|
|
1.IntroductionThe incidence of malignant melanoma has been increasing rapidly worldwide in the last few decades.1 Despite significant improvements in diagnosis, surgical techniques, general patient care, and local and systemic adjuvant therapies, the most fearsome aspect of cancer is that most deaths are due to metastasis.2,3 Currently, the model of pulmonary melanoma metastasis in C57BL/6 mice has been used more and more to study the process of metastasis and to screen novel anti-metastatic drugs as this cancer can be examined over a relatively short period.4–7 However, the biological heterogeneity of cancer cells in the primary neoplasm and metastases and the modification response of a metastatic tumor cell to systemic therapy in the specific organ microenvironment are the main barriers to the treatment of metastases. This reveals that understanding the process of metastasis on the systemic, cellular and molecular levels are the pressing goals of cancer research.2,8–10 Many methods, such as biochemical,11,12 cell biology13,14 and statistical macroscopy,7,15 are classified according to different monitored objectives and have been employed to inspect the physiological changes of pulmonary melanoma metastasis for studying the process of metastasis and evaluating the drug effects. However, these methods can’t provide high spatial resolution of the pulmonary tissue. Histological examination is the gold standard for cancer diagnosis as it can reveal tissue and cellular morphologic features with submicron spatial resolution to identify the pathologic stages of the cancer tissue. Nonetheless, the procedure of histological biopsy is both invasive and time-consuming as it requires hematoxylin and eosin (HE) stain and physical sectioning. Therefore, new techniques that not only provide high spatial resolution but also monitor the physiological changes of pulmonary melanoma metastasis without complicated sample processing are urgently needed. Nonlinear optical microscopy (NOM) based on multiphoton absorption and second harmonic generation (SHG) has been widely employed to study the structure and function of cells and tissues due to its unique features of submicron resolution, greater penetration depth, low photo-damage and inherent optical sectioning ability.16 In particular, NOM can provide the visualization of cellular and subcellular structures using plentiful intrinsic chromophores without any pretreatment for the tissue.17 Intracellular nicotinamide adenine dinucleotide (NADH), triphosphopyridine nucleotide (NADPH) and flavin adenine dinucleotide (FAD) can take part in cellular energy metabolism and induce multiphoton autofluorescence (MAF), shedding light on associated carcinogenesis.18,19 In addition, SHG signals arising from collagen fibers can reveal the remodeling of the extracellular matrix (ECM) in the tissue.20,21 SHG imaging can evaluate strategies for altering the aging, atherosclerosis and tumor effectively.22–24 In a recent study, it has been demonstrated that the diagnosis based on the morphologic features obtained by NOM agreed with histological examination for 88.6% of the specimens.25 Unfortunately, to the best of our knowledge, NOM has not been employed in the research of pulmonary melanoma metastasis to monitor the physiological changes of pulmonary tissue during the whole process of colonization, which is the one of two important steps of metastasis.8 In addition, although qualitative differences in morphologic features and quantitative measurement of the MAF and SHG tissue images are feasible enough to evaluate the pathologic stages of the cancer tissue,26–28 special challenges remain for NOM to study the process of pulmonary melanoma metastasis. First, the diameter of intact pulmonary metastasis melanoma nodules in C57BL/6 mice is usually several millimeters in size in the middle and late stages. Conventional NOM cannot provide morphologic features of such a large region with high resolution due to the limited field of view of hundreds of square microns. Second, accuracy of the quantitative analysis of the tissue is important to evaluate the pathological states. However, it requires large area imaging rather than imaging of a small portion of the tumor tissue. We report here on monitoring the process of pulmonary melanoma metastasis ex vivo using a home-built large area nonlinear optical microscope (LA-NOM) and intrinsic fluorophores in C57BL/6 mice. Lung tissue at different time points after a tail vein injection of cancer cells are characterized by MAF and SHG imaging in a volume of . Combined with the morphological analysis of MAF and SHG images, quantitative analysis by the MAF and SHG intensity ratio (MAFIR and SHGIR) between pathological tissue and normal tissue, and the MAF to SHG index (MAFSI) are employed to evaluate physiological changes of pulmonary melanoma metastasis. Our results demonstrate that LA-NOM succeeds in evaluating the pathological changes of pulmonary melanoma metastasis in C57BL/6 mice. 2.Materials and Methods2.1.Sample PreparationThe cell line was grown in tissue culture media (RPMI 1640, Gibco, USA) supplemented with heat-inactivated 10% fetal bovine serum (HyClone, Australian), penicillin (Gibco, USA), streptomycin (Gibco, USA) in a humidified atmosphere of 5% and 95% air at 37°C. Cells were harvested by trypsin/ethylene diamine tetraacetic acid (EDTA) and washed with serum-free RPMI 1640 medium three times. Cell pellets were resuspended in ice-cold phosphate buffered saline (PBS) with cells per 0.5 ml. Female C57BL/6, 6- to 8-week-old mice from Slac Laboratory Animal (Shanghai, China) were used for this research work. The mice were kept under pathogen-free conditions at a constant temperature of 24°C to 26°C and humidity of 30% to 50%, and allowed food and water ad libitum. All animal experiments were approved by the Animal Experimentation Ethics Committee of Huazhong University of Science and Technology. A PBS of 0.5 ml containing B16 cells was injected into C57BL/6 syngeneic mice intravenously. The mice were sacrificed and the lungs excised at different time points on days 3, 5, 7, 10, 12, 15, 18, 20, and 25 after tail vein injections. Two mice were sacrificed at each time point and a total of 20 mice (2 normal mice and 18 mice injected B16 cells) were imaged. In each mouse, two specimens were taken from the fresh lung tissue after removing excess blood. One specimen was placed into PBS waiting for nonlinear optical imaging. The other specimen was fixed in 4% paraformaldehyde for HE stain and histological examination. Before the appearance of nodules, the specimens were randomly chosen from the fresh tissue. After the nodules appeared, the specimens were excised from the regions including as many nodules as possible. 2.2.Imaging Instrumentation and ProtocolCombined MAF and SHG imaging was performed on the home-built LA-NOM in this study, as shown in Fig. 1. Ti:Sapphire laser (Maitai BB, Spectra-Physics) was used as the excitation source (, repetition rate: 80 MHz and tunable range: 710 to 980 nm). The excitation beam was scanned by two perpendicular galvanometer mirrors (Model 6215, Cambridge Technology). Serial images were obtained by translating the specimen using a sample translation stage (ES111, Prior Scientific) after each optical scanning imaging and combined into a large area imaging section. Axial scanning was achieved by a piezoelectric stage (P-563.3CD, Physik Instrumente). After splitting from the excitation laser by a long wave pass dichroic mirror (FF665, Semrock) and through a short wave pass emission filter (FF680/SP, Semrock), the autofluorescence and SHG signals were separated into two simultaneous detection channels by a dichroic mirror (FF409, Semrock). Two filters ( and , Semrock) and PMTs (H7422-40 and H7421-40, Hamamastu) were used to detect SHG and autofluorescence, respectively. Fig. 1Setup of large area nonlinear optical microscopy. ND: neutral density filter; D: diaphragm; DCM: dichroic mirror; SP: short-pass filter; F: band-pass filter; O: objective lens; PZT stage: piezoelectric stage; PMT: photomultiplier tube. 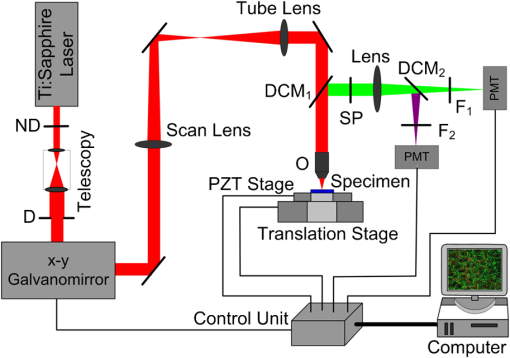 The specimens were placed into a home-built chamber, which was specially tailored for LA-NOM to image the fresh tissue. A two-photon excitation laser light (wavelength of 760 nm) was used for LA-NOM imaging of the lung specimens. The excitation beam was focused and the emitted signals were collected through a low magnification apochromat objective (UPLSAPO , Olympus) to achieve a large field of view and high spatial resolution. The lateral and axial resolution was 0.9 μm and 2.1 μm, respectively. The imaging area of each section was composed of small area optical scanning images with (). The dwell time per pixel was 24 μs and the time per small optical scanning image was 1.6 s. The axial image stacks were generated using a 2 μm step size. For each specimen, three image stacks were acquired at different regions. Six image stacks were obtained at each time point and a total of 60 image stacks were achieved in this study. To perform the quantitative comparison, all of the specimens were imaged with the same average power of 7.8 mW. All imaging was completed within four hours after the animal sacrifice. 2.3.Quantification of MAF and SHG Images2.3.1.MAFIR and SHGIRTo quantitatively analyze the variations of MAF and SHG intensity during the process of cancer cells colonization, MAFIR and SHGIR are employed in the study, defined as the ratio of MAF and SHG intensity in pathological tissue to that in normal tissue. The calculation steps of MAFIR are: First, the maximal MAF intensity value in all of the 60 MAF image stacks was chosen and used to normalize all the image stacks. Second, at each time point, the sum of MAF intensity in each image stack was calculated by adding the MAF intensity of all the pixels throughout the whole imaging volume. Then the average MAF intensity at each time point was obtained by the datum of six image stacks. At last, MAFIRs were calculated by the average MAF intensity of normal tissue and pathological tissue at different time points. Using the same method the SHGIR can be also calculated. 2.3.2.MAFSIIn order to eliminate the individual differences between the different mice as much as possible, MAFSI is used to quantify the relative intensity variation between cellular MAF and ECM SHG in tissue. For each two-dimension nonlinear optical image, MAFSI was calculated according to the formula , in which and were the MAF and SHG intensity cumulated of all pixels in the same imaging section, respectively. As a result, the more MAFSI value approaching the maximum value of 1, the higher content level of the MAF presenting in the imaging section. 3.Results3.1.Morphological AnalysisLarge-area and high-resolution MAF, SHG and merged MAF and SHG images of normal C57BL/6 mice pulmonary tissue are shown in the first row of Fig. 2(a)–2(c). The imaging depth is 2 μm below the lung tissue surface. The magnified views of marked areas in Fig. 2(a)–2(c) are shown in Fig. 2(d)–2(f), respectively. Morphologically, observation from MAF images in the first row of Fig. 2(a) and 2(d) shows that alveoluses have uniform structure and are evenly distributed in the normal tissue. As illustrated in the first row of Fig. 2(b) and 2(c), collagen fibers are continuously distributed with high density in the normal tissue. In addition, image-merged MAF and SHG in the first row of Fig. 2(f) shows that the collagen fibers are round in the vicinity of the alveoli regularly in the normal tissue. Fig. 2MAF and SHG imaging for normal pulmonary tissue and pathological pulmonary tissue of C57BL6 mice on the time of days 3, 10, 15, 25 after tail vein injection. (a)–(c) are MAF, SHG and merged MAF and SHG large area images (). The arrows indicate the intact nodules. Scale bar is 400 μm. (d)–(f) are magnified views of marked areas in (a)–(c), respectively. The magnified MAF images reveal that the uniform structure of alveolus has been destroyed gradually and cancer cells appeared after day 15, while the continuous collagen have been ruptured into point-point distribution in the pulmonary metastasis nodules. The arrowheads show cancer cells. Scale bar is 40 μm. (g) HE images of pulmonary tissue at different time points. Scale bar is 40 μm. 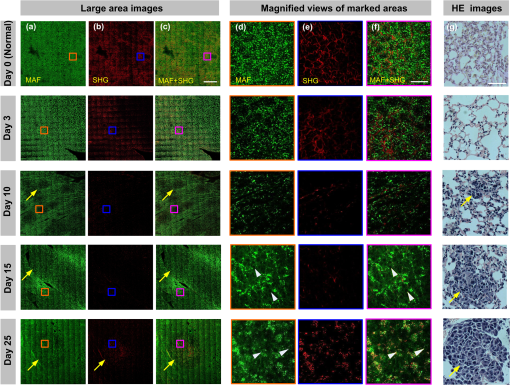 Unlike in the normal tissue, the morphologic features of pathological tissue become disorderly with the time-lapse after tail vein injection. The representative results of day 3, day 10, day 15, and day 25 are also shown in Fig. 2. As illustrated in Fig. 2, the feature of lung tissue at day 3 is similar with the normal tissue, except the low intensity of the MAF and SHG. After day 10, the small nodules appear in the lung tissue, while the profile of alveolus becomes blurry and the collagen fibers become discontinuous. Particularly after day 15, the cancer cells are clearly observed in the MAF image, as indicated by the arrowheads in Fig. 2. MAF and SHG images of intact nodules are shown by the arrows, especially at day 25, which reveals that the structures of alveolus are disappeared and the collagen fibers ruptured into point-point distribution in the pulmonary metastasis nodules. In order to validate the MAF and SHG imaging, HE images at different time points are also shown in the Fig. 2. Compared with normal tissue, the alveolar units disrupt in early stage and disappear in the late stage. At day 10, the cancer cells can be easily distinguished from normal cells for their large and anomalous nuclei. With the time elapsed, the volume of cancer cell nuclei increase rapidly, especially in the late stage. At day 25, the cancer cell nuclei are much larger than the normal cell nuclei and take up the major space of tissue. In addition, the normal lung tissue is abundant in collagen fiber, while the metastasis tissues are rich in vessels and cancer cell nuclei. 3.2.Quantitative AnalysisThe process of the pulmonary melanoma metastasis reflected by morphologic feature can also be quantified. The MAFIR and SHGIR of pathological tissue and normal tissue are shown in the Fig. 3. Combined with Fig. 2, three stages are divided by the time points of nodules appearing in microscope and being macroscopical. As illustrated in Fig. 3, both of the MAF and SHG intensity decrease at early stage from day 0 to day 7. The MAF intensity increases in the middle stage from day 10 to day 15 and decreases again in late stage after day 15, while the SHG intensity reduces to and maintains in a certain level after day 10. The two-tailed student’s -test is performed on the MAFIR and SHGIR matrixs () at different stages, as shown in Fig. 3. The MAFIRs have significant difference, while the SHGIRs cannot be distinguished between each other in each stage. Nevertheless, the results demonstrate that MAFIR provided by LA-NOM can succeed in monitoring the process of the metastasis. Fig. 3MAFIR and SHGIR of pathological pulmonary tissue at different time points. All the MAF and SHG intensity in imaging volume of at different time points were normalized according to the intensity of normal pulmonary tissue. ; error bars indicate standard deviation; asterisk, double asterisk, calculated using a two-tailed student’s -test for the MAFIR and SHGIR in early (days 3, 5, and 7), middle (days 10, 12, and 15, marked by shadow rectangle) and late (days 18, 20, and 25) stages, respectively. 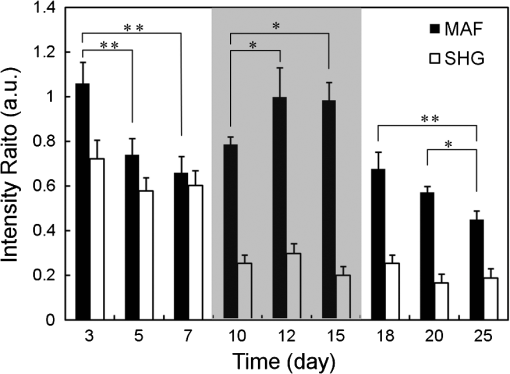 Layer-resolved MAFSI as a function of depth for the specimens excised at different time points are shown in Fig. 4. The markers represent mean of MAFSI data and the curves are the fitting results. The results reveal that both the surface of normal tissue and pathological tissue are rich in collagen fibers and the density of the collagen fiber decreases with the depth in the tissue. The results of -test shown in Fig. 4(a) reveal that MAFSI can be effectively employed to monitor the pathologic stages of lung tissue in early stage without nodules. However, in the middle stage (from day 10 to 15), the MAFSIs have no significant difference at any depth. After day 15, MAFSIs decrease in the tissue surface, but maintain a certain value at depth below 20 μm. The MAFSIs have significant difference at depth of , which can be used to monitor the physiological changes of pulmonary melanoma metastasis in late stage. Fig. 4Layer-resolved MAFSI as a function of depth in normal pulmonary tissue and pathological pulmonary tissue. A two-tailed student’s -test is performed on the MAFSI matrix () at each depth point between different time points. (a)–(c) The MAFSI versus depth in early stage (days 0, 3, 5, and 7), middle stage (days 10, 12, and 15) and late stage (days 18, 20, and 25). The shadow rectangles in (a) and (c) indicate that the curves can be significantly distinguished by the value of -test at special depth in the tissue. Error bars indicate standard error of mean; asterisk, double asterisk, triple asterisk, . 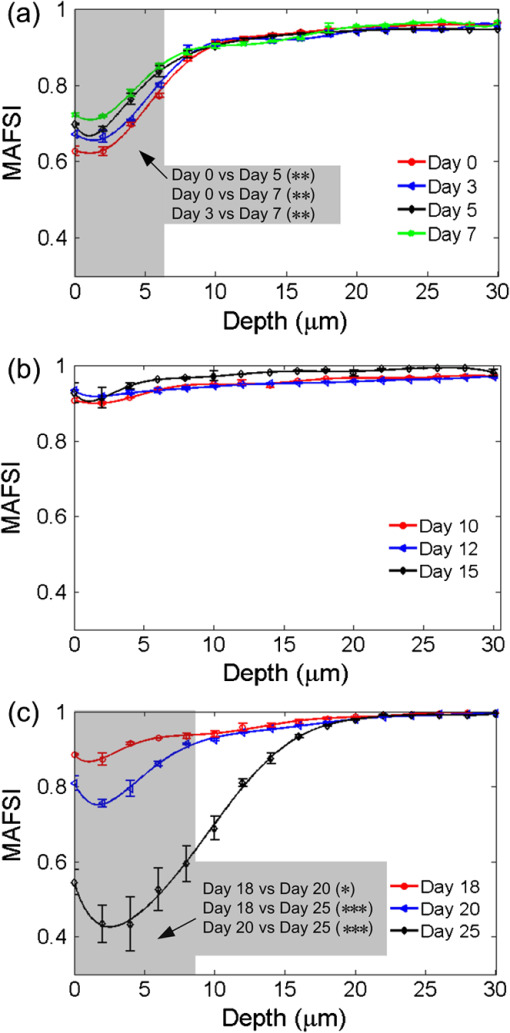 4.DiscussionThe process of cancer metastasis consists of a long series of sequential and interrelated steps: tumor cells detach from the primary tumor, intravasate into the blood vessels or lymphatics, circulate in the body, adhere to vessel wall of distant organs, extravasate vessels, establish a new microenvironment and proliferate at the new site.2 It has been demonstrated that degradation of collagen in ECM can promote invasion and metastasis of cancer cells.29 With the malignant melanoma cells metastasizing into lung tissue, the ECM will be remodeled resulting in the transformation of density and distribution of collagen fibers,18,23,30 which leads to the SHGIR decrease as shown in Fig. 3. The reduction of MAFIR from day 3 to day 7 in Fig. 3 may be induced by the decrease of the normal cells in the tissue, which is also revealed by the HE images from day 3 to day 7. At the middle stage, the proliferation of cancer cells and imposing metabolic stress of the tissue—which are enhanced by the remodeled tissue structure, growth factors released by tissue stroma and no pressure from the surrounding microenvironment—lead to the MAFRI rising from day 10 to day 15.9,31 Eventually, unconstrained proliferation of cancer cell without migration results in large nodules within a “capsule” of ECM, which can induce the cell apoptosis due to the limited availability of nutrients and oxygen.9,32 It has been demonstrated that excessive proliferative signaling can also trigger cell senescence and/or apoptosis.33–35 In addition, the large nucleus of the cancer cell colonizing in the lung tissue cannot provide MAF, which is another reason of MAFRI reduction in later staged after day 18. According to the definition of MAFSI, the inflection point of each curve in Fig. 4 reveals that the density of collagen fibers at the depth of is much higher than that at any other depth. In Fig. 4(a)–4(c), the changes of MAFSIs versus time, especially in the tissue surface, are primarily induced by the cooperation of MAF and SHG intensity discussed above. In order to achieve large area images, the specimens were pressed lightly by a cover slip in the chamber. Our results reveal that this treatment has little influence on the morphologic features of lung tissue. Furthermore, quantitative parameters mentioned above can effectively eliminate this influence, mostly because MAFIR (SHGIR) is calculated using the MAF (SHG) intensity of the whole images stack and MAFSI represents the relative density of cellular chromophores and collagen fibers in each imaging section. The imaging depth of NOM depends on the tissue properties of absorption and scattering, the excitation wavelength, the excitation laser power on the tissue, and the MAF and SHG signals-detection efficiency.36 Generally, the imaging depth of NOM can be up to 100 micrometers in the fresh lung tissue.21,37 In our study, we used the excitation wavelength of 760 nm, which is the optimal excitation wavelength for NAD(P)H and FAD.38 Non-descanned detection, the most efficient fluorescence collection scheme,39 and GaAsP photocathode PMT with high quantum efficiency ( QE at peaking wavelength) used in the home-built system can provide high MAF and SHG signals-detection efficiency. Although, the detailed tissue structural information degrade with the increasing imaging depth, our home-built LA-NOM can allow for imaging depth of in the lung tissue. However, the density of collagen fibers decreases with the depth and maintains a low level in deep lung tissue below 20 μm. Therefore, the MAF and SHG images were only obtained up to 30 μm below the tissue surface in this study. In the middle and late stage of cancer, the macroscopic nodules can be easily discriminated from the lung tissue and its number and volume also can reflect the process of cancer. However, there is no nodule in the early stage of cancer. The physiological changes of the tissue should be evaluated during the whole process of pulmonary melanoma metastasis, especially during the early stage of cancer.2,8,10 In addition, metastasis is a cell- and tissue-driven process, for which cancer cells and ECM interact and react throughout the progression of the disease.9 Therefore, monitoring the progress of pulmonary melanoma metastasis by label-free imaging with submicron spatial resolution is of significance. For monitoring the process of metastasis, in vivo imaging that can eliminate the individual differences between the mice induced by the intrinsic properties of the tumor cells and responses of the host2 can show more natural information than ex vivo imaging. Two techniques can achieve in vivo imaging with subcellular resolution; video-rate microscopy combined with a special window chamber and endoscopy.37,40–42 Nevertheless, to apply these new methods to diagnosis and therapy, understanding the process of metastasis at the cellular level by using conventional full-format microscope is absolutely a prerequisite. The pilot study is helpful for the future in vivo applications of those new methods, such as endoscopy. 5.ConclusionIn summary, we achieved label-free monitoring the progress of pulmonary melanoma metastasis of C57BL/6 mice ex vivo by LA-NOM. Microscopic morphologic changes during the process of pulmonary melanoma metastasis were depicted by MAF and SHG images at different time points. In addition, quantitative parameters, such as MAFRI, SHGRI, and MAFSI were used to quantify the pathological changes of pulmonary melanoma metastasis based on MAF and SHG intensity, especially in early and late stage after tail vein injection. Combining morphological analysis and quantitative analysis of the tissue, our results demonstrate that LA-NOM can be used to monitor the process of pulmonary melanoma metastasis, which indicates that LA-NOM can provide a powerful tool for the research in tumor pathophysiology and therapy evaluation. AcknowledgmentsThis work was supported by National Major Scientific Research Program of China (No. 2011CB910401), National Natural Science Foundation of China (No. 61178077), Program for New Century Excellent Talents in University (No. NCET-08-0216) and Youth Science and Technology Program (No. 201271031424). The authors also thank the Analytical and Testing Center (Huazhong University of Science and Technology) for spectral measurements. ReferencesD. S. Rigel, J. Russak and R. Friedman,
“The evolution of melanoma diagnosis: 25 years beyond the ABCDs,”
CA Cancer J. Clin., 60
(5), 301
–316
(2010). Google Scholar
I. J. Fidler,
“Timeline—the pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited,”
Nat. Rev. Cancer, 3
(6), 453
–458
(2003). http://dx.doi.org/10.1038/nrc1098 NRCAC4 1474-175X Google Scholar
J. H. Lee et al.,
“Biological factors, tumor growth kinetics, and survival after metastasectomy for pulmonary melanoma,”
Ann. Surg. Oncol., 16
(10), 2834
–2839
(2009). http://dx.doi.org/10.1245/s10434-009-0583-5 1068-9265 Google Scholar
E. S. Hwang et al.,
“In vivo tumor suppression activity by T cell-specific T-bet restoration,”
Int. J. Cancer, 127
(9), 2129
–2137
(2010). http://dx.doi.org/10.1002/ijc.25238 IJCNAW 0020-7136 Google Scholar
C. Martinez et al.,
“Experimental model of pulmonary metastasis treatment with IFN alpha,”
Cancer Lett., 225
(1), 75
–83
(2005). http://dx.doi.org/10.1016/j.canlet.2004.11.047 CALEDQ 0304-3835 Google Scholar
H. Prydz et al.,
“Ex vivo and in vivo delivery of anti-tissue factor short interfering RNA inhibits mouse pulmonary metastasis of B16 melanoma cells,”
Clin. Cancer Res., 12
(13), 4055
–4061
(2006). http://dx.doi.org/10.1158/1078-0432.CCR-05-2482 CCREF4 1078-0432 Google Scholar
H. Z. Yang et al.,
“Blocking TLR2 activity attenuates pulmonary metastases of tumor,”
PLOS One, 4
(8), e6520
(2009). http://dx.doi.org/10.1371/journal.pone.0006520 1932-6203 Google Scholar
C. L. Chaffer and R. A. Weinberg,
“A perspective on cancer cell metastasis,”
Science, 331
(6024), 1559
–1564
(2011). http://dx.doi.org/10.1126/science.1203543 SCIEAS 0036-8075 Google Scholar
P. Friedl and S. Alexander,
“Cancer invasion and the microenvironment: plasticity and reciprocity,”
Cell, 147
(5), 992
–1009
(2011). http://dx.doi.org/10.1016/j.cell.2011.11.016 0092-8674 Google Scholar
N. Sethi and Y. Kang,
“Unravelling the complexity of metastasis—molecular understanding and targeted therapies,”
Nat. Rev. Cancer, 11
(10), 735
–748
(2011). http://dx.doi.org/10.1038/nrc3125 NRCAC4 1474-175X Google Scholar
G. Kuttan and C. Manesh,
“Effect of naturally occurring allyl and phenyl isothiocyanates in the inhibition of experimental pulmonary metastasis induced by B16F-10 melanoma cells,”
Fitoterapia, 74
(4), 355
–363
(2003). http://dx.doi.org/10.1016/S0367-326X(03)00055-8 FTRPAE 0367-326X Google Scholar
G. Kuttan and C. Guruvayoorappan,
“Effect of amentoflavone on the inhibition of pulmonary metastasis induced by B16F-10 melanoma cells in C57BL/6 mice,”
Integr. Cancer Ther., 6
(2), 185
–197
(2007). http://dx.doi.org/10.1177/1534735407302345 ICTNAY 1534-7354 Google Scholar
M. H. Franket et al.,
“Isolation of tumorigenic circulating melanoma cells,”
Biochem. Biophys. Res. Commun., 402
(4), 711
–717
(2010). http://dx.doi.org/10.1016/j.bbrc.2010.10.091 BBRCA9 0006-291X Google Scholar
Y. Fang et al.,
“Anticancer properties of 10-hydroxycamptothecin in a murine melanoma pulmonary metastasis model in vitro and in vivo,”
Toxicol. In Vitro, 25
(2), 513
–520
(2011). TIVIEQ Google Scholar
W. G. Seo et al.,
“Suppressive effect of Zedoariae rhizoma on pulmonary metastasis of B16 melanoma cells,”
J. Ethnopharmacol., 101
(1–3), 249
–257
(2005). http://dx.doi.org/10.1016/j.jep.2005.04.037 JOETD7 0378-8741 Google Scholar
W. Denk, J. H. Strickler and W. W. Webb,
“Two-photon laser scanning fluorescence microscopy,”
Science, 248
(4951), 73
–76
(1990). Google Scholar
W. W. Webb et al.,
“Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation,”
Proc. Natl. Acad. Sci., 100
(12), 7075
–7080
(2003). http://dx.doi.org/10.1073/pnas.0832308100 0369-3236 Google Scholar
N. D. Kirkpatrick, M. A. Brewer and U. Utzinger,
“Endogenous optical biomarkers of ovarian cancer evaluated with multiphoton microscopy,”
Cancer Epidem. Biomar., 16
(10), 2048
–2057
(2007). http://dx.doi.org/10.1158/1055-9965.EPI-07-0009 CEBPE4 1055-9965 Google Scholar
C. C. Wang et al.,
“Label-free discrimination of normal and pulmonary cancer tissues using multiphoton fluorescence ratiometric microscopy,”
Appl. Phys. Lett., 97
(4), 043706
(2010). http://dx.doi.org/10.1063/1.3460913 APPLAB 0003-6951 Google Scholar
L. M. Loew and P. J. Campagnola,
“Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms,”
Nat. Biotechnol., 21
(11), 1356
–1360
(2003). http://dx.doi.org/10.1038/nbt894 NABIF9 1087-0156 Google Scholar
M. C. Schanne-Klein et al.,
“Three-dimensional investigation and scoring of extracellular matrix remodeling during lung fibrosis using multiphoton microscopy,”
Microsc. Res. Tech., 70
(2), 162
–170
(2007). http://dx.doi.org/10.1002/jemt.v70:2 MRTEEO 1059-910X Google Scholar
M. B. Lilledahl et al.,
“Characterization of vulnerable plaques by multiphoton microscopy,”
J. Biomed. Opt., 12
(4), 044005
(2007). http://dx.doi.org/10.1117/1.2772652 JBOPFO 1083-3668 Google Scholar
C. D. Davies et al.,
“Second-harmonic generation in collagen as a potential cancer diagnostic parameter,”
J. Biomed. Opt., 13
(5), 054050
(2008). http://dx.doi.org/10.1117/1.2983664 JBOPFO 1083-3668 Google Scholar
X. Jian, S. Zhuo and J. Chen,
“Diagnostic application of multiphoton microscopy in epithelial tissues,”
J. Innov. Opt. Health Sci., 4
(2), 159
–163
(2011). Google Scholar
P. Wilder-Smith et al.,
“In vivo multiphoton fluorescence imaging: a novel approach to oral malignancy,”
Laser Surg. Med., 35
(2), 96
–103
(2004). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
N. Ramanujam et al.,
“Multiphoton microscopy of endogenous fluorescence differentiates normal, precancerous, and cancerous squamous epithelial tissues,”
Cancer Res., 65
(4), 1180
–1186
(2005). http://dx.doi.org/10.1158/0008-5472.CAN-04-3031 CNREA8 0008-5472 Google Scholar
C. C. Wang et al.,
“Early development of cutaneous cancer revealed by intravital nonlinear optical microscopy,”
Appl. Phys. Lett., 97
(11), 113702
(2010). http://dx.doi.org/10.1063/1.3490644 APPLAB 0003-6951 Google Scholar
W. Zheng et al.,
“Diagnostic value of nonlinear optical signals from collagen matrix in the detection of epithelial precancer,”
Opt. Lett., 36
(18), 3620
–3622
(2011). http://dx.doi.org/10.1364/OL.36.003620 OPLEDP 0146-9592 Google Scholar
L. M. Matrisian and C. E. Brinckerhoff,
“Timeline—matrix metalloproteinases: a tail of a frog that became a prince,”
Nat. Rev. Mol. Cell Biol., 3
(3), 207
–214
(2002). http://dx.doi.org/10.1038/nrm763 NRMCBP 1471-0072 Google Scholar
F. Kheradmand, K. J. Greenlee and Z. Werb,
“Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted,”
Physiol. Rev., 87
(1), 69
–98
(2007). http://dx.doi.org/10.1152/physrev.00022.2006 PHREA7 0031-9333 Google Scholar
S. Alexander and P. Friedl,
“Cancer invasion and resistance: interconnected processes of disease progression and therapy failure,”
Trends. Mol. Med., 18
(1), 13
–26
(2012). http://dx.doi.org/10.1016/j.molmed.2011.11.003 TMMRCY 1471-4914 Google Scholar
G. P. Gupta and J. Massague,
“Cancer metastasis: building a framework,”
Cell, 127
(4), 679
–695
(2006). http://dx.doi.org/10.1016/j.cell.2006.11.001 0092-8674 Google Scholar
S. W. Lowe, E. Cepero and G. Evan,
“Intrinsic tumour suppression,”
Nature, 432
(7015), 307
–315
(2004). http://dx.doi.org/10.1038/nature03098 NATUAS 0028-0836 Google Scholar
M. Collado and M. Serrano,
“Senescence in tumours: evidence from mice and humans,”
Nat. Rev. Cancer, 10
(1), 51
–57
(2010). http://dx.doi.org/10.1038/nrc2772 NRCAC4 1474-175X Google Scholar
D. Hanahan and R. A. Weinberg,
“Hallmarks of cancer: the next generation,”
Cell, 144
(5), 646
–674
(2011). http://dx.doi.org/10.1016/j.cell.2011.02.013 0092-8674 Google Scholar
A. K. Dunn et al.,
“Influence of optical properties on two-photon fluorescence imaging in turbid samples,”
Appl. Opt., 39
(7), 1194
–1201
(2000). http://dx.doi.org/10.1364/AO.39.001194 APOPAI 0003-6935 Google Scholar
M. R. Looney et al.,
“Stabilized imaging of immune surveillance in the mouse lung,”
Nat. Methods, 8
(1), 91
–96
(2011). http://dx.doi.org/10.1038/nmeth.1543 NMAEA3 1548-7091 Google Scholar
W. W. Webb, S. H. Huang and A. A. Heikal,
“Two-photon fluorescence spectroscopy and microscopy of NAD(P)H and flavoprotein,”
Biophys. J., 82
(5), 2811
–2825
(2002). http://dx.doi.org/10.1016/S0006-3495(02)75621-X BIOJAU 0006-3495 Google Scholar
W. R. Zipfel, R. M. Williams and W. W. Webb,
“Nonlinear magic: multiphoton microscopy in the biosciences,”
Nat. Biotechnol., 21
(11), 1368
–1376
(2003). http://dx.doi.org/10.1038/nbt899 NABIF9 1087-0156 Google Scholar
L. Fu et al.,
“Nonlinear optical endoscopy based on a double-clad photonic crystal fiber and a MEMS mirror,”
Opt. Express, 14
(3), 1027
–1032
(2006). http://dx.doi.org/10.1364/OE.14.001027 OPEXFF 1094-4087 Google Scholar
Y. Liu et al.,
“Visualization of hepatobiliary excretory function by intravital multiphoton microscopy,”
J. Biomed. Opt., 12
(1), 014014
(2007). http://dx.doi.org/10.1117/1.2710237 JBOPFO 1083-3668 Google Scholar
X. D. Li et al.,
“Scanning all-fiber-optic endomicroscopy system for 3D nonlinear optical imaging of biological tissues,”
Opt. Express, 17
(10), 7907
–7915
(2009). http://dx.doi.org/10.1364/OE.17.008382 OPEXFF 1094-4087 Google Scholar
|


