thereby reduce the ablation efficiency. Thus the optimal laser system should have a repetition rate fast enough to maximize tissue removal, while allowing enough time for the plasma and plume to dissipate.6 High power densities (intensity or irradiance) could promote induced ablation by plasma, resulting in more precise and well-defined cavity edges. Certainly, ultrashort pulses have been identified as an alternative, and several indications to use femtosecond pulse ablation are under investigation.2–5 The advantages of femtosecond microsurgery lasers, including the high structural precision and a small thermally affected area may be more effectively obtained during a microdrilling close to the ablation threshold conditions, i.e., using a minimal fluence necessary for material ablation.13 The laser ablation in the femtosecond regime also has advantages regarding chemical attacks, such as the removal of calcium ion, trainers, which play an important role to keep the cariogenic resistance within those oral cavity tissues.14 Femtosecond lasers can be used to reduce thermal influence due to a lower heat present when compared to lasers with long pulses. A lower heat is present because, when using femtosecond lasers, the fluence needed for achieving micropores is magnitudes smaller than when using ns lasers for the same repetition rate and wavelength. The noncreation of microcracks for short pulses is of great importance in dentistry because microcracks would weaken the tissue and increase external permeability, therefore resulting in new tooth decay.13 A comparative study of the different morphological characteristics of the hard tissue after exposure to the lasers operating in the femtosecond pulse regime was done. The study aims at removing the superficial tissues and making sure to preserve the structure of the sample at the same time. The preservation of the remaining tissue may be important for various surgical applications including implants. The threshold condition for femtosecond laser pulses to ablate bovine femur was also determined in this paper. 2.Materials and MethodsA Ti: Sapphire femtosecond (Libra-S, Coherent, Palo Alto, CA, USA) laser was used in this study emitting pulses of approximately 70 fs, the emission being centered at a wavelength of 801 nm and operating at a pulse repetition rate of 10 to 1000 Hz or in the single shot mode. The pulse duration of 70 fs was measured using a second-order autocorrelator (Coherent, SSA™). In this study, two different kinds of samples were irradiated: dentin from human extracted teeth and bovine femur cortical pieces. These pieces were polished to obtain a smooth and level surface. We used fresh bovine femur and six human teeth (third molar) newly extracted following an orthodontic indication. The bovine bone was cut into six pieces of . The teeth were cut into two parts, excluding the root part and keeping the crown part. All tissues were included in a polyester resin so that the surface of all could be positioned strictly perpendicular to the laser beam. The samples were polished in a politriz machine, using sandpaper water increasing the grain (up to 600 grit). The teeth were polished until the dentin was exposed. The samples were positioned on a sample stage with axis. Using highly reflective coated dielectric mirrors, the beam was passed through a 200-mm focal length lens before reaching the target sample. The samples were irradiated using different energy levels per pulse (EPP) and different operation modes, with the single shot mode or with a repetition rate between 10 and 1000 Hz. Irradiation times were controlled by a mechanical shutter. The laser system was operated without an additional cooling system. After each irradiation, the samples were prepared following a defined routine and then transferred to the scanning electron microscope (SEM) to evaluate the morphological variations of the irradiated hard materials and to measure the diameters of the ablation craters. 3.Results and DiscussionHard tissues were used to determine conditions of the ablation threshold,13 using both simple and multiple pulses for different laser fluences. In order to determine precisely the threshold of the modified structure, the laser beam diameter on the surface of the target and the energy of the laser pulse must be known. For a Gaussian laser beam, the spatial variation of the fluence of the laser can be represented by:14 where is the distance from the beam center, is the beam waist, i.e., the radius of the laser spot on the target surface at of the peak fluence , and is the incident laser pulse energy. When the threshold of the laser fluence is denoted by th, the radius () or diameter () of the cavity ablation can be written in terms of peak fluence as in Ref. 15:To apply Eq. (2) to determine the radius of the spot radius , precise measurements of the diameter are necessary, especially when we consider the typical asymmetry of cavities. For these measurements, the shape of the cavities was almost circular, as seen in the SEM image, although the actual cavities are not perfectly circular. Figure 1 shows the results of the measurements of the diameter of the cavity squared, as a function of the laser fluence peak on a semi-logarithmic scale, for the bovine femur. This chart allows calculating the radius of the laser beam, based on a graph obtained by fitting the experimental data. The radius of the laser spot was estimated to be and for samples irradiated with 1000 to 100 pulses, respectively. For the effective number of pulses and single pulse, the spot radius was estimated to be and , respectively. As it is difficult to judge the diameter for a few short pulses, due to their structures being much less pronounced and highly influenced by statistical processes, therefore the laser spot size changes for the different measurements with different effective number of pulses. Fig. 1Graphs of the diameter squared cavities versus fluence, when the bone was irradiated with the femtosecond laser with: , , , and . 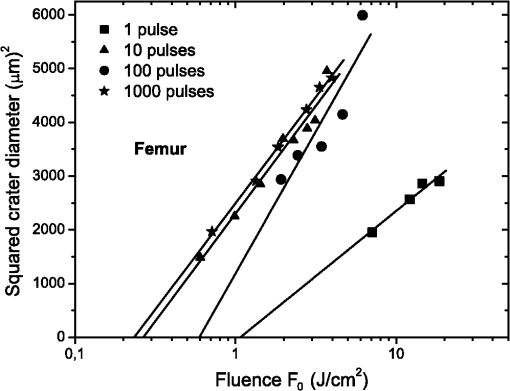 From the lines in Fig. 1, the fluence ablation threshold (th) was determined by locating the value of fluence where the fitted line crosses the -axis for 1, 10, 100, and 1000 pulses; the threshold values obtained were 1.06, 0.59, 0.27, and , respectively. This method to determine the ablation threshold is usually used for other materials, e.g. ceramic dielectrics16,17 and hard dental tissue.7 There are also other geometric methods used to determine the damage threshold due to ablation.18 One of these methods consists in the formation of a superficial damage profile for a sample motion across the laser focus. A simple measurement of the maximal cross-sectional dimension of the damage profile, which depends only on the power of the laser beam, is used to compute the local intensity threshold. Girard et al. determined the ablation threshold for bone tissues to be through direct analysis of the SEM images.5 The value found by Girard et al. was higher than the one we found in this study (), although both studies were performed working with a repetition rate of 1 kHz, but Girard et al. were using a pulse width of 200 fs. Since a longer pulse duration has been used, the ablation threshold is increased because the maximum intensity is reduced for similar fluences, and less electrons are generated from multiphoton processes.5,19 One of the effects of incubation for bovine femur investigated was to study the reduction of the fluence ablation threshold when we increased the number of pulses.16 Ablation was observed in these tissues and shown in Fig. 2, where the threshold decreases continuously when the number of pulses is increased from 1 to 10, 100 or 1000. This reduced threshold during accumulative ablation (th, ) can be represented by:14,16,17 where the exponent characterizes the material’s response to cumulative ablation, based on the following conditions: (1) for , where the incubation effect exists; (2) for , where the fluence at the threshold conditions is independent of the number of pulses; and (3) for , where the material becomes stronger during cumulative ablation,16 i.e., the ablation efficiency decreases.From Eq. (3), the incubation factor was calculated to be for the bovine femur. This result shows that the effect of incubation is also substantial during ablation of the bone using the femtosecond laser. Incubation effects can become significant and may change the structure of the tissue during the process. These effects can result in a lowered ablation threshold for subsequent pulses, thereby changing the overall ablation dynamics. Depending on the geometry of the ablation region and of the laser system operation (for example, repetition rate and exposure time), multipulse exposure may also cause beam distortion and shadowing effects as well as light scattering due to residual debris. In Fig. 3 we show SEM microimages of the bone tissue irradiated with a femtosecond laser, with a varying effective number of pulses of the laser. Fig. 3SEM microimages of bovine femur irradiated with a femtosecond laser in the focused mode: (a) pulse energy , pulse number ; (b) , and detailed in (c); (d) , ; (e) , and detailed in (f). Morphological variations of the femur bone were obtained. 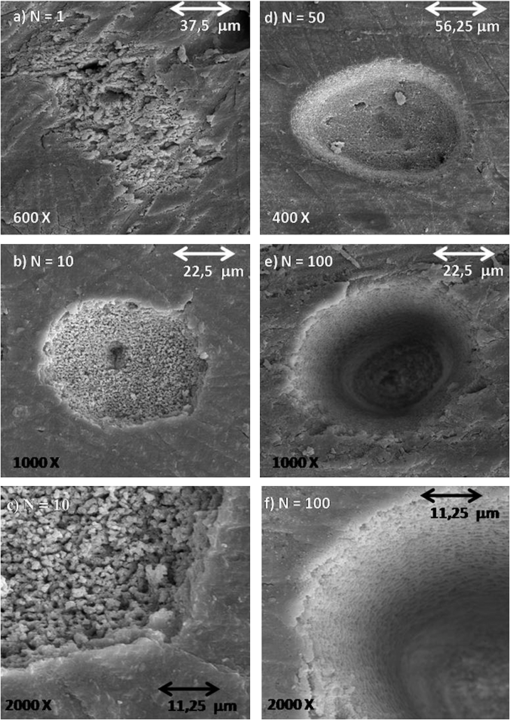 From Fig. 3 we see that it was sufficient to use only one femtosecond pulse [Fig. 3(a)] with a fluence of in order to modify the surface of the tissue. Using an effective number of pulses , we obtained morphological variations of the tissue [see Fig. 3(b)], and details are also shown in Fig. 3(c). From these images we noticed that cavitation occurs, where the edges of the cavity are relatively well-defined boundaries with small exfoliations, when compared to . After a more thorough analysis, we see that an exposure of bone trabeculae [see Fig. 3(c)] takes place, and no evidence of heat damage, i.e., carbonization is visible. This is an important feature, because if we get an only superficial [see Fig. 3(c)] ablation, we can increase the capacity of the cell adhesion to facilitate integration of the bone; for example, in the case of bone implants. When we apply 50 pulses (1 s, 50 Hz) to the tissue, [see Fig. 3(d)] we see that, despite the beam profile not being perfectly round, the cavity does not show signs of charring or redeposition of material on its surface or its inner walls. The same occurs for (1 s, 100 Hz) [Figs. 3(e) and 3(f)], without effect of a thermal (carbonization) or mechanical damage, with well-defined borders, and the internal walls were preserved [see Fig. 3(f)]. As we used the laser as a surgical tool, we know that it has been suggested to work with higher repetition rates in order to obtain a higher pulse ablation rate. In Fig. 4 we present SEM microimages of the femur bovine tissue irradiated with a femtosecond laser in the focused mode using (1 s, 1 kHz). Fig. 4SEM microimages of bovine femur irradiated with a femtosecond laser in the focused mode: (a) , , and detailed in (b); (c) , and detailed in (d). Morphological preservation of the femur bone was obtained. 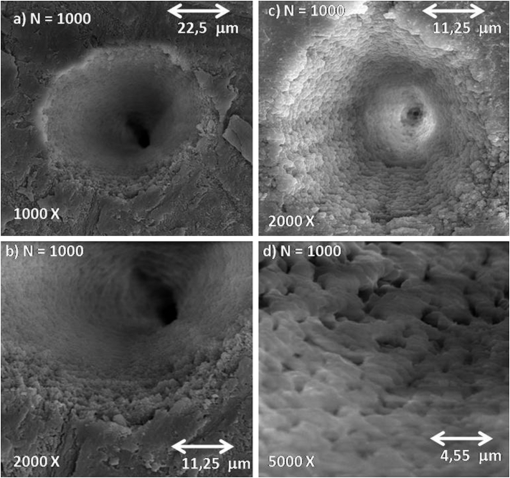 For an energy pulse of 0.178 mJ and a pulse number of 1000 pulses [see Fig. 4(b)], we found that the cavity still shows well-defined borders and no redeposition of material as had occurred when applying the other effective number of pulses (see Fig. 3). From Figs. 3 and 4, it is obvious that the ablated volume grew when the effective number of pulses was increased, but this information was not quantified here. Moreover, despite the higher effective number of pulses (), no heat affected zones emerged and no mechanical damage, e.g. cracks, were seen. The effective number of pulses was increased, for example, from 100 to 1000 pulses, but the ablated morphological cavity did not change much because the time between the pulses continued to be quite long. The mechanisms responsible for the laser ablation were the same. The same can be observed in Fig. 4(c) and in more details in Fig. 4(d): when we vary the fluence of the laser, the internal walls of the cavity show small regions with signs of melting, which in turn may help insulating the tissue. This phenomenon is interesting because, in order to get a precise ablation based on a nonlinear absorption (plasma-mediated ablation), low pulse energies must be used to minimize the mechanical shearing effects.20 When multiple pulses are delivered to one single position, the degree of thermal damage can be influenced by heat accumulation. The effective number of pulses per second should be low enough to avoid the progressive accumulation of residual heat within the tissue. An alternative strategy to avoid the accumulation of heat in a hard tissue involves a scanning laser beam in order to extend the time intervals between the subsequent expositions of each location of the ablated area.6 For bone-processing, other systems are also under evaluation, e.g. excimer lasers, which feature a high UV absorption of electronic transitions of almost all molecules. These excimer lasers have shown a very accurate cutting, but generated zones of thermal damage of approximately 50 μm.21,22 lasers (wavelength 10.8 μm), aiming at the mineral component of the tissue successfully removed the tissue but may generate carbonization.23,24 A Er:YAG laser (wavelength 2.94 μm) with a strong water absorption became popular in the past years for applications in orthopaedical, maxilofacial, and dental surgeries and for possible use in periodontal surgery.23,25,26 Carbonizations were also observed using Nd:YAG lasers,12,26,27 unlike what happened in our study using a femtosecond laser. In Fig. 5 we present micrographs obtained by SEM of human dentin irradiated with a femtosecond laser system, with the laser operating with different effective number of pulses. Fig. 5Microimages obtained by SEM of human dentin irradiated with a femtosecond laser in the focused mode: (a) , ; (b) , ) and (c) in detail; (d) , ; (e) , ; (f) , . Morphological variations of the human dentin were obtained. 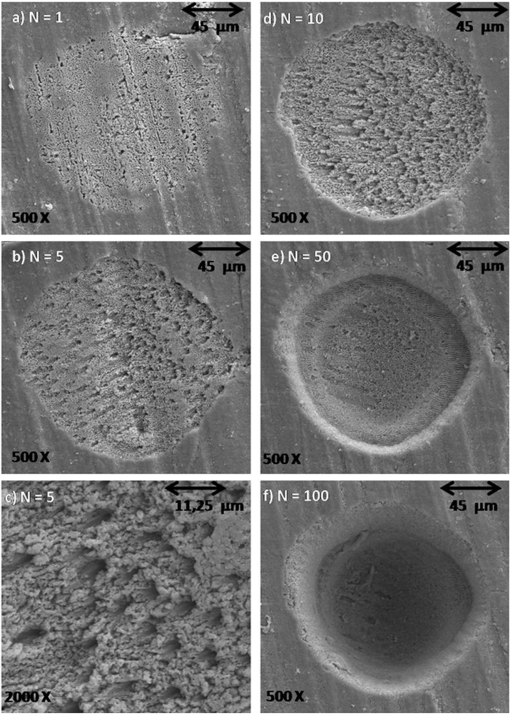 In Fig. 5 we see that using only one femtosecond pulse [see Fig. 5(a)] with a high pulse energy (0.6 mJ) was sufficient to modify the surface of the tissue. After applying five pulses in a range of 1 s per pulse, we evaluate the morphological variations of the tissue [see Fig. 5(b)] and in more detail in Fig. 5(c). From these images we can see that a tissue cavitation occurs, where the edges of the cavity show well-defined boundaries. After a more thorough analysis, we see that the tubules are preserved, an evidence of nonthermal damage on the ablated surface. However, in some regions the tubules were not exposed because there must have been a redeposition of material, due to insufficient energy to eject the material. The phenomena of exposition of these tubules are interesting, as it can be applied in dentistry, e.g. it could help increase the penetrability of adhesive systems used in restoration. For (1 s, 10 Hz), the tubules are also exposed and preserve a healthy tissue (see Fig. 5). When we apply 50 pulses to the tissue, the cavity does not show signs of charring or redeposition of material on its surface or its inner walls. The same occurs for (1 s, 100 Hz) [see Fig. 5(f)] without any presence of thermal (carbonization) or mechanical damage, with well-defined borders and while preserving the internal walls. Similar results were found by Korte et al.13 during laser ablation of dental tissue in the femtosecond regime. For lasers operating in a nanosecond (ns) pulse regime, dental tissue-ablation is less efficient and can be accompanied by collateral damage in the tissue, i.e., mechanical stress and fractures.26 For laser ablation in picoseconds (ps), the destruction of the surrounding material is minimized due to the formation of plasma during the ablation process.12 However, for both schemes (ns and ps) we still found evidence of thermal adjacent tissue damage.12,25 4.ConclusionCharacteristics of laser ablation on biological hard tissues, morphology, selective, and controlled ablation, all of these were addressed in this paper. We determined the conditions of the ablation threshold of bone tissues for different regimes of laser operation. A morphological comparison between the different regimes of operation has been investigated, and special care was taken to preserve the structures. The incubation factor was calculated to be 0.788 for bovine femur bone; these results indicate that the incubation effect is also substantial during the femtosecond laser ablation of hard tissues. The induced-plasma ablation has reduced side effects, e.g. less thermal and mechanical damage, using a superficial femtosecond laser irradiation near threshold conditions. In the femtosecond regime, the morphological characteristics of the cavity were strongly influenced by a change of the effective number of pulses. But even for the highest effective number of pulses and high fluence, we do not see the secondary effect of thermal and mechanical damage in the cavities of hard tissues. Furthermore, in cases of surface treatment, a femtosecond laser proved to be an appropriate tool to process ultraconservative tissues, being efficient removing tissues and not promoting collateral damage, capable of irreversibly modifying the original structure of the tissue. This system of laser ablation in the ultrashort pulse regime proved to be effective and promising for surgical procedures to remove, cut, and modify surfaces of human dentin hard tissues and femur bones. AcknowledgmentsThis research effort is financially supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (Fapesp) and National Counsel of Technological and Scientific Development (CNPq). ReferencesM. H. Niemzet al.,
“Tooth ablation using a CPA-free thin disk femtosecond laser system,”
Appl. Phys. B, 79
(3), 269
–271
(2004). http://dx.doi.org/10.1007/s00340-004-1579-2 APBOEM 0946-2171 Google Scholar
R. F. Z. Lizarelliet al.,
“Selective ablation of dental enamel and dentin using femtosecond laser pulses,”
Laser Phys. Lett., 5
(1), 63
–69
(2008). http://dx.doi.org/10.1002/(ISSN)1612-202X 1612-2011 Google Scholar
A. Z. Freitaset al.,
“Determination of ablation threshold for composite resins and amalgam irradiated with femtosecond laser pulses,”
Laser Phys. Lett., 7
(3), 236
–241
(2010). http://dx.doi.org/10.1002/lapl.v7:3 1612-2011 Google Scholar
G. NicolodelliC. KurachiV. S. Bagnato,
“Femtosecond laser ablation profile near an interface: analysis based on the correlation with superficial properties of individual materials,”
Appl. Surf. Sci., 257
(7), 419
–422
(2011). http://dx.doi.org/10.1016/j.apsusc.2010.10.116 ASUSEE 0169-4332 Google Scholar
B. Girardet al.,
“Effects of femtosecond laser irradiation on osseous tissues,”
Lasers Surg. Med., 39
(3), 273
–285
(2007). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
Y. LiuM. Niemz,
“Ablation of femoral bone with femtosecond laser pulses—a feasibility study,”
Lasers Med. Sci., 22
(3), 171
–174
(2007). http://dx.doi.org/10.1007/s10103-006-0424-8 0268-8921 Google Scholar
D. Bäuerle, Laser Processing and Chemistry, 4th ed.Springer-Verlag, Heidelberg, Berlin
(2011). Google Scholar
R. Esenalievet al.,
“Mechanism of dye-enhanced pulsed laser ablation of hard tissues: implications for dentistry,”
IEEE J. Sel. Top. Quant. Electron., 2
(4), 836
–846
(1996). http://dx.doi.org/10.1109/2944.577306 IJSQEN 1077-260X Google Scholar
L. Goldmanet al.,
“Impact of the laser on dental caries,”
Nature, 203 417
(1964). http://dx.doi.org/10.1038/203417a0 NATUAS 0028-0836 Google Scholar
I. M. Whiteet al.,
“Surface temperature and thermal penetration depth of Nd:YAG laser applied to enamel and dentin,”
Proc. SPIE, 1643 423
–436
(1992). http://dx.doi.org/10.1117/12.137374 PSISDG 0277-786X Google Scholar
J. KrugerW. KautekH. Newesely,
“Femtosecond-pulse laser ablation of dental hydroxyapatite and single-crystalline fluoroapatite,”
Appl. Phys. A, 69
(7), S403
–S407
(1999). http://dx.doi.org/10.1007/s003390051426 APAMFC 0947-8396 Google Scholar
R. F. Z. Lizarelliet al.,
“Characterization of enamel and dentin response to Nd:Y AG picosecond laser ablation,”
J. Clin. Laser Med. Surg., 17
(3), 127
–131
(1999). JCLSEO Google Scholar
F. Korteet al.,
“Towards nanostructuring with femtosecond laser pulse,”
Appl. Phys. A, 77
(2), 229
–335
(2003). APAMFC 0947-8396 Google Scholar
J. Bonseet al.,
“Ultrashort-pulse laser ablation of indium phosphide in air,”
Appl. Phys. A, 72
(1), 89
–94
(2001). http://dx.doi.org/10.1007/s003390000596 APAMFC 0947-8396 Google Scholar
J. M. Liu,
“Simple technique for measurements of pulsed Gaussian-beam spot sizes,”
Opt. Lett., 7
(5), 196
–198
(1982). http://dx.doi.org/10.1364/OL.7.000196 OPLEDP 0146-9592 Google Scholar
H. KimIk-Bu SohnS. Jeong,
“Ablation characteristics of aluminum oxide and nitride ceramics during femtosecond laser micromachining,”
Appl. Surf. Sci., 255
(24), 9717
–9720
(2009). http://dx.doi.org/10.1016/j.apsusc.2009.04.058 ASUSEE 0169-4332 Google Scholar
S. Martinet al.,
“Spot-size dependence of the ablation threshold in dielectrics for femtosecond laser pulses,”
Appl. Phys. A, 77
(7), 883
–884
(2003). http://dx.doi.org/10.1007/s00339-003-2213-6 APAMFC 0947-8396 Google Scholar
R. E. SamadN. D. Vieira Jr.,
“Geometrical method for determining the surface damage threshold for femtosecond laser pulses,”
Laser Phys., 16
(2), 336
–339
(2006). http://dx.doi.org/10.1134/S1054660X06020228 LAPHEJ 1054-660X Google Scholar
B. H. ChristensenP. Balling,
“Modeling ultrashort-pulse laser ablation of dielectric materials,”
Phys. Rev. B, 79
(15), 155424
–10
(2009). http://dx.doi.org/10.1103/PhysRevB.79.155424 PRBMDO 1098-0121 Google Scholar
A. Vogelet al.,
“Intraocular Nd:YAG laser surgery: light-tissue interaction, damage range, and the reduction of collateral effects,”
IEEE J. Quant. Electron., 26
(12), 2240
–2260
(1990). http://dx.doi.org/10.1109/3.64361 IEJQA7 0018-9197 Google Scholar
Y. Nakamuraet al.,
“Morphological changes of rat mandibular bone with ArF excimer laser in vivo,”
J. Clin. Lasers Med. Surg., 17
(4), 145
–149
(1999). PLDHA8 1549-5418 Google Scholar
M. L. Walteret al.,
“Photoablation of bone by excimer laser radiation,”
Lasers Surg. Med., 25
(2), 153
–158
(1999). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
N. M. FriedD. Fried,
“Comparison of Er:YAG and 9.6-μm TE lasers for ablation of skull tissue,”
Lasers Surg. Med., 28
(4), 335
–343
(2001). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
M. Frentzenet al.,
“Osteotomy with 80-µs laser pulses—histological results,”
Lasers Med. Sci., 18
(2), 119
–124
(2003). http://dx.doi.org/10.1007/s10103-003-0264-8 0268-8921 Google Scholar
M. Abu-Serriahet al.,
“Removal of partially erupted third molars using an erbium (Er):YAG laser: a randomised controlled clinical trial,”
Br. J. Oral Maxillofac. Surg., 42
(3), 203
–208
(2004). Google Scholar
B. H. KivançÖ. İ. A. UlusoyG. Görgül,
“Effects of Er:YAG laser and Nd:YAG laser treatment on the root canal dentin of human teeth: a SEM study,”
Lasers Med. Sci., 23
(3), 247
–252
(2008). http://dx.doi.org/10.1007/s10103-007-0474-6 0268-8921 Google Scholar
R. F. Z. Lizarelliet al.,
“A comparative study of nanosecond and picosecond laser ablation in enamel: morphological aspects,”
J. Clin. Laser Med. Surg., 18
(3), 151
–157
(2000). JCLSEO Google Scholar
|