|
|
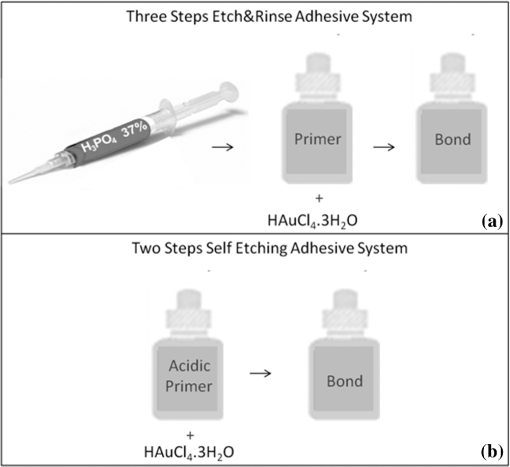
1.IntroductionContemporary restorative procedures are based on the adhesive properties of tooth colored resin-based materials.1 Even though several studies revealed excellent immediate and short-term bonding effectiveness of dental adhesives,2 bond stability for a long time period still remains an important concern.3 Stable bonding is created by full impregnation of the dentin substrate by blends of resin monomers, thus avoiding different degrees of incomplete impregnation4,5 and producing a compact and homogenous hybrid layer.1 This Hybrid layer is created by a mixture of the dentin organic matrix, residual hydroxyapatite crystallites, resin monomers, and the solvent. Each of these individual components may be affected by aging and/or degradation phenomena within the hybrid layer.1 There are two known degradation patterns: degradation of resin (hydrolysis) and degradation of exposed collagen fibrils (disorganization).6 Hybrid layer degradation may increase the water content of the bonded interface which has been claimed as one of the major causes for collagen breakdown.1 A variety of photo-induced synthetic methods for producing metal nanoparticles and nanostructures have been developed to obtain monometallic and bimetallic nanoparticles, and composite materials. In particular, it is known that direct photoreduction of results in formation of gold nanoparticles in aqueous solution7 and polymer matrices.8 Gold nanoparticles are key materials in nanoscience and nanotechnology due to their strong absorption and scattering derived from an intense localized plasmonic resonance.9–11 This plasmonic resonance can be tuned over a broad spectral range in various gold nanostructures (such as nanorods and nanoshells) Gold is biocompatible and nanoparticles can be fabricated with excellent colloidal stability. Because of these features, gold nanoparticles have extensively been used for bioimaging. In particular, multiplexed dark-field imaging as well as near-infrared-enabled deep tissue imaging can be achieved. Additionally, they can be surface-functionalized with biomolecules and used for targeted delivery to specific cells.12–14 Gold nanoparticles have also been explored in the biomedical field to reduce the pore size of collagen, to reduce collagenase biodegradation formation of multiple cross-links with the collagen structure.15 Optical coherence tomography (OCT) is a relatively new imaging modality that uses near-infrared broad band light sources to scan tissues. These systems offer cross-sectional and 3D images with resolution on the order of few microns and a depth of penetration of one to two millimeters. It generates a signal based on the presence of refractive index mismatches and scattering events within the imaged volume. In particular, an agent that modifies the scattering properties of a sample can improve the image contrast. Contrast agents could lead to improvement of in situ OCT image quality to a degree comparable to biopsy, reducing the need to remove, section, and stain tissues.10 Here, we demonstrate the potential use of gold nanoparticles as contrast agents for OCT in dentistry. Spherical gold nanoparticles were produced inside the hybrid layer and tubules by a photothermal reduction in situ. Gold ions were dispersed in the primer of several dental bonding systems. A new procedure was developed to simultaneously reduce the gold ions and photopolymerize the adhesive system. Furthermore, OCT was used to visualize dentin structures in a non-invasive and non-destructive way, and to identify the gold nanoparticle distribution within the tissue. 2.Methodology2.1.Dental Bonding Agent PreparationTwo commercially available dental bonding agents (DBAs) were modified in this study. Adper Scotchbond Multi-Purpose from 3M ESPE Corporation is a three-step etch and rinse adhesive which removes the smear layer completely and demineralizes the subsurface of dentin with phosphoric acid. Adhese from Ivoclair Vivadent is a two-step self-etch adhesive, which incorporates the smear layer into the hybrid layer via the use of an acidic primer, and does not require further rinsing. In spite of this difference, for both agents, the bond and the primer are in different vials. Therefore, the gold precursor can be added only to the primer and the bond is applied as a final solvent-free coating. Figure 1 shows the steps for the preparation of the modified adhesive systems. The gold precursor () was purchased from Sigma-Aldrich. Due to the hygroscopic nature of the gold precursor, it was kept in a glove box with a dry argon atmosphere. The gold precursor was dissolved in a small amount of acetone. The DBA primer/gold precursor solution was mixed in a glove box and stirred for 20 min, then ultrasonicated for 5 min. 2.2.Tooth PreparationHuman third molars were collected from the Teeth Bank of the Universidade Federal de Pernambuco. The teeth were stored in distilled water until the start of the experiment. The occlusal one-third of each tooth was removed by means of a diamond saw (Isomet 1000) under water lubrication. The teeth were sectioned perpendicularly to the longitudinal axis, 1 to 1.5 mm into the sample, removing the enamel to obtain a superficial dentin. The dentin surfaces were polished with 600-grit SiC paper (DP 10 from Panambra) for one minute under running water to create a standardized smear layer, as described in Ref. 16, and stored in water for one week. The sectioned teeth were randomly distributed into four groups corresponding to the adhesive materials used: Adper Scotch Bond Multi-Purpose and Adhese were each tested with and without the gold nanoparticle precursor. Both adhesives were applied following the manufacturers’ instructions. In our procedure, warm air () was used to improve the evaporation of the solvent as suggested previously,17 as well as to accelerate the reduction of gold nanoparticles in the primer of the experimental adhesive systems. Light curing for 60 s from a commercially available light source (Ultrablue LED/DMC) was used to photopolymerize the DBA concurrently with formation of the gold nanoparticles. 2.3.Scanning Electron Microscopy ImagingScanning electron microscopy (SEM) was used to characterize the gold nanoparticle formation. A Hitachi S-4000 field-emission scanning electron microscope in the back scattered mode and secondary electron emission mode was used. 2.4.OCT ImagingSamples were imaged by the OCT technique. A commercial spectral optical coherence tomography instrument, the Ganymede system from Thorlabs, was used to produce cross-sectional and volumetric optical imaging of the samples. The system is based on a Michelson interferometer configuration. The main unit contains a super luminescent diode (SLD) light source. A fiber-optic coupler was used to direct the light from the broadband SLD source to the scanner probe. The central wavelength of the SLD was 930 nm, with a spectral width of 100 nm, providing an axial resolution of 5 μm. With an A-scan rate of 29 kHz, the system can produce 29 frames per second (fps) with 512 lines per frame. At this speed, it can rapidly generate 2D and 3D images of the samples. The images were taken by scanning the oclusal surface in a bucco-lingual direction. The long wavelength light penetrated deep into the dentin, and a tomographic image (OCT slice) parallel to the long axis of teeth was obtained. 3.Results and DiscussionIn dental restorative techniques, stable bonds are obtained by full impregnation of the dentin substrates with the adhesive. The stability of the bonded interface is based on the formation of a homogeneous hybrid layer. Preparation of tooth with dental burs creates a smear layer of debris into the dentin. An adhesive system removes or treats the smear layer, creating a hybrid layer of resin bond, collagen fibers, dentin surface structure and intertubular structure. Over the hybrid layer, an adhesive layer forms. Under the hybrid layer, resin tags are established into the tubules.1,4,5 To confirm the formation and distribution of gold nanoparticles in the bonded dentin structure, SEM and OCT were performed. The SEM images in Fig. 2 show the presence of gold nanoparticles within the dentinal tubules. Nine randomly selected teeth were prepared and sectioned, then subjected to SEM evaluation. The fracture of the specimens avoided the formation of a new smear layer. The dentin contains many microscopic tubules of 0.5 to 4.0 µm diameter which extend parallel to each other from the pulp chamber to the amelo- or cement-dentinal junctions.18,19 Gold nanoparticles were observed in the dentin with diameters of 40 to 120 nm. No difference was observed in the production of gold nanoparticles with the two adhesive systems used. In the photoreduction procedure, gold was not expected to interfere with the penetration of the modified DBA into dentinal tubules since the nanoparticles are formed after DBA application and light irradiation. Fig. 2SEM images of gold nanoparticles (arrows) showing penetration into dentinal tubules. Dentin () and tubules () are identified. 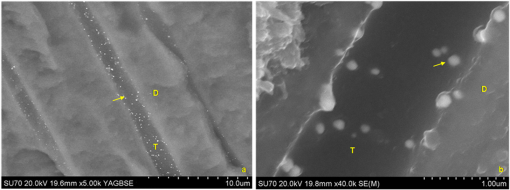 OCT images of the samples with and without gold nanoparticles were obtained. Figure 3 shows a typical OCT cross-sectional image of the dental bonding structure. Hyperintense regions of OCT images represent highly scattering portions and high levels of direct backscatter of light due to the presence of gold nanoparticles within the sampled volume. In Fig. 3, this information is displayed in false colors, using warm and cool colors for higher and lower scattering intensities, respectively. The data displayed in false colors allows better distinguishability of different image sections, since on a colored image, different shades of color and intensity can be identified.20 In Fig. 3, all the regions of the adhesion process are identifiable: adhesive layer, hybrid layer, and resin tags. OCT measurements revealed a hybrid layer thickness of 56 to 89 μm. On the average, Resin Tags extended 150 μm deep into the dentin tissue, with an extreme depth value of 350 μm observed in Fig. 4. As consequence of the localized variation of the dentin structure,21 the resin penetration is not uniformly distributed. Fig. 3OCT images of the adhesion process on dentin using DBAs prepared with gold nanoparticles. The adhesion process regions are identified: adhesive layer (AL), hybrid layer (HL), and resin tags (arrows). 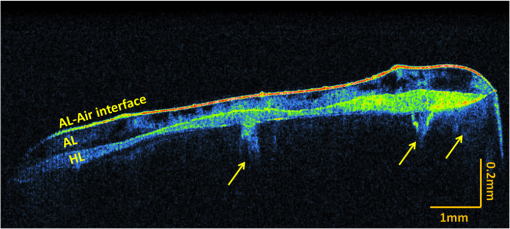 Fig. 4Three-dimensional reconstruction with (a) sectional and (b) volume rendering views. The adhesive layer (AL), hybrid layer (HL), and resin tags (RT) are identified. 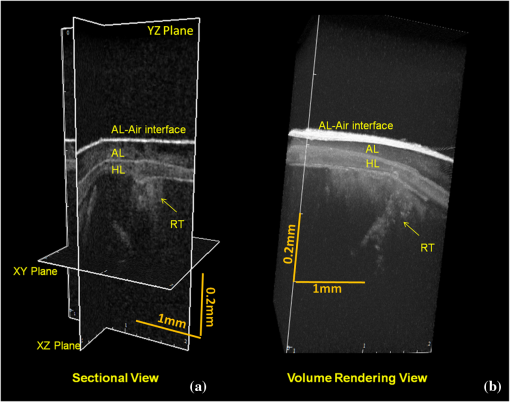 In the OCT images of the dental adhesive systems without nanoparticles, the interface between the adhesive layer and the hybrid layer was not observable. Moreover, we were not able to identify the resin tags and the hybrid layer extension in the samples without gold particles. OCT was also performed in 3D imaging mode. In this mode, the OCT software acquires multiple adjacent ( direction) 2D OCT frames (slices) in the plane, forming a stack of images as a 3D data set. The 3D reconstruction can be examined in a sectional or volume rendering view, as shown in Fig. 4. The sectional view projects optical sections to one plane, or section level, in the , or planes as shown in Fig. 4(a). The volume rendering view is the full 3D volume representation shown in Fig. 4(b). The image background often limits the visibility of structures, but one can get a better understanding of the sample volume configuration by the use of various rendering methods. Selected section planes of the OCT 3D images are shown in Fig. 5. With the and section planes, one can identify the limits of the three bonding regions, while the section exhibits the distribution and characterization of gold nanoparticles in each adhesion layer. The image plane of the adhesive layer shows the formation of gold nanoparticles, but not in a homogenous way. The image plane of the hybrid layer shows a homogenous distribution of the nanoparticles. The section plane of the resin tags shows that the nanoparticles were formed only in some limited regions. Fig. 5OCT sectional reconstruction of each bonding region: adhesive layer, hybrid layer and resin tags (left to right). The plane is and the Plane is . 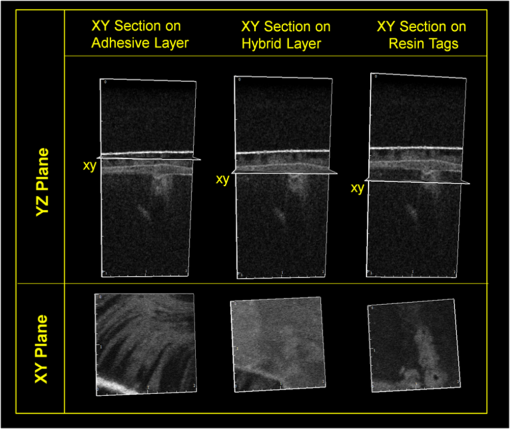 The OCT imaging technique also allowed the identification of cavities surrounded by the demineralized area in the dentin structure, shown as a white spot lesion, that were not observed by the naked eye during tooth preparation. The white spot lesion is the manifestation of earlier local destruction of hard tissue due to a non-continuous demineralization processes. Nanoparticles can have the same dimensions as those of the missing hydroxyapatite and therefore have the potential to infiltrate deep into demineralized area. Figure 6 shows that by OCT characterization, one can identify a high concentration of gold nanoparticles not only at the surface, but also into all demineralized areas surrounding a small cavity in dentin. A 3D image in the attenuation mode exhibits a global view of the dentin sample and is presented in Fig. 6(a). One can identify a cavity in the dentin structure. The extension of the cavity is characterized by a cross-section image in the plane shown in Fig. 6(b). The high-intensity areas surrounding the small cavity are associated with a higher concentration of the nanoparticles. The gold nanoparticle distribution is identified with clip-plane views in the plane from the surface to the internal region, as shown in Fig. 6(c). Fig. 6An OCT image of the dentin structure: (a) 3D attenuation mode, , (b) cross-section in the plane, and (c) clip-plane views in the plane from the surface (left) to the internal region (right). 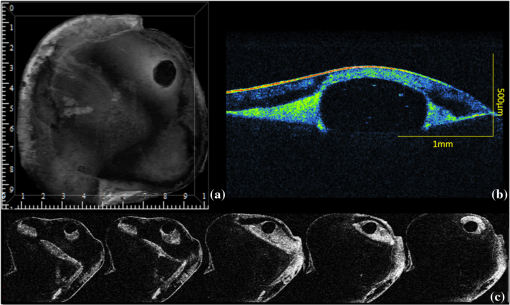 We developed a gold nanoparticle/polymer hybrid material by photo-induced gold reduction in conjunction with current dental bonding agents. The metallic nanoparticles were evaluated as a contrast agent for OCT, and allowed the structural characterization of adhesive and hybrid layers which were not distinguishable in samples without nanoparticles. In the future, contrast agents could be explored to identify dental bonding materials under restorative material layers. Besides serving as contrast agents for optical imaging techniques, gold nanoparticles may be functionalized to provide sites in the surface with specialized functionality. Many works have already presented positive results about crosslinking between type I collagen and gold nanoparticles in biomedicine.15,21 In particular, type I collagen is one of the main components and represents the backbone of the dentin organic matrix,22 thus different approaches to slow or prevent the disintegration of the collagen network is pivotal to increase bonding stability. 4.ConclusionIn summary, we have demonstrated photo-induced gold reduction in dental bonding agents in situ to obtain a gold nanoparticle/polymer hybrid dental bonding material. The formation of the gold nanoparticles was characterized by scanning electron microscopy (SEM) and OCT. With OCT sectional reconstruction, we observed distinct distributions of nanoparticles in each bonding region. The bonding structures were identified and measured, finding a maximum penetration of 350 μm into the dentin tissue. We demonstrated the use of gold nanoparticles as contrast enhancing agent for OCT imaging in dentistry. Contrast agents for OCT produces a better image quality, allowing an improvement in the identification of tooth structures, and therefore extending the application of this technique to numerous diagnostic procedures. AcknowledgmentsThis study was supported by a grant and scholarship from CAPES, by the Center of Excellence in Nanophotonics and Biophotonics (PRONEX/FACEPE/CNPq), INCT Fotonica (CNPq) and the United States Air Force Office of Science and Research. It is also supported by the Photonics National Institute of Science and Technology. ReferencesL. Breschi et al.,
“Dental adhesion review: aging and stability of the bonded interface,”
Dent. Mater., 24
(1), 90
–101
(2008). http://dx.doi.org/10.1016/j.dental.2007.02.009 DEMAEP 0109-5641 Google Scholar
S. Inoue et al.,
“Micro-tensile bond strength of eleven modern adhesives to dentin,”
J. Adhes. Dent., 3
(3), 237
–245
(2001). 1461-5185 Google Scholar
J. De Munck et al.,
“A critical review of the durability of adhesion to tooth tissue: methods and results,”
J. Dent. Res., 84
(2), 118
–132
(2005). http://dx.doi.org/10.1177/154405910508400204 JDREAF 0022-0345 Google Scholar
H. Sano et al.,
“Microporous dentin zone beneath resin-impregnated layer,”
Operat. Dent., 19
(2), 59
–64
(1994). 0361-7734 Google Scholar
Y. Yoshida et al.,
“A novel approach to AFM characterization of adhesive tooth- biomaterials interfaces,”
J. Biomed. Mater. Res., 47
(1), 85
–90
(1999). http://dx.doi.org/10.1002/(ISSN)1097-4636 JBMRBG 0021-9304 Google Scholar
M. Hashimoto et al.,
“In vitro degradation of resin-dentin bonds analyzed by microtensile Bond test, scanning and transmission electron microscopy,”
Biomater., 24
(21), 3795
–3803
(2003). http://dx.doi.org/10.1016/S0142-9612(03)00262-X BIMADU 0142-9612 Google Scholar
M. Sakamoto, M. Fujistuka and T. Majima,
“Light as a construction tool of metal nanoparticles: Synthesis and mechanism,”
J. Photochem. Photobiol. C, 10
(1), 33
–56
(2009). http://dx.doi.org/10.1016/j.jphotochemrev.2008.11.002 1389-5567 Google Scholar
S. Shukla et al.,
“Subwavelength direct laser patterning of conductive gold nanostructures by simultaneous photopolymerization and photoreduction,”
ACS Nano, 5
(3), 1947
–1957
(2011). http://dx.doi.org/10.1021/nn103015g 1936-0851 Google Scholar
J. Zhou et al.,
“Functionalized gold nanoparticles: synthesis, structure and colloid stability,”
J. Colloid Interface Sci., 331
(2), 251
–262
(2009). http://dx.doi.org/10.1016/j.jcis.2008.12.002 JCISA5 0021-9797 Google Scholar
T. S. Troutman,
“Plasmon resonant nanostructures of gold for biomedical applications,”
The University of Arizona,
(2008). Google Scholar
P. N. Prasad, Nanophotonics, John Wiley & Sons, New York
(2004). Google Scholar
H. Zhang and M. E. Meyerhoff,
“Gold coated magnetic particles for solid phase immunoassays: enhancing immobilized antibody binding efficiency and analytical performance,”
Anal. Chem., 78
(2), 609
–616
(2006). http://dx.doi.org/10.1021/ac051720x ANCHAM 0003-2700 Google Scholar
M. Everts et al.,
“Covalently linked Au Nanoparticles to a viral vector: potential for combined photothermal and gene cancer therapy,”
Nano Lett., 6
(4), 587
–591
(2006). http://dx.doi.org/10.1021/nl0500555 NALEFD 1530-6984 Google Scholar
J. Chen et al.,
“Immuno gold nanocages with tailored optical properties for targeted photothermal destruction of cancer cells,”
Nano Lett., 7
(5), 1318
–1322
(2007). http://dx.doi.org/10.1021/nl070345g NALEFD 1530-6984 Google Scholar
L. Castaneda et al.,
“Collagen cross-Linking with Au nanoparticles,”
Biomacromolecules, 9
(12), 3383
–3388
(2008). http://dx.doi.org/10.1021/bm800793z BOMAF6 1525-7797 Google Scholar
R. P. Chappel, P. Spencer and J. D. Eick,
“The effects of current dentinal adhesives on the dentinal surface,”
Quintessence International, 25
(12), 851
–859
(1994). DEDIAY 0011-8567 Google Scholar
C. A. Klein-Júnior et al.,
“Evaporating solvents with a warm air-stream: effects on adhesive layer properties and resin-dentin bond strength,”
J. Dent., 36
(8), 618
–625
(2008). http://dx.doi.org/10.1016/j.jdent.2008.04.014 0300-5712 Google Scholar
S. N. Bhaskar, Histologia e Embriologia Oral de Orban, 10th ed.Ed Artes Médicas, São Paulo
(1989). Google Scholar
D. H. Pashley,
“Clinical correlations of dentin structure and function,”
J. Prosthet. Dent., 66
(6), 777
–781
(1991). http://dx.doi.org/10.1016/0022-3913(91)90414-R JPDEAT 0022-3913 Google Scholar
B. B. C. Kyotoku,
“Desenvolvimento de um Sistema de Imageamento Usando a Tomografia por Coerência Óptica no Domínio Temporal e de Fourier,”
Universidade Federal de Pernambuco,
(2006). Google Scholar
M. A. Haidekker et al.,
“Influence of gold nanoparticles on collagen fibril morphology quantified using transmission electron microscopy and image analysis,”
BMC Med. Imag., 6
(4), 1
–7
(2006). BMIMA3 1471-2342 Google Scholar
C. Lin, W. H. Douglas and S. L. Erlandesen,
“Scanning electron microscopy of type I collagen at the dentin–enamel junction of human teeth,”
J. Histochem. Cytochem., 41
(3), 381
–388
(1993). http://dx.doi.org/10.1177/41.3.8429200 JHCYAS 0022-1554 Google Scholar
|