|
|
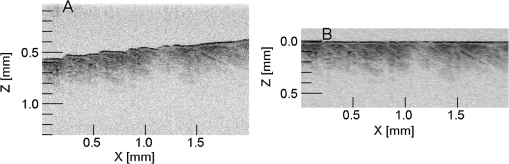
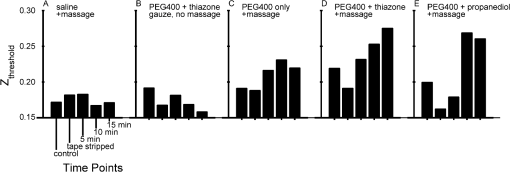
1.IntroductionFor a strong light-scattering tissue like skin, ballistic and quasi-ballistic photons of incident light cannot penetrate deeply into the tissue, which limits the ability of many optical imaging methods to image deeply. Imaging methods such as optical coherence tomography (OCT),1,2 confocal reflectance/fluorescence microscopy,3 second-harmonic generation microscopy,4 and 2-photon microscopy5 are limited by the optical scattering properties of the skin to superficial depths. Optical clearing using high refractive index and hyperosmolarity agents can reduce the scattering of biological tissues. With this approach, better optical imaging depth and contrast have been presented6,7 and deeper optical treatment has been achieved.8,9 An effective way to achieve optical clearing in in vitro experiments is to immerse an excised skin sample in an optical clearing agent (OCA) with high refractive index and hyperosmolarity. By immersion, the OCA directly interacts with the dermis, inducing refractive index matching,10,11 dehydration,12–15 collagen fiber dissociation,16–18 or anisotropy factor increase,19 which all contribute to reducing the effective scattering in skin. However, non-invasive optical clearing of skin in vivo is more difficult. The outermost layer of the skin, the stratum corneum, presents a significant barrier to topically applied OCAs and is hence responsible for the poor optical clearing effect. To break the barrier of the stratum corneum, multiple penetration enhancing methods have been introduced, such as chemical enhancers,16,20 ultrasound,21,22 microneedles,23 flashlamp,24,25 laser fractional ablation,26 and photo-irradiation.27 Also, physical massage is commonly known to enhance penetration of topical agents into skin. Recently, we reported imaging dermal blood flow on rat dorsal skin by optical clearing using PEG-400 with the chemical penetration enhancers thiazone and 1,2-propanediol.28,29 Optical coherence tomography (OCT) has the ability to image tissues in different depth in vivo.30,31 This ability makes OCT a powerful tool to monitor the optical clearing process in tissues. In this paper, OCT imaging was applied to monitor the optical clearing of rat skin in vivo. The optical clearing efficacy was assessed quantitatively by analyzing the OCT reflectance from different depths. The synergistic effect of an OCA (PEG-400), two chemical penetration enhancers (tiazone and 1,2-propanediol), and physical massage on optical clearing was tested. 2.Materials and MethodsA commercial OCT system (model OCP930SR, Thorlabs, Newton, NJ, USA) working at with FWHM, an optical power of 2 mW, a focal length of 20 mm, a maximum image depth of 1.6 mm, a lateral resolution of 20 μm, and an axial resolution of 6.2 μm in air was used in this investigation. The image depth did not exceed 1 mm. The sensitivity drop-off versus depth of the SDOCT system was modest over this 1 mm range, and was ignored. An OCA, PEG-400 (refractive index: 1.47, Tianjin Kermel Chemical Reagent Co., Ltd. Tianjing, China), and two kinds of chemical penetration enhancers, thiazone {[benzisothiazol-3(2H)-one-2-butyl-1,1-dioxide], refractive index: 1.47, Guangzhou Heming Trading Co., Ltd. Guangzhou, China} and 1,2-propanediol (refractive index: 1.43, Tianjin Guangcheng Chemical Reagent Co., Ltd. Tianjing, China) were used in this work. PEG-400 was premixed with each chemical penetration enhancers at a PEG-400: enhancer volume ratio of . Fifteen male 4-week-old Sprague-Dawley rats were obtained from the biological department of Saratov State University. Rats were anaesthetized with zoletil via intramuscular injection. The dorsal hair was shaved, and the residue hair was removed using depilatory cream. The skin was then tape-stripped 5 to 7 times with an adhesive tape to clean the skin surface and to remove the residual epilatory cream, which is important to achieve the optical clearing effect. In humans, usually more than 30 tape-strippings are required to remove stratum corneum.32 The rat skin stratum corneum is also several cell layers thick, and also requires many tape-strippings for removal and enhanced permeability. The middle region of the back lateral to the backbone was chosen for OCAs treatment. Treatment sites were kept the same place for all rats. Four protocols used either PEG-400 with thiazone, PEG-400 with propanediol, PEG-400 only, or saline only. To improve the penetration of the OCAs, a soft massage was applied by moving a small plastic rod back and forth on the site of OCA application throughout the application period. A fifth protocol served as a massage-free control, with PEG-400 plus thiazone applied by using a saturated gauze without massage. Three rats were used for each of the five protocols for a total of 15 rats. For each area of application, B-scan images were taken for the intact skin, skin after stripping, 5, 10, and 15 min after agent application. Prior to each time of imaging, the skin surfaces were wiped clean of OCAs with Kimwipes™ (Shanghai ANPEL Scientific Instrument Co., Ltd. Shanghai, China). OCAs were then re-applied after the imaging. The time of imaging (5 min for handling and image acquisition) was not included in the reported OCA application time. During the experiments, the anesthetized rats were fixed on a platform and the OCT probe was mounted on a rotating arm that when swung into position always measured the same skin site on the rat. Three duplicate images were acquired for each time point. Animal movements during breathing sometimes caused a fluctuation in the images, but subsequent image analysis identified the skin surface hence occasional movements were easily corrected. The acquisition time for each frame was 125 ms, with a 2-s interval between duplicated acquisitions. The mean relative standard deviation of local reflectance between three duplicated acquisitions was 7.5%. The OCT reflectance was calibrated by using a glass-glycerol interface created by a drop of glycerol adjacent to a glass coverslip. Calibration of the OCT system was accomplished by (1) establishing the focus function of the objective lens, and (2) establishing the calibration constant, CALIB, of the system. The focus function is the sensitivity of the OCT reflectance measurement as a function of the distance of the measurement depth () from the focus of objective lens (), . The skin surface defined and was located in the upper third of the OCT imaging range. The was specified by moving a glass/air interface over the axial scanning range of the OCT system. The CALIB was specified by measuring the OCT signal from a glass/glycerol interface at , [counts], which corresponded to a reflectivity of . Then OCT measurements on skin, [counts], were adjusted to yield reflectance , where for a mirror. Since the OCT signal is proportional to the square root of the diffuse reflectance,33 the reflectance was calculated as proportion to the square of OCT signal: After image acquisition, the skin surface was flattened by image analysis to enable averaging of the profile over the whole image. The flattening procedure involved finding the position of the skin surface at each lateral position in the image, and then translating the column of pixels at that to bring the skin surface to a common axial position. Figure 1 shows an example of flattening the skin by image analysis. The flattening procedure is done after first correcting for the focus function of the system, i.e., correcting the axial dependence of the image intensity as a function of distance from the focus of the objective lens. The numerical aperture of the OCT system is low (, after accounting for the refraction at the air/skin surface), so the focal length of the system should not be an issue with regard to the flattening procedure. The flattening procedure will distort the tissue structure somewhat because it shifts pixels so that the surface is aligned at one constant -axis position. But given the one-dimensionality of the skin, and because the study addresses the one-dimensional penetration of light into the skin, such spatial distortion has minimal effect on the deduced light penetration. 3.ResultsFigure 2 shows the typical results for topical application of optical clearing agents by 5 different treatment protocols. A dark color represents a strong OCT reflectance and a light color represents a weaker signal due to lower reflection by the tissue in the focus and/or stronger signal attenuation to/from a particular depth . The OCT signal falls as a function of depth in the dermis. The images did not change significantly after cleaning by tape-stripping with adhesive tape. With optical clearing, the upper image of the dermis becomes lighter and the boundary between the epidermis and dermis becomes less apparent. As optical clearing develops, the superficial layers of the dermis become lighter, which means less scattering is occurring, hence photons can travel deeper to image. For the groups of PEG-400 with both chemical penetration enhancers and soft massage, the most significant optical clearing can be seen, while for the PEG-400 with only soft massage there is less clearing. The effect of optical clearing can be hardly seen for the control groups of massage with saline or PEG-400 without massage. Fig. 2Computer-flattened optical coherence tomography (OCT) images of rat dorsal skin during topical application of different optical clearing agents. 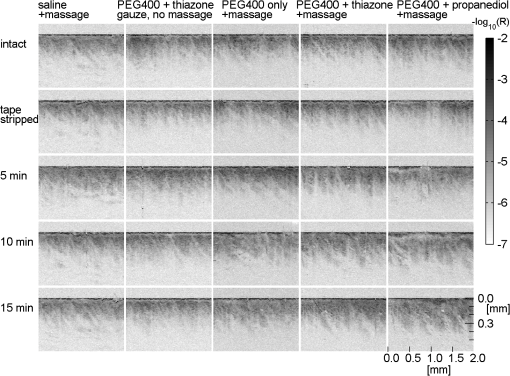 Figure 3 is the depth reflectance, , averaged over all the lateral x positions. The solid line is from the measurements of the intact skin, and the dotted line is from those after applying the mixtures with massage for 15 min. It can be found that the OCT signal from the surface is very strong, but decreases rapidly within 20 μm. After the sharp strong signal of the surface, for the intact skin, the signal of the intact skin initially drops presumably due to the epidermis, then increases due to the superficial dermis which is more strongly scattering. The signal gradually decays versus increasing depth in the dermis. In the experimental group, the superficial signal drops due to clearing and the signal at deeper depths increases due to less attenuation by overlying tissue. The optical clearing reduces the reflectance from the upper dermis layer, e.g., 20 to 100 μm, and enhances the signal from deeper dermis layer, e.g., 100 to 450 μm. To illustrate the increased penetration, a threshold signal of reflectance was chosen and the depth at which the signal dropped below this threshold was noted as . After the 15-min application of OCAs with massage-assisted penetration, increased from 0.219 to 0.275 mm (26% increase) for PEG-400 with thiazone. The increased from 0.197 to 0.262 mm (33% increase) for PEG-400 with 1,2-propanediol. Fig. 3The depth reflectance before and after the topical application of optical clearing agents, showing the average of three duplicate images. (a) Using thiazone as the enhancer. (b) Using propanediol as the enhancer. 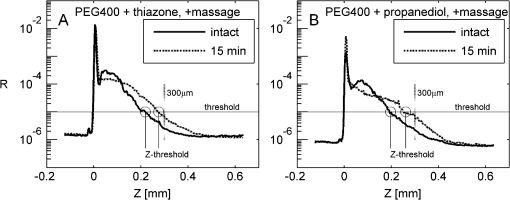 Figure 4 shows the time-resolved reflectance from a 300-μm depth during five different treatment protocols. The SD bars shows the standard deviation for nine experiment repetitions (three repetitions for each site and three rats for each protocol). The two control groups, [Fig. 4(a)] and [Fig. 4(b)], showed no change in reflectance. The reflectance from the 300-μm depth increased with PEG-400 solution and massage but no enhancer after 15 min [Fig. 4(c)]. For PEG-400 with thiazone [Fig. 4(c)] or 1,2-propanediol [Fig. 4(d)], the 300-μm reflectance increased about 3-fold from to after 15 min of topical application of agents with soft massage. Fig. 4Reflectance from a depth of 300 μm after the application of different optical clearing agents. (a) , (b) , (c) , (d) , and (e) . 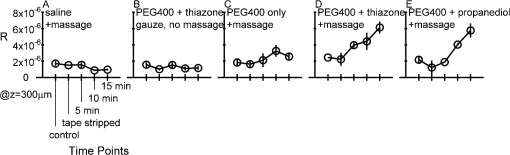 Figure 5 shows bar graphs of the average , i.e., depth at which reflectance drops to , during five different treatment protocols. The application of OCAs [Fig. 5(c) to 5(e)] increased significantly, while the two control groups, [Fig. 5(a)] and [Fig. 5(b)] showed no significant changes. After 15 min of OCAs application assisted with massage, the rose 15% for the OCAs without penetration enhancer and 26% and 31% for PEG-400 with thiazone and 1,2-propanediol, respectively. 4.DiscussionThe OCT signal analysis by averaging over all the lateral positions of three duplicate images gives more information about the depth reflectance, as shown in Fig. 3. The sharp strong reflectance is the specular reflectance from the skin surface and we defined it as in skin. It is well known that the turbid characteristic of skin results mainly from strong scattering of dermis, so the reflectance from the epidermis is weaker than that from the upper part of dermis for intact skin. And the strong scattering of the dermis makes photons diffuse widely, so it is difficult to detect the signal from inner part of the dermis by OCT. After application of OCAs with a soft massage, agents enter into the dermis and diffuse into the inter-fiber medium or even cause the tissue dehydration for a hygroscopic OCA. This makes the index matching of collagen fibers and medium, and then decreases the scattering of the dermis. More ballistic and quasi-ballistic photons of incident light penetrate deeply into the tissue. Therefore, the deeper OCT image was enhanced and the signal from the upper part of the dermis was weakened after optical clearing of skin in vivo. For both the thiazone and 1,2-propanediol groups combined with massage applied for 15 min, the 300-μm signal increased significantly, 3-fold relative to intact skin, and increased 26% and 31%. The PEG-400 group alone combined with massage caused less clearing, the 300-μm signal increased to 1.5-fold relative to intact skin and increased 15%. The OCT reflectance depends on two parameters: (1) the attenuation coefficient as photons travel into/out of the skin, and (2) the local reflectivity from each depth position within the skin. This two parameter description has been described by Samatham et al. for confocal reflectance19 and Levitz et al. for OCT,34 respectively. The results of this paper show that application of OCAs caused the signal from the superficial dermis to decrease while the signal from the inner dermis increased. Such a decrease in superficial reflectance is likely due to a decrease in local reflectivity. The photon pathlength in/out for signal from superficial dermis is rather short, so the attenuation is not strong. The increase in deeper reflectance is likely due the decrease in attenuation since the photon pathlength in/out is rather long. Ghosn et al.35 reported similar changes in skin OCT reflectance caused by topical glucose application. Zhong et al.22 reported that optical clearing on human skin enhanced the OCT signals at all depths. Xu et al.36 found that optical clearing of porcine skin in vitro increased the reflectance from deeper dermis although the OCT signal from superficial skin did not change. These three previous reports and our current paper are all consistent with OCA inducing an increase in local reflectivity and a drop in attenuation. The mechanism of optical clearing is still a topic of investigation. Whether the OCA agents cause refractive index changes that shift the refractive index of collagen fibers, or the OCT agents due to their hygroscopic nature cause changes in scattering due to dessication of collagen fibers is still begin explored. Recently, Samatham et al.19 reported that glycerol caused optical clearing of mouse dermis due to changes in anisotropy rather than the scattering coefficient. The swelling of scatterers in the dermis can lead light scattered in a more forward direction. Alternatively, a hygroscopic OCA induced dehydration may decrease the inter-fiber spacing14 yielding more tightly packed fibers, which allows more constructive interference between scatterers thereby decreasing the scattering coefficient and increasing the anisotropy of scatter. The relative roles of these possible mechanisms are still under investigation by several investigative groups. This report illustrates the importance of massage for improving the penetration of PEG-400. Previous investigation showed that the penetration enhancers can significantly improve the permeability of PEG-400 through the stratum corneum, and disturb the arrangement of lipid layers in the stratum corneum.37 However, when we used a gauze application of PEG-400 with thiazone, the OCT signal rarely changed during the application. Only with sufficient massage during OCAs application can significant optical clearing effect be achieved. Rylander et al.38 reported that tissue compression induced by mechanical force leads to a reduction of scattering in tissues. Thus, the mechanical force of massage itself can yield an optical clearing effect if compression is kept constant. But the lack of clearing by massage plus application of saline showed that mechanical force by massage was not sufficient for optical clearing. The massage provides mechanical force to reduce the epithelial cell barrier function and allow more OCA to penetrate into the skin. 1,2-propanediol is usually used as a kind of solvent, and its ability as a penetration enhancer has been reported earlier.28,37,39 This study shows that 1,2-propanediol is as effective as thiazone in enhancing the optical clearing by PEG-400 based on quantitatively analysis. 5.ConclusionThis study demonstrates that the topical application of (1) a mixture of PEG-400, (2) a chemical penetration enhancer, and (3) physical massage can achieve a good optical clearing effect in 15 min on in vivo rat dorsal skin. All three components applied 15 min together can achieve a 3-fold increase in the OCT reflectance from a 300-μm depth and a 31% increase in the image depth . AcknowledgmentsThe authors are thankful to A. N. Bashkatov, E. A. Genina, N. A. Trunina, and O. V. Glushkovskaya- Semyachkina from SSU for their help in providing experiments. Dan Zhu is thankful for the support of the grants of the National Nature Science Foundation of China (No. 81171376), and the Research Fund for the Doctoral Program of Higher Education of China (No. 20110142110073). Valery V. Tuchin and Dan Zhu appreciate the support of their collaboration by grant NSFC-RFBR for the International Cooperation (No. 30911120074) and RFBR-08-02-92224-NNSF_a (RF—China). Valery V. Tuchin is thankful for the support of grants 224014 Photonics4life-FP7-ICT-2007-2; RF 2.1.1/4989, 2.2.1.1/2950,1.4.09; and RF Governmental contract 02.740.11.0879. RF 1.4.09; RF Governmental contract 02.740.11.0879; and FiDiPro, TEKES (40111/11), Finland. ReferencesM. A. Fox et al.,
“Dermal scatter reduction in human skin: a method using controlled application of glycerol,”
Laser Surg. Med., 41
(4), 251
–255
(2009). http://dx.doi.org/10.1002/lsm.v41:4 LSMEDI 0196-8092 Google Scholar
X. Q. Xu and Q. H. Zhu,
“Sonophoretic delivery for contrast and depth improvement in skin optical coherence tomography,”
IEEE J. Sel. Top. Quantum Electron., 14
(1), 56
–61
(2008). http://dx.doi.org/10.1109/JSTQE.2007.912900 IJSQEN 1077-260X Google Scholar
G. Vargas et al.,
“Use of osmotically active agents to alter optical properties of tissue: effects on the detected fluorescence signal measured through skin,”
Lasers Surg. Med., 29
(3), 213
–220
(2001). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
R. Lacomb et al.,
“Quantitative second harmonic generation imaging and modeling of the optical clearing mechanism in striated muscle and tendon,”
J. Biomed. Opt., 13
(2), 021109
(2008). http://dx.doi.org/10.1117/1.2907207 JBOPFO 1083-3668 Google Scholar
R. Cicchi et al.,
“Contrast and depth enhancement in two-photon microscopy of human skin ex vivo by use of optical clearing agents,”
Opt. Express, 13
(7), 2337
–2344
(2005). http://dx.doi.org/10.1364/OPEX.13.002337 OPEXFF 1094-4087 Google Scholar
R. K. Wang et al.,
“Concurrent enhancement of imaging depth and contrast for optical coherence tomography by hyperosmotic agents,”
J. Opt. Soc. Am. B, 18
(7), 948
–953
(2001). http://dx.doi.org/10.1364/JOSAB.18.000948 JOBPDE 0740-3224 Google Scholar
J. Wang et al.,
“Assessment of optical clearing induced improvement of laser speckle contrast imaging,”
J. Innov. Opt. Health Sci., 3
(3), 159
–167
(2010). http://dx.doi.org/10.1142/S1793545810001052 Google Scholar
G. Vargas, J. K. Barton and A. J. Welch,
“Use of hyperosmotic chemical agent to improve the laser treatment of cutaneous vascular lesions,”
J. Biomed. Opt., 13
(2), 021114
(2008). http://dx.doi.org/10.1117/1.2907327 JBOPFO 1083-3668 Google Scholar
R. J. McNichols et al.,
“Temporary dermal scatter reduction: quantitative assessment and implications for improved laser tattoo removal,”
Lasers Surg. Med., 36
(4), 289
–296
(2005). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
X. Wen et al.,
“Controling the scattering of intralipid by using optical clearing agents,”
Phys. Med. Biol., 54
(22), 6917
–6930
(2009). http://dx.doi.org/10.1088/0031-9155/54/22/011 PHMBA7 0031-9155 Google Scholar
V. V. Tuchin et al.,
“Light propagation in tissues with controlled optical properties,”
J. Biomed. Opt., 2
(4), 401
–417
(1997). http://dx.doi.org/10.1117/12.281502 JBOPFO 1083-3668 Google Scholar
T. Yu et al.,
“Quantitative analysis of dehydration in porcine skin for assessing mechanism of optical clearing,”
J. Biomed. Opt., 16
(9), 095002
(2011). http://dx.doi.org/10.1117/1.3621515 JBOPFO 1083-3668 Google Scholar
X. Xu and R. K. Wang,
“The role of water desorption on optical clearing of biotissue: studied with near infrared reflectance spectroscopy,”
Med. Phys., 30
(6), 1246
(2003). http://dx.doi.org/10.1118/1.1576228 MPHYA6 0094-2405 Google Scholar
X. Wen et al.,
“In vivo skin optical clearing by glycerol solutions: mechanism,”
J. Biophotonics, 3
(12), 44
–52
(2010). http://dx.doi.org/10.1002/jbio.201090004 1864-063X Google Scholar
C. G. Rylander et al.,
“Dehydration mechanism of optical clearing in tissue,”
J. Biomed. Opt., 11
(4), 041117
(2006). http://dx.doi.org/10.1117/1.2343208 JBOPFO 1083-3668 Google Scholar
X. Q. Xu and Q. H. Zhu,
“Evaluation of skin optical clearing enhancement with Azone as a penetration enhancer,”
Opt. Commun., 279
(1), 223
–228
(2007). http://dx.doi.org/10.1016/j.optcom.2007.06.055 OPCOB8 0030-4018 Google Scholar
J. Hirshburg et al.,
“Correlation between collagen solubility and skin optical clearing using sugars,”
Laser Surg. Med., 39
(2), 140
–144
(2007). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
J. Hirshburg et al.,
“Collagen solubility correlates with skin optical clearing,”
J. Biomed. Opt., 11
(4), 040501
(2006). http://dx.doi.org/10.1117/1.2220527 JBOPFO 1083-3668 Google Scholar
R. Samatham, K. G. Phillips and S. L. Jacques,
“Assessment of optical clearing agents using reflectance-mode confocal scanning laser microscopy,”
J. Innov. Opt. Health Sci., 3
(3), 183
–188
(2010). http://dx.doi.org/10.1142/S1793545810001064 Google Scholar
J. Jiang and R. K. Wang,
“Comparing the synergistic effects of oleic acid and dimethyl sulfoxide,”
Phys. Med. Biol., 49
(23), 5283
–5294
(2004). http://dx.doi.org/10.1088/0031-9155/49/23/006 PHMBA7 0031-9155 Google Scholar
H. Q. Zhong et al.,
“In vitro study of ultrasound and different-concentration glycerol-induced changes in human skin optical attenuation assessed with optical coherence tomography,”
J. Biomed. Opt., 15
(3), 036012
(2010). http://dx.doi.org/10.1117/1.3432750 JBOPFO 1083-3668 Google Scholar
H. Q. Zhong et al.,
“Synergistic effect of ultrasound and thiazone-PEG 400 on human skin optical clearing in vivo,”
Photochem. Photobiol., 86
(3), 732
–737
(2010). http://dx.doi.org/10.1111/php.2010.86.issue-3 PHCBAP 0031-8655 Google Scholar
J. Yoon et al.,
“Enhancement of optical skin clearing efficacy using a microneedle roller,”
J. Biomed. Opt., 13
(2), 021103
(2008). http://dx.doi.org/10.1117/1.2907483 JBOPFO 1083-3668 Google Scholar
E. A. Genina et al.,
“Possibility of increasing the efficiency of laser-induced tattoo removal by optical skin clearing,”
Qutantum Electron., 38
(6), 580
–587
(2008). http://dx.doi.org/10.1070/QE2008v038n06ABEH013940 QUELEZ 1063-7818 Google Scholar
V. V. Tuchin et al.,
“Optical clearing of skin using flashlamp-induced enhancement of epidermal permeability,”
Laser Surg. Med., 38
(9), 824
–836
(2006). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
E. A. Genina et al.,
“Fractional laser microablation of skin aimed at enhancing its permeability for nanoparticles,”
Quantum Electron., 41
(5), 396
–401
(2011). http://dx.doi.org/10.1070/QE2011v041n05ABEH014639 QUELEZ 1063-7818 Google Scholar
C. H. Liu et al.,
“Enhancement of skin optical clearing efficacy using photo-irradiation,”
Lasers Surg. Med., 42
(2), 132
–140
(2010). http://dx.doi.org/10.1002/lsm.v42:2 LSMEDI 0196-8092 Google Scholar
J. Wang et al.,
“Improvement of in vivo rat skin optical clearing with chemical penetration enhancers,”
Proc. SPIE, 7883 78830Y
(2011). http://dx.doi.org/10.1117/12.874859 PSISDG 0277-786X Google Scholar
D. Zhu et al.,
“Imaging dermal blood flow through the intact rat skin with an optical clearing method,”
J. Biomed. Opt., 15
(2), 026008
(2010). http://dx.doi.org/10.1117/1.3369739 JBOPFO 1083-3668 Google Scholar
Y. C. Wu et al.,
“Noninvasive optical coherence tomography monitoring of structure and hydration changes of human corneas in different preservation media,”
J. Biomed. Opt., 16
(2), 026015
(2011). http://dx.doi.org/10.1117/1.3541792 JBOPFO 1083-3668 Google Scholar
H. G. Ren et al.,
“Enhancing detection of bladder carcinoma in situ by 3-dimensional optical coherence tomography,”
J. Urol., 184
(4), 1499
–1506
(2010). http://dx.doi.org/10.1016/j.juro.2010.05.087 JOURAA 0022-5347 Google Scholar
H. Pinkus,
“Examination of the epidermis by the strip method of removing horny layers. I. Observations on thickness of the horny layer, and on mitotic activity after stripping,”
J. Invest. Dermatol., 16
(6), 383
–386
(1951). JIDEAE 0022-202X Google Scholar
G. Yao and L. V. Wang,
“ Monte Carlo simulation of an optical coherence tomography signal in homogeneous turbid media,”
Phys. Med. Biol., 44
(9), 2307
–2320
(1999). http://dx.doi.org/10.1088/0031-9155/44/9/316 PHMBA7 0031-9155 Google Scholar
D. Levitz et al.,
“Determination of optical scattering properties of highly-scattering media in optical coherence tomography images,”
Opt. Express, 12
(2), 249
–259
(2004). http://dx.doi.org/10.1364/OPEX.12.000249 OPEXFF 1094-4087 Google Scholar
M. G. Ghosn et al.,
“Monitoring of glucose permeability in monkey skin in vivo using optical coherence tomography,”
J. Biophotonics, 3
(1–2), 25
–33
(2010). http://dx.doi.org/10.1002/jbio.200910075 1864-063X Google Scholar
X. Xu, Q. Zhu and C. Sun,
“Assessment of the effects of ultrasound-mediated alcohols on skin optical clearing,”
J. Biomed. Opt., 14
(3), 034042
(2009). http://dx.doi.org/10.1117/1.3156827 JBOPFO 1083-3668 Google Scholar
A. C. Williams and B. W. Barry,
“Penetration enhancers,”
Adv. Drug Deliv. Rev., 56
(5), 603
–618
(2004). http://dx.doi.org/10.1016/j.addr.2003.10.025 ADDREP 0169-409X Google Scholar
C. G. Rylander et al.,
“Mechanical tissue optical clearing devices: enhancement of light penetration in ex vivo porcine skin and adipose tissue,”
Laser Surg. Med., 40
(10), 688
–694
(2008). http://dx.doi.org/10.1002/lsm.v40:10 LSMEDI 0196-8092 Google Scholar
Z. Zhi et al.,
“Improve optical clearing of skin in vitro with propylene glycol as a penetration enhancer,”
J. of Innov. Opt. Health Sci., 2
(3), 269
–278
(2009). http://dx.doi.org/10.1142/S1793545809000590 Google Scholar
|