|
|
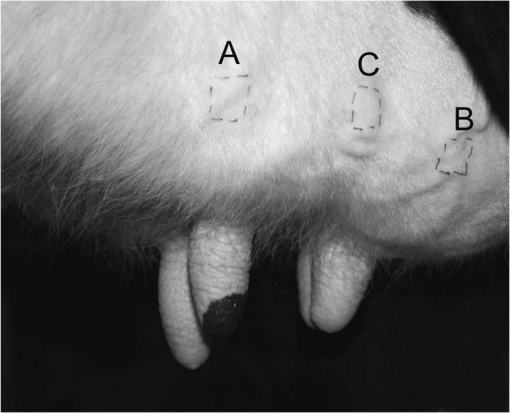
1.IntroductionThe effect of carotenoids on health and productivity in dairy cattle, as well as on the properties of dairy products, has been the focus of scientific interest for a long time. Carotenoids give dairy products a yellowish stain, which consumers relate to a healthy product. Various disorders of cattle such as mastitis, fatty liver syndrome, and infertility have been demonstrated to be associated with low plasma carotenoid concentrations.1,2 The latter phenomenon might be a reflection of oxidative stress,3,4 which occurs when the balance between the reactive oxygen species (ROS) and the antioxidative protective mechanisms of the organism is shifted in favor of the ROS.5,6 Such conditions form a potential hazard for both the well-being of the individual animal and its productivity. Endogenous generation of ROS and RNS (reactive nitrogen species) in the body is inevitable. ROS and RNS, however, are capable of destroying biologically important molecules, e.g., DNA, proteins, carbohydrates, and lipids.7 Therefore, body cells dispose overprotective systems to avoid oxidative damage. These systems comprise various enzymatic and nonenzymatic antioxidative mechanisms.8 Antioxidants have been shown to act synergistically, by forming a protective chain, thus protecting each other against destruction induced by free radicals and other reactive species.9,10 One important group of antioxidants are the carotenoids, which can react with free radicals and in various ways become effective as antioxidants.11–13 Owing to these interactions, the carotenoids could represent marker substances for the entire antioxidative protective system of the organism.3,14 In bovine tissues, among all carotenoids, only -carotene can be found at higher concentrations.15 In addition, 13-cis- and all-trans--carotene and lutein have been detected in the plasma and milk of bovines.16,17 Neither humans nor animals are capable of de novo synthesis of carotenoids and therefore depend on dietary supply.18,19 Until now, high-performance liquid chromatography (HPLC) has been widely used in veterinary medicine for the analysis and quantitative determination of carotenoids.20 Today, the determination of -carotene in blood samples is applied on a routine basis in herd health-monitoring programs. Determination of the carotenoids by HPLC requires sampling of either blood or biopsy materials. Because of the invasive character of the sampling procedures and the costs for the analyses, the latter approach is not suitable for serial analyses.21 In humans, several studies have been undertaken in recent years to detect antioxidants, in particular -carotene and lycopene, in the skin using an optical method based on resonance Raman spectroscopy, which has been demonstrated to deliver fast results, being noninvasive.22 Dermal carotenoid concentrations as determined in human volunteers yielded a wide range of levels.23,24 The latter findings were related to differences in the digestion and metabolism of dietary carotenoids, which could be genetically dependent,25 based on eating habits, lifestyles and, possibly, different stress conditions of volunteers.26 In humans, dermal oxidative stress has been demonstrated following sun irradiation,11,27–29 environmental hazards30, illness or inflammation, and various stress conditions.26,31 Raman spectroscopic measurements are also used in the field of different food products like meat, eggs, and products produced from milk.32,33 These measurements permit quantitative and qualitative determinations of carotenoids.34,35 -Carotene levels in cattle have successfully been determined by Niedorf36 in biopsies obtained from udder skin using reflection spectroscopy. A close correlation of the results was found with those of comparative HPLC measurements ().36 Applying spectroscopy allows higher numbers of individuals to be examined, as well as investigation of the efficacy and kinetics following dietary supply of carotenoid antioxidants and their accumulation in the skin, in vivo26,37–42 and in vitro.43 The purpose of the present study was to apply spectroscopic measurements to monitor the carotenoid concentrations in the skin of cattle by using an innovative, noninvasive technique including a handheld miniaturized spectroscopic system (MSS). 2.Materials and Methods2.1.Study DesignThe study consisted of two different trials. Experiment 1 aimed at the noninvasive in vivo determination of carotenoid concentrations in the bovine udder skin, and experiment 2 included repeated determinations of the carotenoid concentrations in the skin of the same animal. 2.1.1.Experiment 1Twenty-five clinically healthy female cattle, aged between 2 and 11 years, were included in the trial. Twenty cows were of the breed German Holstein and 5 were of the breed German Red Holstein. All animals were kept under the same conditions and were fed at need with a ration consisting of grass silage, hay, and concentrates. The lactation stadium, however, differed individually among the cows. Noninvasive in vivo carotenoid measurements were performed on three pre-determined sites (A, B, C) on the surface of the udder, each located 10 cm proximally to the teat basis. Two sites (A, B) were located perpendicular above the teats, and the third site (C) was located halfway in a direct line between points A and B, as shown in Fig. 1. Each site was subjected to three subsequent measurements. To this end, the MSS was removed from the skin between measurements and then positioned once more on the same skin area. The time interval between the measurements was only a few seconds. Prior to the measurements, the respective skin area was shaved with a disposable razor and the different sites were marked. All measurements were performed on the left side of the udder. 2.1.2.Experiment 2Thirteen cows aged between 3 and 7 years were included in the two parts of experiment 2. Ten animals (9 of the breed German Holstein and 1 of the breed Jersey) were used in part 1. Twelve cows (10 of the breed German Holstein, 1 of the breed German Red Holstein, and 1 of the breed Jersey) were used in part 2. All cows were kept under the same conditions and were fed at need with a ration consisting of silage, hay, concentrates, and straw. During the test period, the animals had not been exposed to any experimental manipulations or any other types of stress; cow were not pregnant, nor had they calved within the last 6 months. No drugs had been administered to the animals during a 3-month period preceding the trial. The carotenoid measurements in the frame of this study were performed as described previously.44 In the part 1, three measurements were performed on 10 animals within four days. Subsequently, in part 2, 12 cows were evaluated as either “sensitive” () or “robust” () according to the results of a behavioral test of the temperament of the cattle.45 The ratings were , no movement; ; ; , very vigorous movement; , twisting of the body and struggling violently. Scores 3, 4, and 5 were categorized as nervous behavior. To verify the objectiveness of evaluation of the temperament test, the 25 animals from experiment 1 were judged by two independent persons simultaneously. The coefficient of concordance was calculated. In addition, stress indicators like tail strokes, attempted escapes, uncoordinated reciprocating movement of the head, calls, urination, and defecation46–50 were recorded. All six animals categorized as being sensitive exhibited at least three stress indicators, whereas animals categorized as being robust exhibited fewer than three stress indicators. The two cows of the breeds German Red Holstein and Jersey belonged to the group of the robust cows, whereas the group of the sensitive cows consisted only of German Holstein breed cows. 2.2.Miniaturized Spectroscopic SystemFor the noninvasive determination of the carotenoid concentration in the bovine udder skin, an LED-based MSS (Opsolution GmbH, Kassel, Germany) was used. The measuring principle of the MSS is based on reflection spectroscopy. Figure 2 shows the of the diffuse reflected spectrum obtained in vivo from the bovine udder skin. The form of the reflection spectrum is determined by the presence of dermal chromophores. Taking into consideration the absorption spectrum of carotenoids, which lies in the blue-green range of the optical spectrum,22 the blue LED-emitted bright spectrum in the range between 440 and 490 nm was used as a source of excitation. The small dip in the reflected spectrum, measured in the range between 458 and 472 nm, is associated with the absorption of carotenoids in the skin sample (see Fig. 2). The value of this dip was recalculated with the relative carotenoid concentration (arbitrary units [a.u.]). Fig. 2Diffuse reflectance spectrum obtained in vivo from bovine udder skin under LED excitation at . 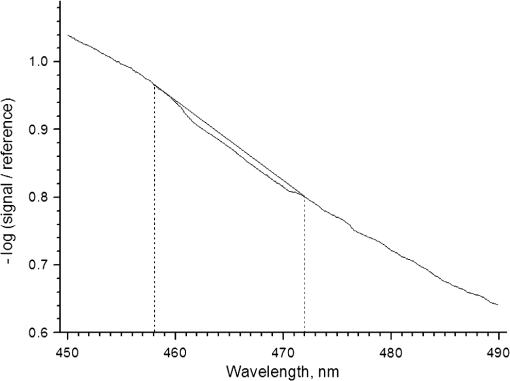 Before in vivo measurements were started, the MSS was calibrated in vitro using bovine skin specimens by the use of resonance Raman spectroscopy, which is specific for the measurement of dermal carotenoids.34 For this purpose, an area of approximately was marked on the perimeter with permanent marker, and the center of this area was further used for both reflectance and Raman measurements. The intensity of the prominent Raman peak at , originating from the carbon–carbon double bond stretch vibration of the backbone of carotenoid molecules under the excitation at 488 nm, was analyzed and compared to the size of the dip in the reflectance spectrum. To exclude the influence of light from outside, the optical measurements were performed under full contact between the skin surface and MSS and Raman devices. A strong linear correlation () was achieved. The spread of the measured with the MSS concentrations of carotenoids did not exceed 10% on bovine udder skin in vitro. A detailed description of MSS has been previously presented by our group.44 2.3.Statistical AnalysisStatistical analysis was carried out using the statistics software package SPSS 18.00 for Windows (IBM, Armonk, NY). The data were tested for normal distribution and, as the latter were partially distributed abnormally, the median was used. The Mann–Whitney -test was applied for independent measurements using two samples, whereas the Friedman test was used for dependent measurements using more than two samples. The Kruskal–Wallis test was applied for comparing more than two different groups. To evaluate correlations between the values, the Spearman correlation test was used. Values with were considered statistically significant. 3.Results3.1.Noninvasive In Vivo Determination of Dermal Carotenoids in Bovine Udder Skin and Reproducibility of Measurements (Experiment 1)Figure 3 shows the results of the measurements on three different sites (A, B, C) on the udder skin of the same animal. The measuring accuracy of the MSS was determined for each animal by calculating the percentage standard deviation from all three measurements performed on the sites A, B, and C. As a result, a measuring accuracy of 12% was established for the MSS applied to the same area of the skin. In addition, the percentage variation of the carotenoid concentrations as determined on three different sites (A, B, C) was determined for each animal. As a result, a mean variation of 17% was determined for the carotenoid values on the different areas of the specific udders. Fig. 3Carotenoid concentrations obtained with the MSS by serial measurements from the three different sites (A, B and C) on the udder skin of an individual animal.  Carotenoid concentrations of the same skin areas showed highly significant differences between individual animals () (Fig. 4). The carotenoid concentration in the skin is represented as . The median () of the relative concentration was . The variation range was 1.06 a.u. A factor of 4 could be revealed between the maximum and the minimum value (Fig. 4). The mean standard variation amounted to 0.05 a.u. The correlation between the daily milk yield and the carotenoid concentration was weak (). Fig. 4Carotenoid concentrations measured by MSS and expressed in arbitrary units on the udder skin of 25 cows (). Cows 1 to 5 were of the breed German Red Holstein and cows 6 to 25 of the breed German Holstein. 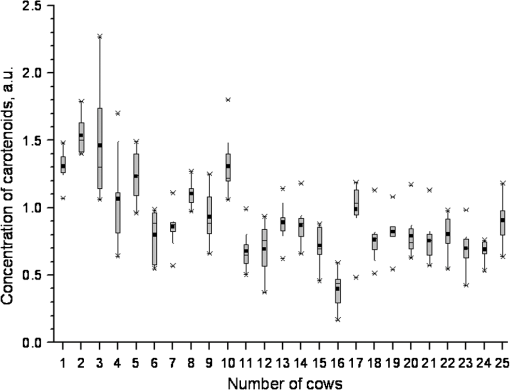 As only one animal was years old, no comparison was made between young and old cattle. Due to the low number of animals, the dependence of the carotenoid concentration on the breed was not computed, either. 3.2.Carotenoid Concentrations in the Udder Skin of Cattle Kept under Standardized Conditions (Experiment 2)Ten healthy cows after undergoing three measurements on the same site of the udder skin within four days exhibited no significant differences in dermal carotenoid concentrations (Fig. 5). During these four days, a mean deviation of 10% was measured for the carotenoid concentrations. Fig. 5Carotenoid concentrations on the udder skin of 10 healthy animals determined on four consecutive days. ; or day 3; . 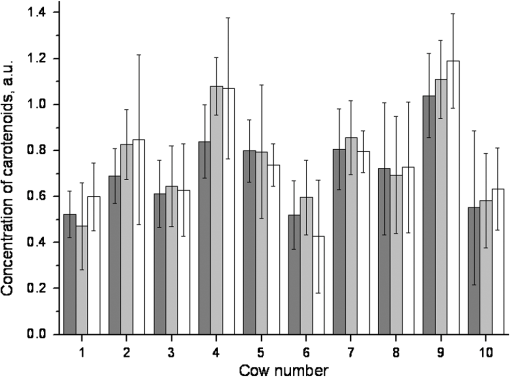 To evaluate differences between observers with respect to the temperament test, the 25 cows from experiment 1 were judged by two persons, independently. The results did not show any significant differences. Using the temperament test, the 12 animals could be clearly categorized as either sensitive () or robust (). The carotenoid concentrations of these animals are given in Fig. 6. Fig. 6Median dermal carotenoid concentrations as expressed in arbitrary units measured on the udder of animals evaluated as either sensitive () or robust () according to the results of a temperament test ().45 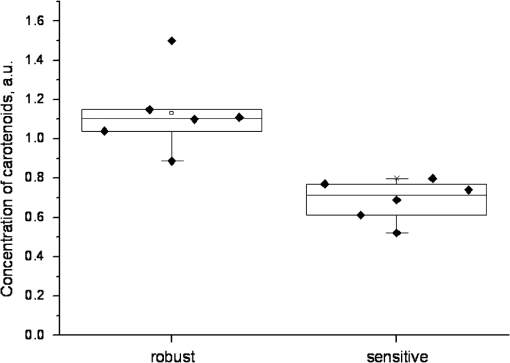 The difference between the medians of the two groups was significant at . The general carotenoid skin udder levels (median of ) of the robust cows amounted to , whereas the sensitive cows exhibited a general carotenoid skin udder level of . 4.DiscussionThe MSS technique, applied in the present study to determine dermal carotenoid concentrations, is suitable for noninvasive measurements of carotenoids in the bovine udder skin. As carotenoids have been demonstrated to represent possible marker substances for the entire antioxidative status in humans,3,26 this noninvasive measurement opens up great perspectives for the use in cattle, to investigate the oxidative stress caused by high milk yield, illness, or environmental stressors. In accordance with observations in humans, the present study demonstrates a huge variation in carotenoid levels as determined by MSS measurements in different cattle. Lotthammer and Ahlswede51 likewise demonstrated significant differences between different individuals in the concentrations of -carotene in blood plasma. Noziere et al.16 found that the -carotene concentrations in the blood plasma of cattle fed with grass silage varied strongly between individual animals, whereas this variability was considerably less profound when the animals were exclusively fed hay. Hesterberg et al.33 showed by the use of resonance Raman spectroscopy that the concentration of carotenoids in the yolks of hen eggs is also influenced by nutrition and housing conditions. Experiments carried out by Niedorf36 on isolated perfused udder skin, using reflection spectroscopic measurements, revealed huge variations in the dermal carotenoid concentrations of organs from different individuals. The animals included in experiment 1 differed regarding their age, breed, and stage of lactation. Calderon et al.17 ascertained that during the nonlactating phase as well as during the first week of lactation, a reduction occurred in the carotenoid concentration of the blood, which gradually increased during a period of 3 months. Similar observations have been reported by various authors.52–54 The influence of age on -carotene concentration in blood is largely unknown. Katsoulos et al.54 observed significantly higher -carotene concentrations in younger animals, while—on the contrary—others had observed either higher concentrations in older cattle55,56 or no age-related differences whatsoever.57,58 A variety of studies revealed breed-related differences with respect to -carotene concentrations in the blood.56,58–61 Tian et al.62 showed statistically significant differences in the -carotene concentration in fat tissues and in color of subcutaneous fat of cattle representing various genotypes. A negative relationship was reported between -carotene status and stearoyl-CoA desaturase activity in cattle, but a positive relationship existed when cattle were fed on the same diet. For this reason, differences in -carotene concentrations were related to genetic differences in intestinal dioxygenase being able to metabolize -carotene to vitamin A.62 Investigations on human skin also resulted in strong differences regarding -carotene and lycopene concentrations as determined on the same site in different volunteers.63,64 In human skin, the distribution and concentrations of carotenoids strongly depend on the state of health of the respective volunteer, his nutritional habits, lifestyle, the investigated area of the body, and the effects of stress factors.26,34,41 Serial measurements on the same individual animal revealed no significant alterations in the dermal carotenoid concentrations during four consecutive days, demonstrating hardly any day-to-day variation over a short period of time. Consequently, the measured values remained relatively stable for each animal under standardized conditions. This could also be verified by measurements in humans. Darvin et al.26 demonstrated that dermal carotenoid concentrations remained stable for weeks, as long as dietary habits did not change. Measurements that had been performed on humans once daily over a period of 1 year demonstrate the effects of stress factors on the carotenoid concentrations in human skin. High levels in dermal carotenoid concentrations correlated well with the intake of food rich in carotenoids by the volunteers, as well as with a reduced number of stress factors that these people were exposed to, as compared to other factors (e.g. smoking, disease). For this reason, the carotenoid concentration in the skin could serve as a suitable marker for enhanced or reduced oxidative processes in the human body.26 Measurements in sensitive and robust cattle, as determined by a standardized temperament test, revealed significant differences in the carotenoid concentrations between the two groups. It remains to be proven in studies with a higher number of animals whether individual differences in temperament, expressed in terms of anxiety, result in reduce intradermal -carotene levels, which might reflect a reduced capacity of such animals to cope with stressful environmental conditions.65 This is of particular interest to livestock farming, as temperament of cattle has been shown to be related to productivity.66,67 Although milk contains only small amounts of the body’s carotenoids, the latter contribute to a high extent to the sensory effect of dairy products through their coloring and antioxidant properties.68 The carotenoid concentration in milk varies depending on breed,69,70 lactation stage,71 genetics,70,71 and nutrition.68 The question to be answered in the near future will be concerned with how strongly the intradermal -carotene status and the levels in milk correlate. Applying MSS for determination of the intradermal carotenoid concentrations in cattle allows noninvasive serial in vivo measurements and opens up new perspectives for a better understanding of the carotenoid metabolism and the effects of stress and disease on the oxidative status of cattle. The noninvasive spectroscopic method for measurement of dermal carotenoids promises a versatile application. 5.ConclusionCarotenoid concentrations were reproducibly measured on bovine udder skin of the same individual animal. The carotenoid concentrations, however, varied significantly between different animals, although all animals had been subjected to the same conditions. Serial measurements on four consecutive days exhibited no significant differences in the intradermal carotenoid concentrations at different time points. Cattle evaluated as robust by a temperament test had significantly higher carotenoid concentrations in their udder skin than animals judged as sensitive (). The present investigations show the feasibility of the determination of carotenoids in bovine skin by applying an optical method based on reflection spectroscopy. ReferencesT. R. Batraet al.,
“Concentration of plasma and milk vitamin-e and plasma beta-carotene of mastitic and healthy cows,”
Int. J. Vitamin Nutr. Res., 62
(3), 233
–237
(1992). IJVNAP 0300-9831 Google Scholar
C. Kawashimaet al.,
“Relationship between plasma beta-carotene concentrations during the peripartum period and ovulation in the first follicular wave postpartum in dairy cows,”
Anim. Reprod. Sci., 111
(1), 105
–111
(2009). http://dx.doi.org/10.1016/j.anireprosci.2008.02.008 ANRSDV 0378-4320 Google Scholar
J. Lademannet al.,
“Carotenoids in human skin,”
Exp. Dermatol., 20
(5), 377
–382
(2011). http://dx.doi.org/10.1111/exd.2011.20.issue-5 EXDEEY 0906-6705 Google Scholar
J. LykkesfeldtO. Svendsen,
“Oxidants and antioxidants in disease: oxidative stress in farm animals,”
Vet. J., 173
(3), 502
–511
(2007). http://dx.doi.org/10.1016/j.tvjl.2006.06.005 1090-0233 Google Scholar
B. Halliwell,
“Biochemistry of oxidative stress,”
Biochem. Soc. Trans., 35
(Pt 5), 1147
–1150
(2007). BCSTB5 0300-5127 Google Scholar
D. R. BickersM. Athar,
“Oxidative stress in the pathogenesis of skin disease,”
J. Invest. Dermatol., 126
(12), 2565
–2575
(2006). http://dx.doi.org/10.1038/sj.jid.5700340 JIDEAE 0022-202X Google Scholar
H. SiesW. Stahl,
“Vitamin-E and vitamin-C, beta-carotene, and other carotenoids as antioxidants,”
Am. J. Clin. Nutr., 62
(6), S1315
–S1321
(1995). Google Scholar
J. J. Thieleet al.,
“The antioxidant network of the stratum corneum,”
Curr. Probl. Dermatol., 29 26
–42
(2001). http://dx.doi.org/10.1159/000060651 APDEBX 0070-2064 Google Scholar
M. Darvinet al.,
“Effect of supplemented and topically applied antioxidant substances on human tissue,”
Skin Pharmacol. Physiol., 19
(5), 238
–247
(2006). http://dx.doi.org/10.1159/000093979 SPPKE6 1660-5527 Google Scholar
M. Wronaet al.,
“Cooperation of antioxidants in protection against photosensitized oxidation,”
Free Radic. Biol. Med., 35
(10), 1319
–1329
(2003). http://dx.doi.org/10.1016/j.freeradbiomed.2003.07.005 FRBMEH 0891-5849 Google Scholar
W. StahlH. Sies,
“Antioxidant activity of carotenoids,”
Mol. Aspects Med., 24
(6), 345
–351
(2003). http://dx.doi.org/10.1016/S0098-2997(03)00030-X MAMED5 0098-2997 Google Scholar
J. Lademannet al.,
“Interaction between carotenoids and free radicals in human skin,”
Skin Pharmacol. Physiol., 24
(5), 238
–244
(2011). http://dx.doi.org/10.1159/000326074 SPPKE6 1660-5527 Google Scholar
A. A. Woodallet al.,
“Oxidation of carotenoids by free radicals: relationship between structure and reactivity,”
BBA Gen. Subjects, 1336
(1), 33
–42
(1997). Google Scholar
S. F. Haaget al.,
“Determination of the antioxidative capacity of the skin in vivo using resonance Raman and electron paramagnetic resonance spectroscopy,”
Exp. Dermatol., 20
(6), 483
–487
(2011). http://dx.doi.org/10.1111/j.1600-0625.2010.01246.x EXDEEY 0906-6705 Google Scholar
K. A. Slifkaet al.,
“A survey of serum and dietary carotenoids in captive wild animals,”
J. Nutr., 129
(2), 380
–390
(1999). JONUAI 0022-3166 Google Scholar
P. Noziereet al.,
“Variations in carotenoids, fat-soluble micronutrients, and color in cows' plasma and milk following changes in forage and feeding level,”
J. Dairy Sci., 89
(7), 2634
–2648
(2006). http://dx.doi.org/10.3168/jds.S0022-0302(06)72340-2 JDSCAE 0022-0302 Google Scholar
F. Calderonet al.,
“Variations in carotenoids, vitamins A and E, and color in cow’s plasma and milk following a shift from hay diet to diets containing increasing levels of carotenoids and vitamin E,”
J. Dairy Sci., 90
(12), 5651
–5664
(2007). http://dx.doi.org/10.3168/jds.2007-0264 JDSCAE 0022-0302 Google Scholar
K. Meyer,
“Colorful antioxidants—Carotenoids: significance and technical syntheses,”
Chem Unserer Zeit, 36
(3), 178
–192
(2002). Google Scholar
J. Lademannet al.,
“Uptake of antioxidants by natural nutrition and supplementation: pros and cons from the dermatological point of view,”
Skin Pharmacol. Physiol., 24
(5), 269
–273
(2011). http://dx.doi.org/10.1159/000328725 SPPKE6 1660-5527 Google Scholar
D. B. Rodriguez-Amaya,
“Food carotenoids: analysis, composition and alterations during storage and processing of foods,”
Forum Nutr., 56 35
–37
(2003). FNOUA6 1660-0347 Google Scholar
D. Talwaret al.,
“A routine method for the simultaneous measurement of retinol, alpha-tocopherol and five carotenoids in human plasma by reverse phase HPLC,”
Clin. Chim. Acta, 270
(2), 85
–100
(1998). http://dx.doi.org/10.1016/S0009-8981(97)00224-6 CCATAR 0009-8981 Google Scholar
M. E. Darvinet al.,
“Noninvasive detection of beta-carotene and lycopene in human skin using Raman spectroscopy,”
Laser Phys., 14
(2), 231
–233
(2004). LAPHEJ 1054-660X Google Scholar
M. E. Darvinet al.,
“Dermal carotenoid level and kinetics after topical and systemic administration of antioxidants: enrichment strategies in a controlled in vivo study,”
J. Dermatol. Sci., 64
(1), 53
–58
(2011). http://dx.doi.org/10.1016/j.jdermsci.2011.06.009 JDSCEI 0923-1811 Google Scholar
L. A. Meinkeet al.,
“Influence on the carotenoid levels of skin arising from age, gender, body mass index in smoking/non-smoking individuals,”
Free Radic. Antiox., 1
(2), 15
–20
(2011). http://dx.doi.org/10.5530/ax.2011 FRARCP 2231-2536 Google Scholar
P. Borel,
“Genetic variations involved in interindividual variability in carotenoid status,”
Mol. Nutr. Food Res., 56 228
–240
(2012). Google Scholar
M. E. Darvinet al.,
“One-year study on the variation of carotenoid antioxidant substances in living human skin: influence of dietary supplementation and stress factors,”
J. Biomed. Opt., 13
(4), 044028
(2008). http://dx.doi.org/10.1117/1.2952076 JBOPFO 1083-3668 Google Scholar
M. E. Darvinet al.,
“Formation of free radicals in human skin during irradiation with infrared light,”
J. Invest. Dermatol., 130
(2), 629
–631
(2010). http://dx.doi.org/10.1038/jid.2009.283 JIDEAE 0022-202X Google Scholar
L. Zastrowet al.,
“The missing link: light-induced (280-1,600 nm) free radical formation in human skin,”
Skin Pharmacol. Physiol., 22
(1), 31
–44
(2009). http://dx.doi.org/10.1159/000188083 SPPKE6 1660-5527 Google Scholar
M. E. Darvinet al.,
“Determination of the influence of IR radiation on the antioxidative network of the human skin,”
J. Biophoton., 4
(1–2), 21
–29
(2011). Google Scholar
V. StoneH. JohnstonM. J. Clift,
“Air pollution, ultrafine and nanoparticle toxicology: cellular and molecular interactions,”
IEEE Trans. Nanobiosci., 6
(4), 331
–340
(2007). http://dx.doi.org/10.1109/TNB.2007.909005 1536-1241 Google Scholar
H. MaedaT. Akaike,
“Nitric oxide and oxygen radicals in infection, inflammation, and cancer,”
Biochem. Moscow, 63
(7), 854
–865
(1998). BIORAK 0006-2979 Google Scholar
D. YangY. Ying,
“Applications of raman spectroscopy in agricultural products and food analysis: a review.,”
Appl. Spectrosc. Rev., 46
(7), 539
–560
(2011). http://dx.doi.org/10.1080/05704928.2011.593216 APSRBB 0570-4928 Google Scholar
K. Hesterberget al.,
“Raman spectroscopic analysis of the carotenoid concentration in egg yolks depending on the feeding and housing conditions of the laying hens,”
J. Biophoton., 5
(1), 33
–39
(2012). Google Scholar
M. E. Darvinet al.,
“Non-invasive in vivo determination of the carotenoids beta-carotene and lycopene concentrations in the human skin using the Raman spectroscopic method,”
J. Phys. D Appl. Phys., 38
(15), 2696
–2700
(2005). http://dx.doi.org/10.1088/0022-3727/38/15/023 0022-3727 Google Scholar
I. V. Ermakovet al.,
“Noninvasive selective detection of lycopene and beta-carotene in human skin using Raman spectroscopy,”
J. Biomed. Opt., 9
(2), 332
–338
(2004). http://dx.doi.org/10.1117/1.1646172 JBOPFO 1083-3668 Google Scholar
F. Niedorf,
“Entwicklung eines Verfahrens zur quantitativen Auswertung nicht-invasiver reflexionsspektroskopischer Messungen von Beta-Carotin in der Haut,”
Institut für Pharmakologie, Toxikologie und Pharmazie der Tierärztlichen, Hochschule, Hannover
(2001). Google Scholar
M. E. Darvinet al.,
“Resonance Raman spectroscopy for the detection of carotenolds in foodstuffs: influence of the nutrition on the antioxidative potential of the skin,”
Laser Phys. Lett., 4
(6), 452
–456
(2007). http://dx.doi.org/10.1002/(ISSN)1612-202X 1612-2011 Google Scholar
U. Blume-Peytaviet al.,
“Cutaneous lycopene and beta-carotene levels measured by resonance Raman spectroscopy: high reliability and sensitivity to oral lactolycopene deprivation and supplementation,”
Eur. J. Pharm. Biopharm., 73
(1), 187
–194
(2009). http://dx.doi.org/10.1016/j.ejpb.2009.04.017 EJPBEL 0939-6411 Google Scholar
X. T. LimaH. C. LimaA. B. Kimball,
“A cross-sectional study of skin carotenoid levels in adult patients with psoriasis,”
J. Invest. Dermatol., 130
(1), S44
(2010). JIDEAE 0022-202X Google Scholar
S. RerksuppapholL. Rerksuppaphol,
“Effect of fruit and vegetable intake on skin carotenoid detected by noninvasive Raman spectroscopy,”
J. Med. Assoc. Thai, 89
(8), 1206
–1212
(2006). JMTHBU Google Scholar
S. T. Mayneet al.,
“Noninvasive assessment of dermal carotenoids as a biomarker of fruit and vegetable intake,”
Am. J. Clin. Nutr., 92
(4), 794
–800
(2010). Google Scholar
M. C. Meinkeet al.,
“Bioavailability of natural carotenoids in human skin compared to blood,”
Eur. J. Pharm. Biopharm., 76
(2), 269
–274
(2010). http://dx.doi.org/10.1016/j.ejpb.2010.06.004 EJPBEL 0939-6411 Google Scholar
S. F. Haaget al.,
“Comparative study of carotenoids, catalase and radical formation in human and animal skin,”
Skin Pharmacol. Physiol., 23
(6), 306
–312
(2010). http://dx.doi.org/10.1159/000313539 SPPKE6 1660-5527 Google Scholar
M. E. Darvinet al.,
“Comparison of two methods for noninvasive determination of carotenoids in human and animal skin: Raman spectroscopy versus reflection spectroscopy,”
J. Biophoton.,
(2012). http://dx.doi.org/10.1002/jbio.201100080 Google Scholar
T. Grandin,
“Behavioral agitation during handling of cattle is persistent over time,”
Appl. Anim. Behav. Sci., 36
(1), 1
–9
(1993). AABSEV Google Scholar
T. Grandin,
“Assessment of stress during handling and transport,”
J. Anim. Sci., 75
(1), 249
–257
(1997). 0021-8812 Google Scholar
T. Grandin,
“The feasibility of using vocalization scoring as an indicator of poor welfare during cattle slaughter,”
Appl. Anim. Behav. Sci., 56
(2–4), 121
–128
(1998). AABSEV Google Scholar
A. M. DepassilleJ. RushenF. Martin,
“Interpreting the behavior of calves in an open-field test: a factor-analysis,”
Appl. Anim. Behav. Sci., 45
(3–4), 201
–213
(1995). AABSEV Google Scholar
S. KondoJ. F. Hurnik,
“Behavioral and physiological-responses to spatial novelty in dairy-cows,”
Can. J. Anim. Sci., 68
(2), 339
–343
(1988). http://dx.doi.org/10.4141/cjas88-038 CNJNAT 0008-3984 Google Scholar
L. Munksgaardet al.,
“Discrimination of people by dairy cows based on handling,”
J. Dairy Sci., 80
(6), 1106
–1112
(1997). http://dx.doi.org/10.3168/jds.S0022-0302(97)76036-3 JDSCAE 0022-0302 Google Scholar
K. H. LotthammerL. Ahlswede,
“Vitamin-A independent effect of beta-carotene on bovine fertility,”
Deut. Tierarztl. Woch., 84
(6), 220
–226
(1977). Google Scholar
J. P. GoffK. KimuraR. L. Horst,
“Effect of mastectomy on milk fever, energy, and vitamins A, E, and beta-carotene status at parturition,”
J. Dairy Sci., 85
(6), 1427
–1436
(2002). http://dx.doi.org/10.3168/jds.S0022-0302(02)74210-0 JDSCAE 0022-0302 Google Scholar
R. ChawlaH. Kaur,
“Plasma antioxidant vitamin status of periparturient cows supplemented with alpha-tocopherol and beta-carotene,”
Anim. Feed Sci. Tech., 114
(1–4), 279
–285
(2004). http://dx.doi.org/10.1016/j.anifeedsci.2003.11.002 AFSTDH 0377-8401 Google Scholar
P. D. Katsouloset al.,
“Long-term fluctuations and effect of age on serum concentrations of certain fat-soluble vitamins in dairy cows,”
Vet. Clin. Path., 34
(4), 362
–367
(2005). VCPADJ Google Scholar
S. J. LeBlancet al.,
“Peripartum serum vitamin E, retinol, and beta-carotene in dairy cattle and their associations with disease,”
J. Dairy Sci., 87
(3), 609
–619
(2004). http://dx.doi.org/10.3168/jds.S0022-0302(04)73203-8 JDSCAE 0022-0302 Google Scholar
W. Braun,
“Studies on the carotenoid and virtamin A levels in cattle,”
J. Nutr., 29 61
–71
(1944). JONUAI 0022-3166 Google Scholar
R. StampferH. Zucker,
“Studies on the relationship between beta-carotene in blood and milk of dairy-cows,”
Z. Tierphysiol. Tierer., 49
(3), 140
–147
(1983). ZTTFAA 0044-3565 Google Scholar
J. H. L. P. MorganG. C. Everitt,
“Some factors affecting yellow fat colour in cattle,”
Proc. N. Zeal. Soc. Anim. Prod., 29 164
–175
(1969). PZAPAD Google Scholar
H. J. Deuelet al.,
“The effect of a high vitamin a intake on the blood and milk carotene of Holstein and Guernsey cows,”
J. Nutr., 23
(6), 567
–579
(1942). JONUAI 0022-3166 Google Scholar
T. S. SuttonP. A. Soldner,
“Seasonal variations in the blood plasma carotene and vitamin-A of adult dairy cattle,”
J. Dairy Sci., 28
(11), 859
–867
(1945). http://dx.doi.org/10.3168/jds.S0022-0302(45)95244-1 JDSCAE 0022-0302 Google Scholar
S.-A. N. K. Newmanet al.,
“Effect of breed on plasma carotene concentration in New Zealand dairy heifers,”
Proc. N. Zeal. Soc. Anim. Prod., 54 119
–120
(1994). PZAPAD Google Scholar
R. Tianet al.,
“Genetic variation in the beta, beta-carotene-,-dioxygenase gene and association with fat colour in bovine adipose tissue and milk,”
Anim. Genet., 41
(3), 253
–259
(2010). http://dx.doi.org/10.1111/age.2010.41.issue-3 69MYSH 0268-9146 Google Scholar
M. E. Darvinet al.,
“In vivo distribution of carotenoids in different anatomical locations of human skin: comparative assessment with two different Raman spectroscopy methods,”
Exp. Dermatol., 18
(12), 1060
–1063
(2009). http://dx.doi.org/10.1111/exd.2009.18.issue-12 EXDEEY 0906-6705 Google Scholar
M. E. Darvinet al.,
“Determination of beta carotene and lycopene concentrations in human skin using resonance Raman spectroscopy,”
Laser Phys., 15
(2), 295
–299
(2005). LAPHEJ 1054-660X Google Scholar
C. Plachta,
“Untersuchungen zum Temperament von AngusDt. und FleckviehDt. Rindern sowie deren reziproken Kreuzung anhand verschiedener Testverfahren unter besonderer Berücksichtigung von Kreuzungseffekten,”
Justus-Liebig-Universität Gießen, Wiesbaden
(2009). Google Scholar
B. D. Voisinetet al.,
“Bos indicus cross feedlot cattle with excitable temperaments have tougher meat and a higher incidence of borderline dark cutters,”
Meat Sci., 46
(4), 367
–377
(1997). http://dx.doi.org/10.1016/S0309-1740(97)00031-4 MESCDN 0309-1740 Google Scholar
B. D. Voisinetet al.,
“Feedlot cattle with calm temperaments have higher average daily gains than cattle with excitable temperaments,”
J. Anim. Sci., 75
(4), 892
–896
(1997). 0021-8812 Google Scholar
C. Agabrielet al.,
“Tanker milk variability according to farm feeding practices: vitamins A and E, carotenoids, color, and terpenoids,”
J. Dairy Sci., 90
(10), 4884
–4896
(2007). http://dx.doi.org/10.3168/jds.2007-0171 JDSCAE 0022-0302 Google Scholar
S. Y. ThompsonK. M. HenryS. K. Kon,
“Factors affecting concentration of vitamins in milk. I.Effect of breed season geographical location on fat-soluble vitamins,”
J. Dairy Res., 31
(1), 1
–25
(1964). Google Scholar
A. M. WinkelmanD. L. JohnsonA. K. H. MacGibbon,
“Estimation of heritabilities and correlations associated with milk color traits,”
J. Dairy Sci., 82
(1), 215
–224
(1999). http://dx.doi.org/10.3168/jds.S0022-0302(99)75226-4 JDSCAE 0022-0302 Google Scholar
S. K. JensenA. K. B. JohannsenJ. E. Hermansen,
“Quantitative secretion and maximal secretion capacity of retinol, beta-carotene and alpha-tocopherol into cows’ milk,”
J. Dairy Res., 66
(4), 511
–522
(1999). Google Scholar
|