|
|
1.IntroductionA pleasant appearance is essential for social acceptance and creates a subjective feeling of well-being, self-confidence, and improved quality of life. Smooth and hair-free legs are a part of aesthetic outer appearance in our cultural system, especially for women. Concerning the removal of unwanted hair, there are several possibilities to deal with. Considering the individual wishes and preferences of women, there are short-, medium-, and long-term solutions for hair removal. In the area of short-term hair removal there are two options available: the hair can be clipped on the surface of the skin by a shaving system. This method is a mechanical process and has to be differentiated from the use of a hair removal cream, which is based on a chemical procedure. Thioglycolic acid is the active ingredient in chemical epilating creams.1 Waxing, sugar epilation, or tweezer epilation are medium-term hair removals and pertain to mechanical elimination of the hair shaft from the hair follicle. For the long-term elimination or permanent destruction of hair follicles, laser hair removal is widely practiced and works with the primary principle of selective photothermolysis.2 For monitoring hair follicle activity, an operator- and patient-friendly, reliable, and sensitive method is indispensable. At present, Trichoscan® is the best officially used method of quantitatively assessing the biological parameters of hair growth.3 Thereby, sequential macro photographs of a target hair area are taken and hair density or hair growth is calculated at given time points after treatment. This is an automated technique that is based on digital photo analysis, originally developed for image analysis of skin dermoscopy used instead of conventional photography. To render detection of even blond, gray, or fine hairs possible, hair shafts have to be dyed with a coloring solution to enhance the visibility.4 This procedure is time consuming, as patients need to return, in order to visualize hair growth after three days to differentiate growing from nongrowing hair follicles. Nowadays, modern spectroscopic and optical methods like reflectance confocal microscopy (RCM) and optical coherence tomography (OCT) are available, which allow a noninvasive analysis of the skin surface and the hair structure5–8 under in vivo conditions. One of the main tasks of RCM in the field of dermatology has up to now been the evaluation of skin diseases, like basal cell carcinoma, actinic keratosis, Bowen’s disease, and vascular lesions.9–11 On the basis of producing horizontal optical investigations of the skin at a cellular resolution, the opportunity to evaluate the hair structure from a different point of view is provided.10 OCT, however, allows real-time cross-sectional evaluation similar to histological sections and has been established for more than 10 years in clinical skin research.12–16 The application and comparison of these techniques in the field of hair removal provide a new experience and may furnish the proof of the efficiency of the various possibilities of hair removal. The aim of this study was to compare RCM and OCT as noninvasive, reliable, and alternative methods to Trichoscan®. This may be useful in the evaluation and monitoring of hair growth in clinical indications. 2.Material and Methods2.1.VolunteersSix healthy women aged between 25 and 50 years (mean age SD) participated in the study. Exclusion criteria were a history of skin disease, pregnancy or lactation, and known or suspected intolerance or hypersensitivity to the investigational products or any of their ingredients. All participants had given their written consent. The study was conducted in accordance with the Declaration of Helsinki and prior approval for the study had been obtained from the Ethics Committee of the Charité—Universitätsmedizin Berlin. 2.2.TrichoscanTrichoscan® Professional (TRICHOLOG GmbH, Freiburg, Germany) with the camera FotoFinder® (FotoFinder Systems GmbH, Bad Birnbach, Germany) is a modification of the conventional semi-invasive trichogram and allows a non invasive, painless measurement with highly reliable and validated results.17 This method combines standard epiluminescence microscopy with automatic digital image analysis, providing a high amount of information in a quantitative form regarding biological parameters of hair growth18 in an area of . These parameters were analyzed and differentiated by mathematical approximation.19 2.3.Reflectance Confocal MicroscopyThe RCM Vivascope 1500 (Lucid Inc. Rochester, NY, USA and MAVIG GmbH, Munich, Germany) was used to visualize hair shafts in the hair follicles on the first study visit. This method is based on the reflectance, scattering, and absorption of monochromatic light by endogenous chromophores of cellular microstructures.20 A near-infrared diode laser with a wavelength of 830 nm is focused on the follicle and the tissue in a selected layer and is examined by a raster scanner. The different refraction indices of cell structure and skin layers show determined reflection patterns that are translated into grayscale for imaging.21 The lateral resolution of 0.5 to 1 μm and axial resolution of 3 to 5 μm, with an optical penetration depth of 250 to 300 μm, is comparable to routine histology.20 More detailed information of this technique and the device used has been described previously.20–23 2.4.Optical Coherence TomographyThe OCT measurements were carried out using the Spectral Domain OCT system Callisto® (Thorlabs GmbH, Germany). This is the optical analogue of ultrasound, but instead of longitudinal ultrasound waves, infrared light, capable of imaging at a maximum detection depth up to 1.7 mm in the sample, is used. The axial resolution in air amounts to 7.0 μm and improves by the refraction index of the skin to 5.0 μm. The Callisto® operates at a maximum axial scan rate of 1.2 kHz, which results in 2 frames per second.24,25 The Spectral Domain OCT utilizes a light source with low temporal coherence to measure optical path length. Light from a broadband SLD with a center wavelength of 930 nm is divided into a sample and a reference arm of a fiber-based Michelson interferometer setup. The light from the sample fiber is refocused by an objective into the skin and hair follicle. The reflected light is recoupled into the sample arm fiber and then combined with the light that has traveled a fixed optical path length and returned from the reference arm. The resulting interferogram is relayed to a detector for conversion into an electrical signal.26,27 OCT extracts spectral information by distributing different optical frequencies onto the spectrometer detector. The information can be acquired within a single exposure, without a moving part. Therefore, it allows a high mechanical stability and low phase noise.24 2.5.Study DesignThe study was carried out as an open-label, single-center study according to a randomized Latin square design. The study was performed on the lower legs of healthy female volunteers with at least a hair length of 2 mm. Before application, the women had to undergo a skin test to exclude an allergic reaction. Before starting the hair removal, the hair was colored using a conventional black dye. The lower leg was rinsed with warm water at 35 to 40°C before shaving. After covering the examined skin area with a layer of women’s shaving foam based on no soap moisturization, a commercially available razor was pulled over the covered area until all the shaving cream had been removed. Finally, the leg was rinsed and patted dry. The length of the growing hair was measured with the Trichoscan®, OCT, and RCM methods immediately after removal. After three and five days, the lengths of the same growing hairs were again measured with Trichoscan® and OCT. 3.Results and DiscussionThe optical methods applied in the present study provide images at different section planes of the skin. The Trichoscan® and RCM measurements provide horizontal images parallel to the skin surface, whereas OCT produces perpendicular sections similar to histological sections obtained from biopsies. Typical images are presented in Fig. 1 [Fig. 1(a): Trichoscan®, Fig. 1(b): RCM, Fig. 1(c): OCT]. Whereas Trichoscan® is limited to the surface, RCM can penetrate the skin up to 200 μm. In this study, OCT detects at a depth of approximately 1000 μm. Trichoscan® and OCT images of the lower leg at baseline, day 3 and day 5 are shown in Figs. 2 and 3. An RCM image at baseline is presented in Fig. 4. The measured hair lengths of Trichoscan® and OCT are demonstrated in Fig. 5. Here, RCM values are not included, as RCM measurement could only be evaluated at baseline and this measuring method does not determine the total length of the hair. Instead, the gap between the skin surface and the clipped end of the hair, which was cut in the infundibulum, was measured. Fig. 1Hair images obtained by different optical methods: (a) Trichoscan®, day 3 image of the scalp; (b) reflectance confocal microscopy (RCM), hair of the lower leg; (c) optical coherence tomography (OCT), hair of the lower leg; the epidermis (EP), the dermis (DE), the hair follicle (HF) and hair (HA). 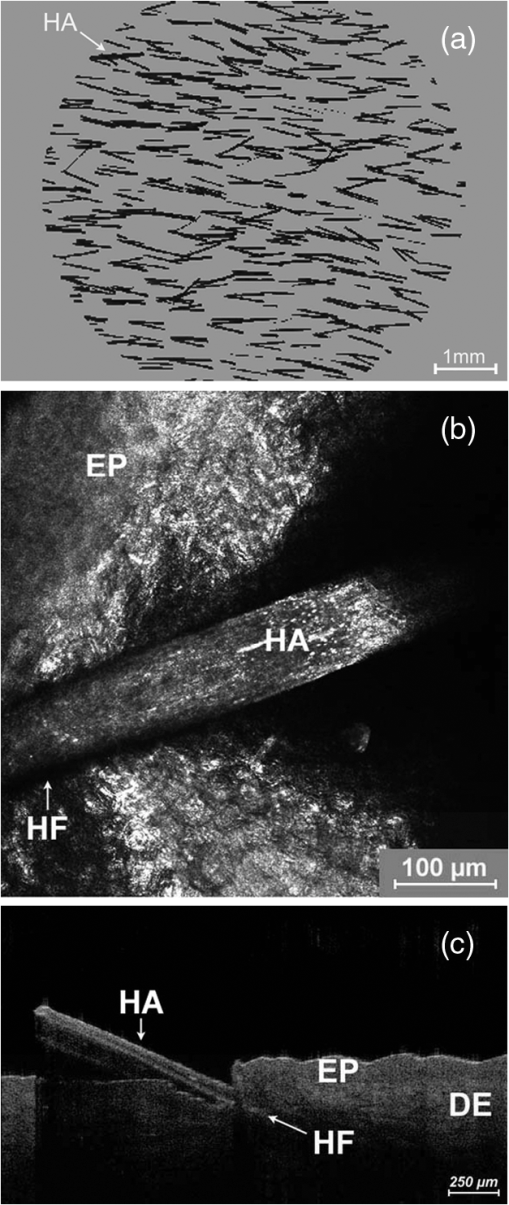 Fig. 2Hair growth measurement images obtained by Trichoscan®, (a) baseline post-shave treatment of the lower leg, (b) day 3, and (c) day 5, analysis of the images, dark discoloration: remains of dye, hair (HA). 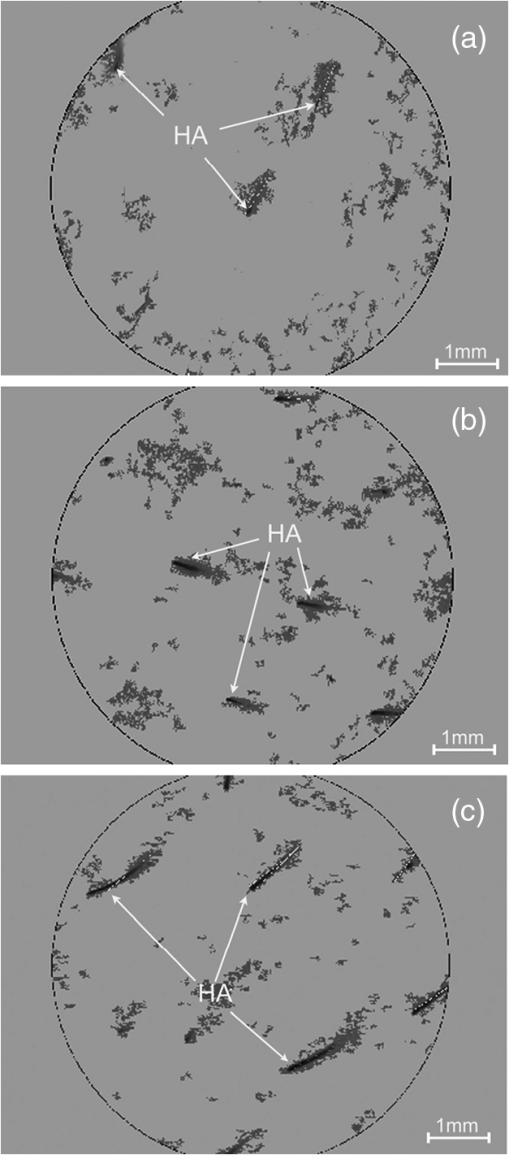 Fig. 3Hair growth measurement images obtained by optical coherence tomography (OCT), (a) baseline post-shave treatment of the lower leg, (b) day 3 and (c) day 5, the epidermis (EP), the dermis (DE), the hair follicle (HF), and hair (HA). 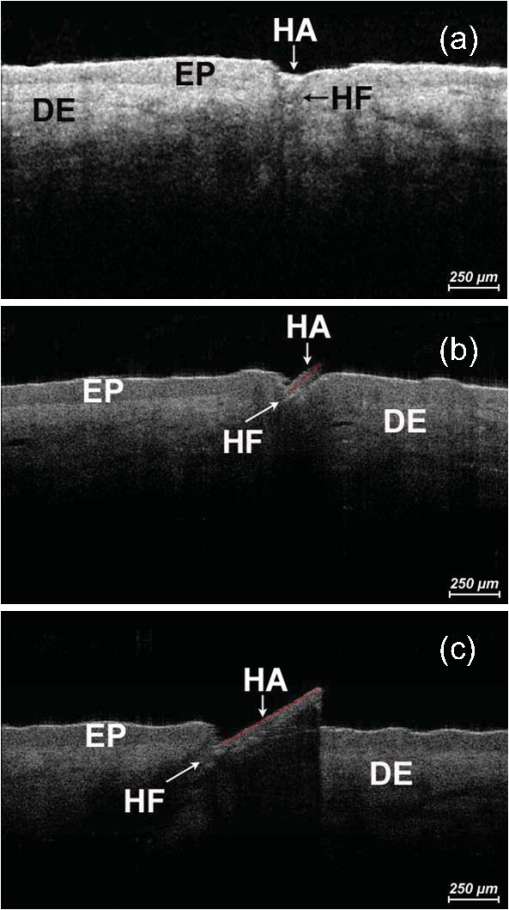 Fig. 4Hair growth measurement image obtained by reflectance confocal microscopy (RCM), baseline post-shave treatment of the lower leg, epidermal cells (EC), hair follicle (HF), and hair (HA). 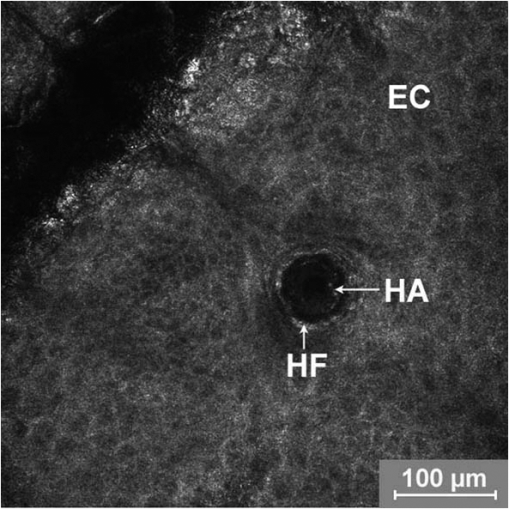 Fig. 5Hair lengths (μm) measured by Trichoscan® (Tricho) and optical coherence tomography (OCT), TP 1: baseline, TP 2: day 3, TP 3: day 5. Significant differences of are marked with an asterisk (*). 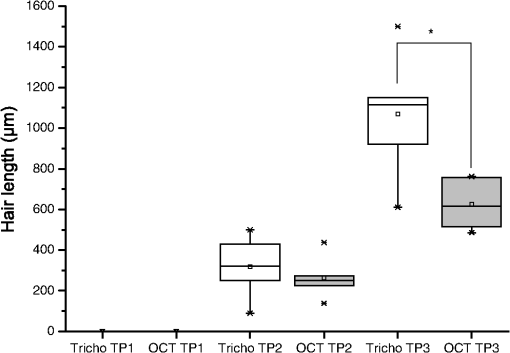 All three methods, Trichoscan®, RCM, and OCT, are suited to obtain useful and exact hair evaluation results. Trichoscan® is promoted as a precise tool for measurement of hair growth parameters, which can be sufficiently emphasized in this study. This tool produces high quality and reproducible digital images, because the images are always taken with same distance between the lens and the skin surface.28 It is particularly suited for measurements on skin surfaces with a high amount of hairs like the scalp [Fig. 1(a)]. In areas of sparse hair growth such as the lower leg, as shown in Fig. 2, this method is increasingly more suitable, because the hairs are located in juxtaposition and do not overlap. It is easier to examine hair length in a single step, if the hair is in an orderly manner. Another advantage of this tool is the ability to measure not only hair length, but also other parameters like density, diameter, growth rate, vellus, and terminal hair density. RCM and OCT unfortunately do not feature these properties and therefore only hair growth results are in comparison. Although, the Trichoscan® method is relatively easy to operate, some investigations had dye remnants, which resulted in artifacts. As a consequence, not only a strict quality control of incoming images had to be carried out, but in some cases even a separate examination needed to be performed. Reliable results include a careful performance, frequent practice, and long experience, and are therefore not accessible for untrained users. In order to reduce the artifacts, different light sources should be used and associated with a new software development, so that in the near future, a hair dye will no longer be necessary.18 RCM is the method with the highest possible magnification in noninvasive imaging of the epidermis, upper dermis, and distal parts of hair follicles and hairs. This precise diagnostic tool enables a cell differentiation, thus which layer is presented and even the damage to the cell structure by mechanical manipulation, possibly by certain hair removal methods. Intensity and velocity of blood flow and aggregation of inflammatory cells are important indicators for the detection of skin damage and can be performed by RCM.11 By moving the laser focus on the skin surface into deeper parts of the skin, the end of the shaved hair can be detected (Fig. 4). The displacement length of the focus from the skin surface to the hair end is the measurement of the depth at which the hair was cut in the hair follicle. The deeper the hair is clipped in the hair follicle, the longer it takes until it reappears on the skin surface. RCM can only be reasonably applied immediately after hair removal. If the hair protrudes from the hair follicle upon the skin surface, its position is generally not perpendicular to the skin, so that it cannot be measured by displacing the focus in parallel to the skin surface. Image size and detection depth are in comparison to RCM, which achieves a higher solution, the advantages of OCT.29 High precision of measurement and good image quality are characteristics of OCT.24 This technique generates perpendicular images of the skin similar to histological sections. This enables not only the examination of different skin components like the epidermis and upper dermis, but also the imaging of hair follicle and hair length [Fig. 1(c)].30 OCT produces useful images immediately after hair removal in the record of the hair follicle and the exact result, as to how deep the hair is clipped [Fig. 3(a)]. In the images (Fig. 3) both the clipped hair in the hair follicle and later the hair outside the hair follicle can be well recognized. Consequently, this technique is well suited to show the hair removal procedure and growth rate. This method is not influenced by the color of the hair in comparison to Trichoscan® and shows exact values of single hair follicles without any artifacts; it is thus interesting for scientific investigations with its current technology, not useful for daily routine. The disadvantage of OCT is that the hair follicle and its hair length are difficult to detect, because the position of the hair is mostly not perpendicular. Therefore, small differences in length can be seen on day 5 (TP3) between OCT and Trichoscan® (Fig. 5). Although, a field of view in 3-D images can be measured, the slow measuring speed induces camera shakes and the result is often not evaluable. A longer observation time is required to be able to detect the same hair, its follicle, and length from baseline to day 5. It is therefore advisable to apply an OCT system with a faster imaging capability; however, this would be accompanied by a loss of high contrast between the skin layers.24 In addition, it should be possible to investigate a larger area and higher number of skin appendages in a short period of time. 4.ConclusionsThe results of the study demonstrated the potential of Trichoscan®, RCM, and OCT for an unambiguous evaluation of the hair growth after shaving, the short-term mechanical procedure. All three optical methods provided equal results. Modern optical techniques, like RCM and OCT, allow precise assessment of hair growth and may also serve as a sensitive parameter in clinical trials, but do not replace the specially developed technique Trichoscan® for hair growth measurement. These diagnostic tools can be seen as auxiliary examination methods for hair growth. For the investigation of the hair clipping quality, RCM is the method with the highest possible magnification. OCT provides assistance for single hair measurement and purveys exact values. The best measuring method depends on the target and the task of the investigation. Future studies concerning medium- and long-term hair removal could provide further insights. ReferencesJ. N. Leeet al.,
“The effects of depilatory agents as penetration enhancers on human stratum corneum structures,”
J. Invest. Dermatol., 128
(9), 2240
–2247
(2008). http://dx.doi.org/10.1038/jid.2008.82 JIDEAE 0022-202X Google Scholar
O. A. Ibrahimiet al.,
“Laser hair removal,”
Dermatol. Ther., 24
(1), 94
–107
(2011). http://dx.doi.org/10.1111/dth.2011.24.issue-1 1396-0296 Google Scholar
R. Hoffmann,
“A 4-month, open-label study evaluating the efficacy of eflornithine 11.5% cream in the treatment of unwanted facial hair in women using TrichoScan,”
Eur. J. Dermatol., 18
(1), 65
–70
(2008). EJDEE4 1167-1122 Google Scholar
M. Olszewskaet al.,
“Methods of hair loss evaluation in patients with endocrine disorders,”
Endokrynol. Pol., 61
(4), 406
–411
(2010). EDPKA2 0423-104X Google Scholar
J. Lademannet al.,
“Penetration and storage of particles in human skin: Perspectives and safety aspects,”
Eur. J. Pharm. Biopharm., 77
(3), 465
–468
(2011). http://dx.doi.org/10.1016/j.ejpb.2010.10.015 EJPBEL 0939-6411 Google Scholar
U. Blume-Peytaviet al.,
“Follicular and percutaneous penetration pathways of topically applied minoxidil foam,”
Eur. J. Pharm. Biopharm., 76
(3), 450
–453
(2010). http://dx.doi.org/10.1016/j.ejpb.2010.06.010 EJPBEL 0939-6411 Google Scholar
J. Lademannet al.,
“Which skin model is the most appropriate for the investigation of topically applied substances into the hair follicles?,”
Skin Pharmacol. Phys., 23
(1), 47
–52
(2010). http://dx.doi.org/10.1159/000257263 1660-5527 Google Scholar
J. Lademannet al.,
“Hair follicles—an efficient storage and penetration pathway for topically applied substances. Summary of recent results obtained at the Center of Experimental and Applied Cutaneous Physiology, Charite—Universitatsmedizin Berlin, Germany,”
Skin Pharm. Physiol., 21
(3), 150
–155
(2008). http://dx.doi.org/10.1159/000131079 1660-5527 Google Scholar
M. Ulrichet al.,
“Evaluation of Bowen‘s disease by in vivo reflectance confocal microscopy,”
Br. J. Dermatol., 166
(2), 451
–453
(2012). http://dx.doi.org/10.1111/j.1365-2133.2011.10563.x BJDEAZ 1365-2133 Google Scholar
M. Ulrichet al.,
“Peritumoral clefting in basal cell carcinoma: correlation of in vivo reflectance confocal microscopy and routine histology,”
J. Cutan. Pathol., 38
(2), 190
–195
(2011). http://dx.doi.org/10.1111/cup.2011.38.issue-2 JCUPBN 0303-6987 Google Scholar
S. Astneret al.,
“Preliminary evaluation of benign vascular lesions using in vivo reflectance confocal microscopy,”
Dermatol. Surg., 36
(7), 1099
–1110
(2010). http://dx.doi.org/10.1111/j.1524-4725.2010.01590.x 1076-0512 Google Scholar
T. GambichlerV. JaedickeS. Terras,
“Optical coherence tomography in dermatology: technical and clinical aspects,”
Arch. Dermatol. Res., 303
(7), 457
–473
(2011). http://dx.doi.org/10.1007/s00403-011-1152-x ADMFAU 0003-9187 Google Scholar
H. Streseet al.,
“Application of optical methods to characterize textile materials and their influence on the human skin,”
J. Biomed. Opt., 16
(4), 046013
(2011). http://dx.doi.org/10.1117/1.3562978 JBOPFO 1083-3668 Google Scholar
N. Garcia Bartelset al.,
“Optical coherent tomography: promising in vivo measurement of hair shaft cross section,”
J. Biomed. Opt., 16
(9), 096003
(2011). http://dx.doi.org/10.1117/1.3626210 JBOPFO 1083-3668 Google Scholar
J. Enfieldet al.,
“In-vivo dynamic characterization of microneedle skin penetration using optical coherence tomography,”
J. Biomed. Opt., 15
(4), 046001
(2010). http://dx.doi.org/10.1117/1.3463002 JBOPFO 1083-3668 Google Scholar
K. G. Phillipset al.,
“Dermal reflectivity determined by optical coherence tomography is an indicator of epidermal hyperplasia and dermal edema within inflamed skin,”
J. Biomed. Opt., 16
(4), 040503
(2011). http://dx.doi.org/10.1117/1.3567082 JBOPFO 1083-3668 Google Scholar
U. Blume-Peytaviet al.,
“Unwanted facial hair: affects, effects and solutions,”
Dermatology, 215
(2), 139
–146
(2007). http://dx.doi.org/10.1159/000104266 DERMEI 0742-3217 Google Scholar
R. Hoffmann,
“Trichoscan: what is new?,”
Dermatology, 211
(1), 54
–62
(2005). http://dx.doi.org/10.1159/000085581 DERMEI 0742-3217 Google Scholar
R. Hoffmann,
“TrichoScan: a novel tool for the analysis of hair growth in vivo,”
in J. Invest. Derm. Symp. Proc.,
109
–115
(2003). Google Scholar
M. Ulrichet al.,
“Noninvasive diagnostic tools for nonmelanoma skin cancer,”
Br. J. Dermatol., 157
(suppl 2), 56
–58
(2007). http://dx.doi.org/10.1007/s00105-009-1878-y HAUTAW 1432-1173 Google Scholar
S. AstnerM. Ulrich,
“Confocal laser scanning microscopy,”
Hautarzt, 61
(5), 421
–428
(2010). http://dx.doi.org/10.1007/s00105-009-1878-y HAUTAW 1432-1173 Google Scholar
M. Ulrichet al.,
“Actinic keratoses: non-invasive diagnosis for field cancerisation,”
Brit. J. Dermatol., 156
(3), 13
–17
(2007). http://dx.doi.org/10.1111/bjd.2007.156.issue-s3 BJDEAZ 1365-2133 Google Scholar
M. Rajadhyakshaet al.,
“In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology,”
J. Invest. Dermatol., 113
(3), 293
–303
(1999). http://dx.doi.org/10.1046/j.1523-1747.1999.00690.x JIDEAE 0022-202X Google Scholar
A. G. Podoleanu,
“Optical coherence tomography,”
Brit. J. Radiol., 78
(935), 976
–988
(2005). http://dx.doi.org/10.1259/bjr/55735832 BJRAAP 0007-1285 Google Scholar
J. Welzel,
“Optical coherence tomography,”
Hautarzt, 61
(5), 416
–420
(2010). http://dx.doi.org/10.1007/s00105-009-1879-x HAUTAW 1432-1173 Google Scholar
J. Lademannet al.,
“Application of optical non-invasive methods in skin physiology: a comparison of laser scanning microscopy and optical coherent tomography with histological analysis,”
Skin Res. Technol., 13
(2), 119
–132
(2007). http://dx.doi.org/10.1111/srt.2007.13.issue-2 0909-752X Google Scholar
A. Alexet al.,
“Multispectral in vivo three-dimensional optical coherence tomography of human skin,”
J. Biomed. Opt., 15
(2), 026025
(2010). http://dx.doi.org/10.1117/1.3400665 JBOPFO 1083-3668 Google Scholar
R. HoffmannD. Van Neste,
“Recent findings with computerized methods for scalp hair growth measurements,”
in J. Invest. Derm. Symp. Proc.,
285
–288
(2005). Google Scholar
J. Welzel,
“Optical coherence tomography in dermatology: a review,”
Skin Res.Technol., 7
(1), 1
–9
(2001). Google Scholar
J. Lademannet al.,
“Application of optical coherent tomography for skin diagnostics,”
Laser Phys., 15
(2), 288
–294
(2005). LAPHEJ 1054-660X Google Scholar
|

