|
|
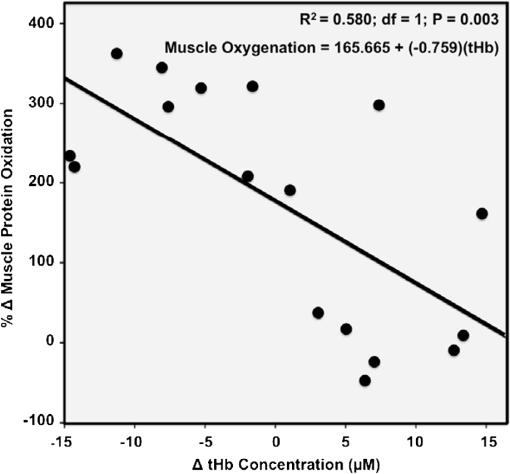
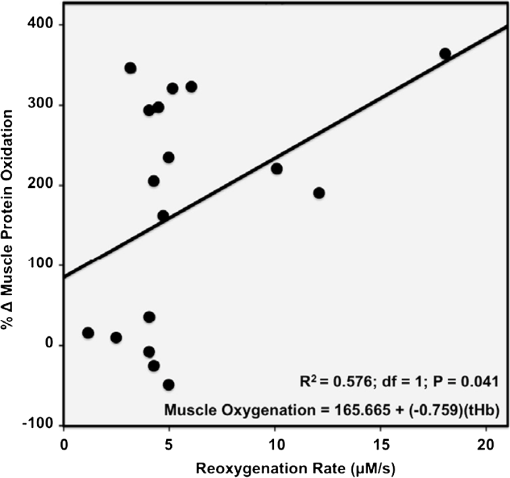
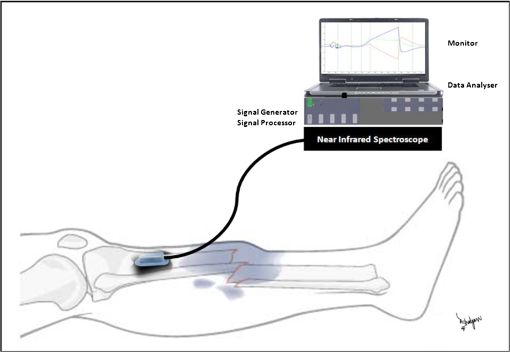
1.IntroductionPostsurgical complications are common following orthopedic procedures that use limb tourniquets to maintain a bloodless surgical field in the extremities. These complications include muscle paresis, impaired wound healing, infection, compartment syndrome, deep vein thrombosis, and increased frequencies of limb pain, swelling, and neuromuscular dysfunction.1–10 The principal causes of tourniquet-induced neuromuscular dysfunction appear to be direct pressure on the nerve and skeletal muscle directly underlying the tourniquet, muscle and microvascular oxidative injury distal to the tourniquet, and reperfusion-associated oxidative stress and inflammation distal and proximal to the tourniquet. Among several variables related to tourniquet cuff inflation that apparently contribute to the development of ischemic muscle injury, the duration of ischemia has been identified as the primary factor.4,11 Several studies have reported that, as long as the duration of ischemia does not exceed 3 h, skeletal muscle escapes irreversible damage due to ischemia and the reperfusion that subsequently occurs (I/R) upon tourniquet release.12–19 The safe time limit for tourniquet use in humans remains controversial, however.10,20–22 The current clinical standard for maximum continuous tourniquet time during lower-extremity orthopedic surgery is 90 min.4,6,11,23,24 This benchmark is based primarily on animal studies, but the clinical relevance of such studies is open to question.2,16,23,25 Surgeons would be better able to balance the advantages of a bloodless operative field against the risks of prolonging muscle recovery and rehabilitation owing to ischemic muscle injury if there were practical, noninvasive monitoring methods that helped determine safe surgical tourniquet time and pressure on a patient-by-patient basis. One potential solution to this problem is near-infrared spectroscopy (NIRS), a noninvasive optical method for the real-time monitoring of tissue oxygenation and hemodynamics.26,27 The science of NIRS hinges on some of the fundamental principles of optics and photonics as they relate to the transmission of light through living tissues and the absorption of light by tissue chromophores. NIRS units use lasers or diodes that transmit pulses of multiple wavelengths of light into tissues and optical sensors that detect returning photons. When NIR light is transmitted through tissue, some is irretrievably lost due to scattering and some is absorbed by compounds other than the chromophores of interest. Only a small proportion of the original photons transmitted can be detected returning from the tissue.28,29 The changes in absorption at discrete wavelengths generate raw optical data that can be converted by mathematical software algorithms into real-time concentration changes for each chromophore using a modification of the Beer—Lambert law.28 The principal chromophores of interest in physiological and clinical studies using NIRS are oxygenated () and deoxygenated (HHb) species of hemoglobin, which each have a distinct extinction coefficient (absorption characteristic) across the NIR spectrum.30,31 NIRS has been validated and used by many investigators to monitor regional tissue oxygenation, hemodynamics, and metabolism in health and disease.30,32 In fact, the use of NIRS for monitoring skeletal muscle hemodynamics during tourniquet use has previously been validated in animals using a rat model.33 However, the application of NIRS in monitoring human limb muscle ischemia is limited to studies regarding limb muscle oxygenation during short-duration venous and arterial occlusion.34–38 Accordingly, the purpose of this clinical study was to investigate leg muscle oxygenation, hemodynamics, and oxidative injury (measured according to protein oxidation) during orthopedic surgical tourniquet use. Specifically, using NIRS we monitored skeletal muscle oxygenation and hemodynamics distal to the tourniquet during surgery, and then examined ischemia-related muscle biopsy protein oxidation using biochemical Western blotting (WB) analyses. We also aimed to establish the feasibility of NIRS for monitoring clinical muscle ischemia over extended periods and to develop a NIRS-based approach for predicting tourniquet-associated muscle oxidative injury. We hypothesized that oxidative damage to muscle proteins distal to the tourniquet would be correlated both with longer tourniquet times and with a slower rate of muscle reoxygenation following tourniquet release. We also hypothesized that NIRS would be a feasible method for noninvasive monitoring of prolonged skeletal muscle ischemia in the clinical setting. 2.Materials and Methods2.1.ParticipantsA convenience sample of patients with closed ankle fractures requiring emergency surgery was recruited upon admission to a level 1 trauma hospital. Inclusion criteria were adults with unilateral ankle fractures, no major comorbidity,39 and no additional previous or current injuries to either limb that might affect the reliability of NIRS measurements. The study received institutional clinical research ethics board approval. Informed written consent was obtained from all volunteers before their participation. All procedures complied with the Declaration of Helsinki. 2.2.Experimental OverviewAll patients received standard general anesthesia. After surgical preparation and positioning of the lower limbs, a pair of NIRS probes (Oxymon M-III, Artinis, the Netherlands), each with 4-cm interoptode distances, which provided a 2-cm penetration depth,40 were fixed with surgical tape over the tibialis anterior muscles (TAs), bilaterally (the TA is the muscle overlying the shin on the anterior surface of the lower limb, below the knee) (Fig. 1). The differential path-length factor (DPF) of the NIRS instrument was set at 4.32 The injured limb was elevated to reduce blood pooling, and then a thigh tourniquet (Zimmer ATS-2000, IN) was inflated to a pressure of 300 mmHg.41 Using the NIRS apparatus, chromophore concentrations of oxygenated () and deoxygenated (HHb) hemoglobin were measured bilaterally in the TA muscles before and during tourniquet inflation and after tourniquet release until returned to baseline. Mean systemic arterial pressure, heart rate, and arterial oxygen saturation were obtained from the upper extremity using an automated blood pressure cuff and a pulse oximeter (AS3000, ADS, NJ). Muscle biopsies were collected from the peroneus tertius muscle (PT) distal to the tourniquet (1) immediately after tourniquet inflation (pre), and (2) toward the end of surgery, immediately before tourniquet deflation (post). The tourniquet was released when the surgeon no longer required arterial obstruction. Fig. 1A NIRS instrument monitors tibialis anterior muscle oxygenation and hemodynamics during lower-limb trauma surgery.  The TA was chosen for NIRS monitoring because it is the most superficial muscle within the anterior compartment of the leg, and it provides sufficient surface area for NIRS probe attachment. During ankle surgery, there is no direct surgical access to the TA, but there is direct access to the PT, which is also in the anterior compartment. So, to minimize the impact on the patient, the PT was selected for surgical biopsy. In addition to their similar anterior compartment location, the TA and PT are comparable in their functionally classified motor unit type distributions,42,43 so we assumed that biochemical assays from the PT would serve as appropriate comparisons to NIRS measurements from the adjacent TA. 2.3.Near-Infrared SpectroscopyOxygenation and hemodynamics were continuously monitored bilaterally, in both TAs, using a four-channel continuous-wave (CW) near-infrared spectroscope (Oxymon M-III, Artinis, the Netherlands). The Oxymon is designed as a plug-and-play instrument. It is a continuous-wave NIRS using wavelengths in 780 and 855 nm with a sampling rate of 50 Hz to 10 s.30 NIRS principles and the calculation of NIRS-derived parameters have been described elsewhere44–47 and are summarized below. In this study, we measured changes in and HHb chromophore concentrations in the TA muscles throughout surgery, from at least 10 min before tourniquet inflation until returned to baseline values following tourniquet deflation. Tourniquet time (duration of tourniquet inflation) and and HHb concentration changes, along with several NIRS variables, were calculated for each subject based on the data collected during tourniquet inflation and after deflation. These variables included the following. (1) Total hemoglobin (tHb), the sum of and HHb concentrations, demonstrates changes in local blood volume in the tissue being monitored.32 (2) Hb difference (), the difference between and HHb concentration changes, is used as an index of tissue oxygenation.45 (3) Reactive hyperemia reveals transient increases in tHb upon reperfusion and is used to evaluate the effect of ischemia on vascular function.48 (4) Recovery time, the time required for to recover to preischemic levels from maximum deoxygenation at the end of the ischemic period, is considered to be an index of tissue oxygen consumption during reperfusion.49 (E) Reoxygenation rate, the rate of increase in concentration during the first 3 sec of reperfusion,32,45 is used to evaluate the speed at which recovery starts upon reperfusion, which is linked to microvascular function.32,49 To control for possible general measurement errors, simultaneous data were also collected from the same location on the control side. Real-time data were sampled at 10 Hz and recorded by the NIRS instrument for further offline analysis using dedicated software (Oxysoft, Artinis, the Netherlands). Changes in tissue oxygenation, deoxygenation, and local blood volume were estimated from changes in , HHb, , and tHb. In addition to NIRS monitoring, any tourniquet adjustments or other changes in surgical setup, including limb repositioning, that might alter the NIRS sampling during the experiments were recorded. Furthermore, surgical fields were visually inspected during the operations to check for the possibility of blood loss. 2.4.Biopsy Collection and OxyBlot AnalysisImmediately upon collection, biopsies were embedded in freezing medium, frozen in liquid nitrogen-cooled isopentane, and stored at until processing. Protein oxidation was measured by WB for reactive carbonyl derivatives using the commercially available OxyBlot protein oxidation detection kit, according to the manufacturer’s instructions (Millipore, Billerica, MA) and as previously described.50,51 Carbonyl groups of myofibrillar protein side chains were derivatized to 2,4-dinitrophyenylhydrazone (DNP). DNP-derivatized protein samples were separated using polyacrylamide gel electrophoresis, after photometric total protein determination of each sample to ensure equal protein loading across lanes. Separated proteins were electrotransferred from the gels to nitrocellulose membranes. Membranes were treated with (1) primary antibodies against DNP, (2) horseradish peroxidase—conjugated secondary antibodies against the primary antibodies, and (3) chemiluminescence reagents. Images of chemiluminescence-treated membranes were digitally captured and analyzed (GelDoc 2000, BioRad, Hercules, CA). This computerized analysis method background-corrects the chemiluminescence images and then uses the chemiluminescent signal intensity of each lane to measure that lane’s volume of oxidized protein using integrated densitometry. Samples from all subjects were run on both pre and post gels. On each post gel, randomly selected pre samples were run as controls that were used to normalize the post to the pre gels. Thus, pre—post differences in the degree of protein oxidation for each subject were calculated from the background-corrected signal intensities of the pre and post gels using the average, normalized pre—post ratio (pairwise comparisons) of integrated densitometry values. 2.5.Statistical MethodsSubject characteristics were summarized using descriptive statistics. Statistical differences in average protein oxidation between the pre and post biopsies were assessed using a paired student’s t-test. During ischemia (from tourniquet inflation to deflation) in both experimental and control TAs, statistical differences between , HHb, and tHb chromophore concentration changes were assessed using paired student’s t-tests. We then used a linear regression model to investigate the effect of each of the following variables on changes in protein oxidation observed in the comparison of pre and post-biopsy samples: age, sex, body mass index (BMI), tourniquet time, , HHb, tHb, , hyperemia interval, recovery time, and reoxygenation rate. Each of these independent variables was entered individually and sequentially into the simple linear regression model. Data were analyzed using SPSS software (SPSS for Windows, Rel.11.0.1. 2001, SPSS Inc., Chicago, IL). Values are reported as . Statistical significance was accepted at . 3.Results3.1.Descriptive CharacteristicsSeventeen patients (13 women, 4 men) with unilateral ankle fractures were included in this study. The mean participant age was (range 19 to 69) years. The mean participant BMI was . 3.2.TourniquetA tourniquet application pressure of 300 mmHg was maintained in all subjects. A bloodless surgical field was obtained in all subjects. The average tourniquet time (duration) was (range 20.7 to 73.8) min. 3.3.CardiovascularDuring tourniquet application, mean arterial pressure increased . Pulse rate and showed no significant changes. 3.4.Near-Infrared SpectroscopyDuring ischemia, TA chromophore concentration changes for , HHb, and tHb were significantly different in the experimental leg compared to the control leg () and were similar across subjects. Figure 2 shows HHb, , tHb, and changes in the TA distal to the tourniquet (left side) compared to the control TA (right side) in a single representative subject before, during, and after thigh tourniquet inflation. After tourniquet inflation in all subjects, the ischemic TA demonstrated a progressive increase in HHb () and a progressive decrease in (). These HHb and changes began to reverse immediately after tourniquet deflation. During tourniquet inflation, tHb increased in eight subjects () and decreased in nine subjects (). showed a significant decrease () during tourniquet application. In control TAs, no significant changes in NIRS variables were observed before, during, or after tourniquet application. Fig. 2Chromophore concentration changes for and HHb, and NIRS variables of tHb and in the tibialis anterior muscle distal to the tourniquet (left side) compared to the control tibialis anterior muscle (right side) in a single representative subject before, during, and after thigh tourniquet inflation. Changes in tHb, as the sum of and HHb concentrations, reflect changes in local blood volume. Changes in (the difference between changes of and HHb concentrations) indicate local tissue oxygenation in the tibialis anterior muscle. A: Tourniquet inflation time; B. Tourniquet release time; C: Point of maximum hyperemia; D: Point of maximum recovery. 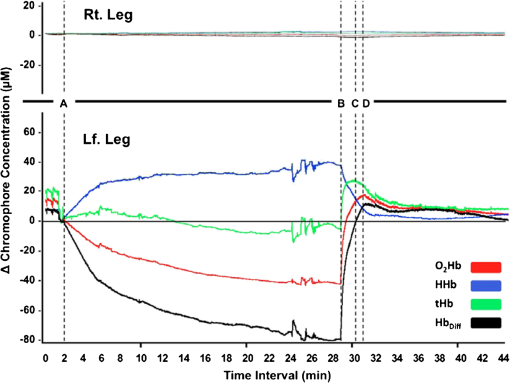 After tourniquet release, values for recovery time and reoxygenation rate of hemoglobin were, respectively, and . The decrease rate was significantly higher among male subjects (). 3.5.Muscle BiopsyOn average, of tourniquet-induced ischemia was associated with a increase in total myofibrillar protein oxidation () (Fig. 3). Interestingly, however, there was no statistically measurable relationship observed between tourniquet duration and pre—post biopsy changes (, ). Fig. 3Raw oxidized protein volume in peroneus tertius samples at the beginning (pre) and end (post) of tourniquet inflation. The raw contents of carbonylated proteins in peroneus tertius muscle biopsy homogenates were determined by integrated densitometry of Western blots prepared using the commercially available OxyBlot method. The mean values for pre (white bar) and post (gray bar) samples were calculated after correcting the raw values to the loading control sample that was run on all gels. The percent difference between pre and post (black bar) indicates the increase in protein oxidation that occurred during the ischemic period. As indicated, the pre—post difference was significant at . 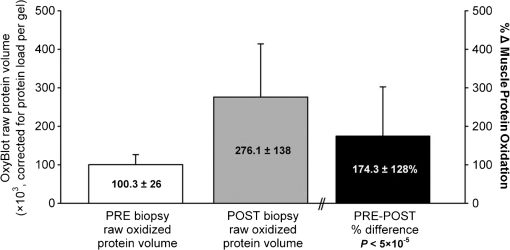 No correlations were found between pre—post protein oxidation and either the age (, ) or the BMI (, ) of the subjects. We observed statistically significant differences between men and women in the average pre—post protein oxidation increase (not shown: 51% greater increase in men, , ). However, the statistical power for this comparison was low due to the small number of men in our sample (). 3.6.OxyBlot Versus NIRS RegressionLinear regression was conducted to assess relationships between each of several NIRS variables and the degree of pre—post protein oxidation. For all subjects, we found that changes in both and tHb were significantly negatively correlated with the average increase in protein oxidation. Specifically, linear regressions showed that a 1-μM increase in resulted in a 6.1% decrease in the degree of pre—post protein oxidation (, ) (Fig. 4), and a 1-µM increase in tHb resulted in an 11.8% decrease in the degree of pre—post protein oxidation (, ) (Fig. 5). In contrast, for all subjects, reoxygenation rate was significantly positively correlated with the average protein oxidation increase. Specifically, for each unit increase in reoxygenation rate the protein oxidation increase was 18.1% greater (, ) (Fig. 6). Significant associations were not observed between the average protein oxidation increase and changes in NIRS-derived indices of either hyperemia interval (, ) or recovery time (, ). Finally, protein oxidation was not correlated with either HHb or ( and , respectively). Fig. 4Scatter plot and regression line showing the correlation between and pre—post changes in muscle protein oxidation. 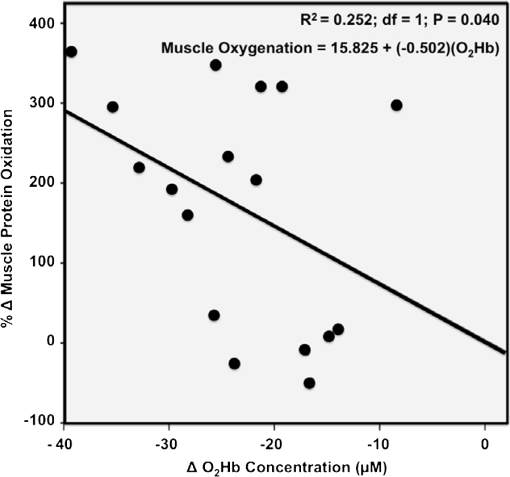 4.DiscussionWe have demonstrated that NIRS is a feasible method for the noninvasive monitoring of skeletal muscle ischemia in the orthopedics setting. Specifically, our data show that tourniquet-induced muscle ischemia lasting 21 to 74 min during lower extremity surgery, without reperfusion, is associated with significant limb muscle protein oxidation. Underlying this association between ischemia and muscle oxidative damage are significant negative correlations between tourniquet-induced muscle protein oxidation and local muscle blood volume changes measured using NIRS. However, our data do not support our hypotheses that during tourniquet-induced lower-extremity ischemia, muscle protein oxidation would be correlated with both (1) longer tourniquet duration and (2) a slower muscle reoxygenation rate after tourniquet release. Rather, we demonstrate that faster muscle reoxygenation is correlated with an increased degree of muscle protein oxidation, which nevertheless emphasizes that muscle injury sustained during prolonged ischemia is linked to altered microvascular function. Harvey Cushing first introduced the pneumatic tourniquet in 1904, and many subsequent investigations have explored the relationship between tourniquet-induced I/R and skeletal muscle injury. Importantly, our findings demonstrate that, on average, 43 min of tourniquet-induced ischemia leads to significant oxidative muscle tissue damage. This is in contrast to previous studies demonstrating that ischemia alone does not lead to protein carbonylation.52,53 Our data complement those of Appell and colleagues,22,54,55 who demonstrated in healthy young subjects undergoing ACL reconstruction that only 15 min of tourniquet-induced ischemia, without reperfusion, causes muscle oxidative damage characterized by myofiber edema and capillary basement membrane thickening. We were surprised to observe that the large increase in tourniquet-associated muscle protein oxidation we report here is not statistically correlated with the average tourniquet duration of 43 min. This finding may be clinically important because it indicates that, at least for tourniquet applications up to 1.25 h, increases in protein oxidation are not determined solely by the tourniquet time. Additional factors that may influence tourniquet-associated muscle protein oxidation include the type and severity of trauma; the length of delay between the initial fracture and surgical repair, since muscle unloading is associated with muscle oxidative stress and protein carbonylation;3,56 cigarette smoking that generates systemic inflammation and oxidative stress, including in limb muscles;57 exercise levels58 and associated myofiber types;59 nutritional status;60 and inflammation and sepsis associated with traumatic muscle and bone injury.61,62 Reactive hyperemia and increased local blood volume (tHb) after tourniquet release are well documented.48 In the control TAs, we observed no muscle tHb changes during surgery, but in the surgical limbs we observed tHb increases in nine patients and tHb decreases in eight patients. These tHb changes during tourniquet use were unexpected observations. The gradual muscle tHb increases observed distal to the tourniquet indicate that in these cases tourniquet pressures were likely insufficient to induce complete limb arterial occlusion. In contrast, the gradual decreases in muscle local blood volume (ΔtHb) observed distal to the tourniquet indicate that there was blood loss from the surgical field in these cases. Regression analyses of NIRS variables demonstrated significant negative relationships between muscle protein oxidation and both ΔtHb and . Taken together, these findings suggest that muscle oxidative damage distal to the tourniquet is determined partly by changes in local oxygenated blood volume. This observation may suggest that muscles are in fact protected against ischemic injury (i.e., protein oxidation) when there is arterial leakage into the limb at the tourniquet site, but that oxidative injury is intensified when blood loss distal to the tourniquet, at the surgical field, leads to decreased limb muscle blood volume. Our data show a negative relationship between ΔtHb and the pre—post increase in contractile protein oxidation, which suggests that using a pneumatic tourniquet pressure that permits a very small arterial leak, rather than completely occluding arterial flow, may protect the muscle tissue distal to the tourniquet against ischemia-associated oxidative injury. Consequently, a possible next step from this study might be to develop a “smart” pneumatic tourniquet integrated with a NIRS system, which would control cuff pressure according to real-time muscle ΔtHb values distal to the tourniquet site. In fact, the actual amount of tourniquet pressure required for complete arterial occlusion varies across individuals41 because, for a given tourniquet cuff pressure, patients with larger limb circumferences, higher BMIs, or uncontrolled hypertension sometimes do exhibit a small amount of blood leakage at the tourniquet site.63 Further studies with larger sample sizes, additional methods of tourniquet pressure selection, and preferably control trial designs are required to confirm our observation that there is a negative correlation between muscle oxidative damage and changes in local oxygenated blood volume. Our data also show that, distal to the tourniquet, the degree of muscle protein oxidation is positively correlated with changes in reoxygenation rate. Previous reports demonstrate that I/R injuries are characterized by inflammation and vascular damage, leading to pooling of the oxygen-carrying red blood cells.22,64,65 We speculate that, during ischemia, this combination of increased vascular permeability and red blood cell pooling contributes to faster reoxygenation during reperfusion, as measured using NIRS.32,45 Theoretically, this would explain how the degree of protein oxidation could be linked to the reoxygenation rate observed during reperfusion. Thus, with further study, using NIRS to measure the reoxygenation rate could assist surgeons in predicting the degree of tourniquet-induced muscle protein oxidation and, subsequently, to select the most appropriate therapeutic options that have the potential to attenuate I/R injury and improve recovery following surgery. Such NIRS-based clinical monitoring of tourniquet ischemia may be particularly important because the muscle contractile protein oxidation we report here is likely to have long-lasting consequences.66,67 Specifically, recent in vitro evidence suggests that oxidative damage to muscle contractile proteins dramatically increases these proteins’ susceptibility to irreversible destruction by caspase-3,67 a protein-degrading enzyme that is activated in skeletal muscle following tourniquet use in humans.64 Animal models also show that posttourniquet recovery of muscle contractile protein composition requires .68 Clinically, then, if oxidative protein damage is not mitigated, surgical tourniquet use will likely exacerbate functional deficits and prolong postsurgical recovery. There are limitations to this study. Although NIRS data have been used extensively for studying muscle oxygenation and hemodynamics, this technique should be considered a noninvasive tool only for estimating changes in muscle oxygenation and blood volume.69 A possible limitation of the protein oxidation/local blood volume correlation we observed is that it is based on two different muscles: the TA and PT were used to collect NIRS and biopsy data, respectively. The TA has a slightly faster motor unit composition than the PT42,43 and so, theoretically, protein oxidation should be greater in the TA than the PT because faster muscles are lower in endogenous antioxidants.70 Additionally, the small sample size in the current study limits the statistical power for finding significant relationships between muscle protein changes and study variables, including ischemia duration and recovery time. Finally, since our cross-sectional study did not include patient follow-up to assess our participants’ postoperative conditions and their leg muscle rehabilitation, we cannot directly compare tourniquet-associated protein oxidation to patient outcomes. Further investigations will both clarify the long-term consequences of tourniquet use for skeletal muscle and establish clear parameters for using NIRS to predict the extent of tourniquet-associated muscle damage. In summary, this study establishes NIRS as an advantageous and feasible technique for noninvasive monitoring of muscle oxygenation and hemodynamics in the clinical setting. We also demonstrate that large increases in muscle protein oxidation occur during tourniquet-induced ischemia, even without reperfusion. The findings of this study provide a foundation for future clinical investigations. One such investigation may be the development of a NIRS prototype for continuous monitoring of skeletal muscle oxygenation and hemodynamics during prolonged surgical procedures. Another important follow-up may be to investigate the application of NIRS for the early diagnosis of critical muscle ischemic conditions, such as acute compartment syndrome in high-risk trauma patients (Fig. 7).71 ReferencesD. W. ShentonS. A. SpitzerB. M. Mulrennan,
“Tourniquet-induced rhabdomyolysis: a case report,”
J. Bone Joint Surg. Am., 72
(9), 1405
–1406
(1990). Google Scholar
L. R. Mohleret al.,
“Intermittent reperfusion fails to prevent post tourniquet neurapraxia,”
J. Hand Surg. Am., 24
(4), 687
–693
(1999). http://dx.doi.org/10.1053/jhsu.1999.0687 Google Scholar
C. G. MurphyD. C. WinterD. J. Bouchier-Hayes,
“Tourniquet injuries: pathogenesis and modalities for attenuation,”
Acta Orthop. Belg., 71
(6), 635
–645
(2005). 0001-6462 Google Scholar
A. OdinssonV. Finsen,
“Tourniquet use and its complications in Norway,”
J. Bone Joint Surg. Br., 88
(8), 1090
–1092
(2006). http://dx.doi.org/10.1302/0301-620X.88B8.17668 0301-620X Google Scholar
G. Konradet al.,
“Tourniquets may increase postoperative swelling and pain after internal fixation of ankle fractures,”
Clin. Orthop. Relat. Res., 433 189
–194
(2005). http://dx.doi.org/10.1097/01.blo.0000151849.37260.0a CORTBR 0009-921X Google Scholar
K. C. Saunderset al.,
“Effect of tourniquet time on postoperative quadriceps function,”
Clin. Orthop. Relat. Res., 143 194
–199
(1979). CORTBR 0009-921X Google Scholar
J. J. DobnerA. J. Nitz,
“Postmeniscectomy tourniquet palsy and functional sequelae,”
Am. J. Sports Med., 10
(4), 211
–214
(1982). http://dx.doi.org/10.1177/036354658201000404 AJSMDO Google Scholar
N. MaffulliV. TestaG. Capasso,
“Use of a tourniquet in the internal fixation of fractures of the distal part of the fibula: a prospective randomized trial,”
J. Bone Joint Surg., 75
(5), 700
–703
(1993). JBJSB4 0021-9355 Google Scholar
D. M. Danielet al.,
“Effects of tourniquet use in anterior cruciate ligament reconstruction,”
Arthroscopy, 11 307
–311
(1995). http://dx.doi.org/10.1016/0749-8063(95)90008-X ARTHE3 0749-8063 Google Scholar
A. Wakaiet al.,
“Pneumatic tourniquets in extremity surgery,”
J. Am Acad. Orthop. Surg., 9
(5), 345
–351
(2001). 1067-151X Google Scholar
E. Artacho-PdrulaR. Rolan-VillalobosR. Vaamonde-Lemos,
“Capillary and fiber size interrelationships in regenerating rat soleus muscle after ischemia: a quantitative study,”
Acta Anat., 142
(1), 70
–76
(1991). http://dx.doi.org/10.1159/000147163 ACATA5 0001-5180 Google Scholar
K. Harriset al.,
“Metabolic response of skeletal muscle to ischemia,”
Am. J. Physiol., 250
(2 P2 2), H213
–H220
(1986). AJPHAP 0002-9513 Google Scholar
J. P. Idstromet al.,
“Purine metabolism after in vivo ischemia and reperfusion in rat skeletal muscle,”
Am. J. Physiol., 258
(6 Pt 2), H1668
–H1673
(1990). AJPHAP 0002-9513 Google Scholar
J. Blebeaet al.,
“Quantitative histochemical evaluation of skeletal muscle ischemia and reperfusion injury,”
J. Surg. Res., 43
(4), 311
–321
(1987). http://dx.doi.org/10.1016/0022-4804(87)90087-4 JSGRA2 0022-4804 Google Scholar
S. PasupathyS. Homer-Vanniasinkam,
“Ischaemic preconditioning protects against ischaemia/reperfusion injury: emerging concepts,”
Eur. J. Vasc. Endovasc. Surg., 29
(2), 106
–115
(2005). http://dx.doi.org/10.1016/j.ejvs.2004.11.005 1078-5884 Google Scholar
R. A. Pedowitzet al.,
“Effects of reperfusion intervals on skeletal muscle injury beneath and distal to a pneumatic tourniquet,”
J. Hand Surg. Am., 17
(2), 245
–255
(1992). http://dx.doi.org/10.1016/0363-5023(92)90400-J Google Scholar
H. HaljamaeE. Enger,
“Human skeletal muscle energy metabolism during and after complete tourniquet ischemia,”
Ann. Surg., 182
(1), 9
–14
(1975). http://dx.doi.org/10.1097/00000658-197507000-00002 ANSUA5 0003-4932 Google Scholar
R. HudaD. R. SolankiM. Mathru,
“Inflammatory and redox responses to ischaemia/reperfusion in human skeletal muscle,”
Clin. Sci. (Lond.), 107
(5), 497
–503
(2004). http://dx.doi.org/10.1042/CS20040179 Google Scholar
M. Mathruet al.,
“Inhaled nitric oxide attenuates reperfusion inflammatory responses in humans,”
Anesthesiology, 106
(2), 275
–282
(2007). http://dx.doi.org/10.1097/00000542-200702000-00015 ANESAV 0003-3022 Google Scholar
A. E. Flatt,
“Tourniquet time in hand surgery,”
Arch. Surg., 104
(2), 190
–192
(1972). http://dx.doi.org/10.1001/archsurg.1972.04180020070013 ARSUAX 0004-0010 Google Scholar
D. ChiuH. H. WangM. R. Blumenthal,
“Creatine phosphokinase release as a measure of tourniquet effect on skeletal muscle,”
Arch. Surg., 111
(1), 71
–74
(1976). http://dx.doi.org/10.1001/archsurg.1976.01360190073013 ARSUAX 0004-0010 Google Scholar
H. J. Appellet al.,
“Skeletal muscle damage during tourniquet-induced ischaemia: the initial step towards atrophy after orthopaedic surgery?,”
Eur. J. Appl. Physiol. Occup. Physiol., 67
(4), 342
–347
(1993). http://dx.doi.org/10.1007/BF00357633 EJAPCK 1432-1025 Google Scholar
A. A. Sapegaet al.,
“Optimizing tourniquet application and release times in extremity surgery: a biochemical and ultrastructural study,”
J. Bone Joint Surg. Am., 67
(2), 303
–314
(1985). Google Scholar
J. L. BollmanE. V. Flock,
“Changes in phosphate of muscle during tourniquet shock,”
Am. J. Physiol., 142 290
–292
(1944). AJPHAP 0002-9513 Google Scholar
R. B. Heppenstallet al.,
“A comparative study of the tolerance of skeletal muscle to ischemia: tourniquet application compared with acute compartment syndrome,”
J. Bone Joint Surg. Am., 68
(6), 820
–828
(1986). Google Scholar
S. Terakadoet al.,
“Early occurrence of respiratory muscle deoxygenation assessed by near-infrared spectroscopy during leg exercise in patients with chronic heart failure,”
Jpn. Circ. J., 63
(2), 97
–103
(1999). http://dx.doi.org/10.1253/jcj.63.97 JCIRA2 0047-1828 Google Scholar
W. Moallaet al.,
“Respiratory muscle deoxygenation and ventilatory threshold assessments using near infrared spectroscopy in children,”
Int. J. Sports Med., 26
(7), 576
–582
(2005). http://dx.doi.org/10.1055/s-2004-830332 IJSMDA 0172-4622 Google Scholar
D. T. DelpyM. Cope,
“Quantification in tissue near-infrared spectroscopy,”
Phil. Trans. R. Soc. Lond. B, 352 649
–659
(1997). http://dx.doi.org/10.1098/rstb.1997.0046 PTRBAE 0962-8436 Google Scholar
M. FerrariL. MottolaV. Quaresima,
“Principles, techniques and limitations of near infrared spectroscopy,”
Can. J. Appl. Physiol., 29
(4), 463
–487
(2004). http://dx.doi.org/10.1139/h04-031 1066-7814 Google Scholar
B. Shadganet al.,
“Sternocleidomastoid muscle oxygenation and hemodynamic response to incremental inspiratory threshold loadeing measured by near infrared spectroscopy,”
Respir. Physiol. Neurobiol., 178
(2), 202
–209
(2011). http://dx.doi.org/10.1016/j.resp.2011.06.001 RPNEAV 1569-9048 Google Scholar
R. Boushelet al.,
“Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease,”
Scand. J. Med. Sci. Sports, 11
(4), 213
–222
(2001). http://dx.doi.org/10.1034/j.1600-0838.2001.110404.x SMSSEO Google Scholar
M. C. P. Van Beekveltet al.,
“Muscle tissue oxygenation as a functional tool in the follow up of dermatomyositis,”
J. Neurol. Neurosurg. Psychiatr., 73
(1), 93
–94
(2002). http://dx.doi.org/10.1136/jnnp.73.1.93 JNNPAU 0022-3050 Google Scholar
J. G. Kimet al.,
“Hemodynamic changes in rat leg muscles during tourniquet-induced ischemia-reperfusion injury observed by near-infrared spectroscopy,”
Physiol. Meas., 30
(7), 529
–540
(2009). http://dx.doi.org/10.1088/0967-3334/30/7/001 PMEAE3 0967-3334 Google Scholar
C. Casavolaet al.,
“Blood flow and oxygen consumption with near-infrared spectroscopy and venous occlusion: spatial maps and the effect of time and pressure of inflation,”
J. Biomed. Opt., 5
(3), 269
–276
(2000). http://dx.doi.org/10.1117/1.429995 JBOPFO 1083-3668 Google Scholar
L. M. Gentilello,
“Near-infrared spectroscopy versus compartment pressure for the diagnosis of lower extremity compartmental syndrome using electromyography-determined measurements of neuromuscular function,”
J. Trauma, 51
(1), 1
–8
(2001). http://dx.doi.org/10.1097/00005373-200107000-00001 JOTRA5 0022-5282 Google Scholar
G. Yuet al.,
“Time-dependent blood flow and oxygenation in human skeletal muscles measured with noninvasive near-infrared diffuse optical spectroscopies,”
J. Biomed. Opt., 10
(2), 24
–27
(2005). http://dx.doi.org/10.1117/1.1884603 JBOPFO 1083-3668 Google Scholar
H. Gómezet al.,
“Use of non-invasive NIRS during a vascular occlusion test to assess dynamic tissue saturation response,”
Intensive Care Med., 34
(9), 1600
–1607
(2008). http://dx.doi.org/10.1007/s00134-008-1145-1 ICMED9 0342-4642 Google Scholar
B. Shadganet al.,
“Wireless near-infrared spectroscopy of skeletal muscle oxygenation and hemodynamics during exercise and ischemia,”
Spectroscopy, 23 233
–241
(2009). http://dx.doi.org/10.3233/SPE-2009-0391 SPECET 0887-6703 Google Scholar
M. E. Charlsonetet al.,
“A new method of classifying prognostic comorbidity in longitudinal studies: development and validation,”
J. Chronic Dis., 40
(5), 373
–383
(1987). http://dx.doi.org/10.1016/0021-9681(87)90171-8 JOCDAE 0021-9681 Google Scholar
S. Perrey,
“Non-invasive NIR spectroscopy of human brain function during exercise,”
Methods, 45
(4), 289
–299
(2008). http://dx.doi.org/10.1016/j.ymeth.2008.04.005 MTHDE9 1046-2023 Google Scholar
S. Noordinet al.,
“Surgical tourniquets in orthopaedics,”
J. Bone Joint Surg. Am., 91
(12), 2958
–2967
(2009). http://dx.doi.org/10.2106/JBJS.I.00634 Google Scholar
R. P. DumT. T. Kennedy,
“Physiological and histochemical characteristics of motor units in cat tibialis anterior and extensor digitorum longus muscles,”
J. Neurophysiol., 43
(6), 1615
–1630
(1980). JONEA4 0022-3077 Google Scholar
L. Jamiet al.,
“Distribution of physiological types of motor units in the cat peroneus tertius muscle,”
Exp. Brain Res., 48
(2), 177
–184
(1982). http://dx.doi.org/10.1007/BF00237213 0014-4819 Google Scholar
M. C. Van der Sluijset al.,
“A new and highly sensitive continuous wave near infrared spectrophotometer with multiple detectors,”
Proc. SPIE, 3194 63
–72
(1997). http://dx.doi.org/10.1117/12.301097 PSISDG 0277-786X Google Scholar
B. Grassiet al.,
“Blood lactate accumulation and muscle deoxygenation during incremental exercise,”
J. Appl. Physiol., 87
(1), 348
–355
(1999). JAPYAA 0021-8987 Google Scholar
P. J. Kirkpatricket al.,
“Defining thresholds for critical ischemia by using near-infrared spectroscopy in the adult brain,”
J. Neurosurg., 89
(3), 389
–394
(1997). http://dx.doi.org/10.3171/jns.1998.89.3.0389 JONSAC 0022-3085 Google Scholar
I. Tachtsidiset al.,
“Investigation of in vivo measurement of cerebral cytochrome-c-oxidase redox changes using near-infrared spectroscopy in patients with orthostatic hypotension,”
Physiol. Meas., 28
(2), 199
–211
(2007). http://dx.doi.org/10.1088/0967-3334/28/2/008 PMEAE3 0967-3334 Google Scholar
N. R. FahmyD. G. Patel,
“Hemostatic changes and postoperative deep-vein thrombosis associated with use of a pneumatic tourniquet,”
J. Bone Joint Surg. Am., 63
(3), 461
–465
(1981). Google Scholar
B. Chanceet al.,
“Recovery from exercise-induced desaturation in the quadriceps muscles of elite competitive rowers,”
Am. J. Physiol., 262
(3 Pt 1), C766
–C775
(1992). AJPHAP 0002-9513 Google Scholar
A. N. Kavaziset al.,
“Mechanical ventilation induces diaphragmatic mitochondrial dysfunction and increased oxidant production,”
Free Radic. Biol. Med., 46
(6), 842
–850
(2009). http://dx.doi.org/10.1016/j.freeradbiomed.2009.01.002 FRBMEH 0891-5849 Google Scholar
M. A. Zergerogluet al.,
“Mechanical ventilation-induced oxidative stress in the diaphragm,”
J. Appl. Physiol., 95
(3), 1116
–1124
(2003). JAPYAA 0021-8987 Google Scholar
F. Hammersenet al.,
“The ultrastructure of microvessels and their contents following ischemia and reperfusion,”
Prog. Appl. Microcirc., 13 1
–26
(1989). PAMIEH Google Scholar
P. C. Grisottoet al.,
“Indicators of oxidative injury and alterations of the cell membrane in the skeletal muscle of rats submitted to ischemia and reperfusion,”
J. Surg. Res., 92
(1), 1
–6
(2000). http://dx.doi.org/10.1006/jsre.2000.5823 JSGRA2 0022-4804 Google Scholar
H. J. Appellet al.,
“Administration of tourniquet. Prevention II. of postischemic oxidative stress can reduce muscle edema,”
Arch. Orthop. Trauma Surg., 116
(1–2), 101
–105
(1997). http://dx.doi.org/10.1007/BF00434111 AOTSEF 1434-3916 Google Scholar
J. A. Duarteet al.,
“Administration of tourniquet. I. Are edema and oxidative stress related to each other and to the duration of ischemia in reperfused skeletal muscle?,”
Arch. Orthop. Trauma Surg., 116
(1–2), 97
–100
(1997). http://dx.doi.org/10.1007/BF00434110 AOTSEF 1434-3916 Google Scholar
J. M. LawlerW. SongS. R. Demaree,
“Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle,”
Free Radic. Biol. Med., 35
(1), 9
–16
(2003). http://dx.doi.org/10.1016/S0891-5849(03)00186-2 FRBMEH 0891-5849 Google Scholar
M. Flück,
“Hypoxaemia enhanced peripheral muscle oxidative stress in COPD,”
Thorax, 60
(10), 797
–798
(2005). http://dx.doi.org/10.1136/thx.2005.047738 THORA7 0040-6376 Google Scholar
M. C. Gomez-CabreraE. DomenechJ. Viña,
“Moderate exercise is an antioxidant: upregulation of antioxidant genes by training,”
Free Radic. Biol. Med., 44
(2), 126
–131
(2008). http://dx.doi.org/10.1016/j.freeradbiomed.2007.02.001 FRBMEH 0891-5849 Google Scholar
R. K. Chanet al.,
“Reperfusion injury to skeletal muscle affects primarily type II muscle fibers,”
J. Surg. Res., 122
(1), 54
–60
(2004). http://dx.doi.org/10.1016/j.jss.2004.05.003 JSGRA2 0022-4804 Google Scholar
R. T. Hepple,
“Why eating less keeps mitochondria working in aged skeletal muscle,”
Exerc. Sport Sci. Rev., 37
(1), 23
–28
(2009). http://dx.doi.org/10.1097/JES.0b013e3181877dc5 ESSRB8 0091-6331 Google Scholar
M. G. SchlagK. A. HarrisR. F. Potter,
“Role of leukocyte accumulation and oxygen radicals in ischemia-reperfusion-induced injury in skeletal muscle,”
Am. J. Physiol. Heart Circ. Physiol., 280
(4), H1716
–H1721
(2001). 0363-6135 Google Scholar
R. HudaD. R. SolankiM. Mathru,
“Inflammatory and redox responses to ischaemia/reperfusion in human skeletal muscle,”
Clin. Sci. (Lond.), 107
(5), 497
–503
(2004). http://dx.doi.org/10.1042/CS20040179 Google Scholar
Y. IshiiH. NoguchiY. Matsuda,
“A new tourniquet system that determines pressure in synchtony with systolic blood pressure,”
Arch. Orthop. Trauma Surg., 128
(3), 297
–300
(2008). http://dx.doi.org/10.1007/s00402-007-0446-0 AOTSEF 1434-3916 Google Scholar
M. Schoenet al.,
“Ischemic preconditioning prevents skeletal muscle tissue injury, but not nerve lesion upon tourniquet-induced ischemia,”
J. Trauma, 63
(4), 788
–797
(2007). http://dx.doi.org/10.1097/01.ta.0000240440.85673.fc JOTRA5 0022-5282 Google Scholar
R. Tamaset al.,
“Hemorheological, morphological, and oxidative changes during ischemia-reperfusion of latissimus dorsi muscle flaps in a canine model,”
Microsurgery, 30
(4), 282
–288
(2010). 0738-1085 Google Scholar
S. K. PowersA. N. KavazisJ. M. McClung,
“Oxidative stress and disuse muscle atrophy,”
J. Appl. Physiol., 102
(6), 2389
–2397
(2007). http://dx.doi.org/10.1152/japplphysiol.01202.2006 JAPYAA 0021-8987 Google Scholar
A. J. Smuderet al.,
“Oxidation enhances myofibrillar protein degradation via calpain and caspase-3,”
Free Radic. Biol. Med., 49
(7), 1152
–1160
(2010). http://dx.doi.org/10.1016/j.freeradbiomed.2010.06.025 FRBMEH 0891-5849 Google Scholar
D. Awerbucket al.,
“Skeletal muscle form and function after 4 hr ischemia-hypothermia,”
J. Surg. Res., 57
(4), 480
–486
(1994). http://dx.doi.org/10.1006/jsre.1994.1173 JSGRA2 0022-4804 Google Scholar
R. BoushelC. A. Piantadosi,
“Near-infrared spectroscopy for monitoring muscle oxygenation,”
Acta Physiol. Scand., 168
(4), 615
–622
(2000). http://dx.doi.org/10.1046/j.1365-201x.2000.00713.x APSCAX 0001-6772 Google Scholar
J. Hollanderet al.,
“Superoxide dismutase gene expression in skeletal muscle: fiber-specific adaptation to endurance training,”
Am. J. Physiol., 277
(3 Pt 2), R856
–R862
(1999). AJPHAP 0002-9513 Google Scholar
B. Shadganet al.,
“Diagnostic techniques in acute compartment syndrome of the leg,”
J. Orthop. Trauma, 22
(8), 581
–587
(2008). http://dx.doi.org/10.1097/BOT.0b013e318183136d JORTE5 0890-5339 Google Scholar
|