|
|
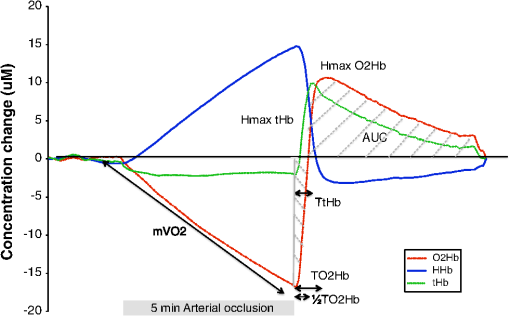
1.IntroductionMicrovascular function is structurally dependent of microvascular density and capillary recruitment, and refers to small vessels (less than 150 μm) including arterioles, capillaries, and venules.1 Microvascular dysfunction is thought to contribute to insulin resistance and hypertension associated with obesity, and potentially links central adiposity with cardiovascular disease risk.1 An impairment of microvascular function (measured by hyperemic velocity) was found to be predictive of future cardiovascular events in apparently healthy men.2 Microvascular function assessed by fingertip temperature variation upon brachial artery occlusion was shown to correlate with coronary artery calcification, myocardial perfusion, and insulin resistance in asymptomatic individuals.3–5 Recently, assessment of microvascular function using near-infrared spectroscopy (NIRS) during a 5 min brachial artery postocclusive reactive hyperemia (PORH) was performed in healthy adults and in patients with peripheral arterial disease and Chronic Heart Failure (CHF).6–10 It was also shown by our group that microvascular function measured by NIRS during PORH was impaired in patients with metabolic syndrome and coronary heart disease and that the degree of impairment was related to the number of cardiovascular risk factors.11 However, only one previous small study performed with six healthy subjects has documented the reproducibility of lower limb NIRS PORH parameters.8 Thus, studies reporting the reproducibility of NIRS parameters in evaluating microvascular function during forearm PORH are lacking. Such data would be of great interest since forearm PORH evaluation by NIRS is a simple, noninvasive, low-cost, and a less uncomfortable technique than lower limb microvascular function evaluation. This makes forearm PORH evaluation by NIRS the method of choice to evaluate microvascular function in situations outside lower limb peripheral vascular disease. Furthermore, the inter-observer reproducibility of NIRS parameters measured during PORH has also not been established. The aims of this study were therefore: 1. to evaluate the intra-subject reproducibility, and 2. to evaluate the inter-observer reproducibility of forearm NIRS parameters measured during PORH in young healthy males. 2.Methods2.1.SubjectsTwenty-four young healthy male subjects were recruited in the study. Participants were included if they were healthy nonsmoking men, aged between 18 and 50 years, and exempt from any cardiovascular risk factors or diseases. Exclusion criteria were , diagnosed (treated/untreated) hypertension (), abdominal obesity (), diabetes mellitus, and/or previous cardiovascular disease (coronary heart disease and/or heart failure). Subjects taking any pharmacological drugs, vitamin C, or omega-3 polyunsaturated fatty acid supplements were also excluded from the study to control for potential impact on vascular function. Participants were told to refrain from any strenuous exercise and alcohol consumption the day prior to testing. This study was approved by the Montreal Heart Institute Ethics Committee and all subjects provided written informed consents. 2.2.Study ProtocolEach subject underwent a medical history, a physical examination with measurement of height, weight, waist circumference, and body composition with bio-electrical impedance (Tanita, model BC418, Japan). A blood draw was also done to measure fasting blood glucose, insulin, and lipid profile. Microvascular function assessment by NIRS during forearm PORH was repeated on two different occasions separated by a minimum of 7 and a maximum of 30 days ( days). Assessments were performed in the early morning (8:30 to 9:00 am) while subjects were overnight-fasted and resting in a supine position in a quiet, dimly lit and temperature controlled room (22°C to 25°C). An automated pneumatic cuff inflator (Hokanson model E20) was positioned above the right elbow and NIRS optodes were positioned below the elbow on the right brachio-radialis muscle with an inter-optode distance of 45 mm. The optodes were attached to the skin of participants with adhesive stickers so the angle and position of the optodes were kept constant. Optodes were connected to a continuous-wave NIRS (Oxymon, Artinis Medical Systems, Nijmegen, Netherlands) that generated light at 905, 850, and 770 nm, which differentiated between oxy- and deoxyhemoglobin/myoglobin ( and , respectively).12,13 For convenience, both hemoglobin and myoglobin will be referred to as hemoglobin since their respective spectrum cannot be distinguished. The changes in absorption at the discrete wavelengths were converted into concentration changes of and HHb (μM) using a modified Lambert—Beer law in which a path-length factor was incorporated to correct for scattering of photons in the tissue.14 The sum of both signals represented total hemoglobin (tHb). To correct for scattering of photons in the tissue, a differential path-length factor of 4.0 was used for the calculation of absolute concentration changes.13,15 NIRS acquisition was done continuously during a 2 min preocclusive rest period, 5 min occlusion (pressure cuff inflated at 100 mm Hg over the systolic blood pressure), and a 5 min postocclusive period.7,11 NIRS signals were sampled at 10 Hz, displayed in real time and stored on a hard drive for off-line software analysis (Oxysoft, Artinis Medical Systems, Netherlands). Figure 1 shows typical NIRS signals for , HHb, and tHb that are used for off-line NIRS parameters analysis. (1) Muscle oxygen consumption () () was measured by evaluating the rate of decrease of during arterial occlusion [].7,11–13 Concentration changes of were expressed in and converted to .12,13 A value of was used for muscle density.12,13 The following NIRS parameters were measured during PORH: (2) ½ time recovery of the ( ) (s): time after release of the cuff until the initial preocclusion values are reached, (3) time to maximal () (s): time between the release of the cuff and the maximum value of is reach, (4) maximal amplitude of (Hmax ) (μm): maximal amplitude of postocclusion signal, (5) maximal amplitude of tHb (Hmax tHb) (μm): peak value of postocclusive tHb, (6) time to maximal tHb (TtHb) (s): time between the release of the cuff and the maximum value of tHb is reach, (7) increase rate to max was calculated by dividing maximal amplitude of by time to maximal , (8) increase rate to max was calculated by dividing HRmax of tHb by time to Hmax of tHb, (9) postocclusion area under the curve of (AUC ) (arbitrary unit; a.u.) is the area under the 5 min postocclusion curve, (10) post-occlusion area under the curve of HHb (AUC HHb) (a.u.) is the area under the 5 min postocclusion HHb curve, (11) post-deflation area under the curve of tHb (AUC tHb) (a.u.) is the area under the 5 min postocclusion tHb curve.7,11 2.3.Statistical AnalysisData were analyzed using Statview software (SAS, USA, version 5.0) and are presented as except where otherwise indicated. Normal distribution of the data was verified by a Shapiro-Wilk test and data were transformed logarithmically when this criteria was not met. For continuous variables, statistical differences between tests were evaluated by a one-way ANOVA with repeated measure. A was considered significant. Intra-subject reproducibility was obtained by comparing NIRS parameters evaluated on two different occasions, in the same subjects. Inter-observer reproducibility was obtained by having two observers familiar with the methodology calculate each NIRS parameter for the same NIRS evaluation of every subject. The relative reproducibility, defined by the degree to which individuals maintain their rank order in a sample with repeated measurements,16,17 was assessed by the intraclass correlation coefficient (ICC).16 An was defined as poor, an ICC between 0.21 to 0.40 was defined as fair, an ICC between 0.41 and 0.60 was defined as satisfactory, an ICC between 0.61 and 0.80 was defined as good, and an was defined as an excellent agreement between both evaluations.18 Absolute reproducibility was evaluated with coefficient of variation (CV) [] and standard error of measurement (SEM) calculated as recommended by Hopkins [].16 SEM represents the degree to which repeated measurements vary for a given individual (i.e., trial-to-trial noise) and will be presented as a percentage of the average of both evaluations.16,17 3.Results3.1.Baseline CharacteristicsBaseline characteristics of the study population are given in Table 1. Subjects were healthy young males, without any cardiovascular risk factors or diseases. Study subjects had normal body composition and blood glucose, insulin, and lipid levels. Table 1Baseline characteristics of study subjects (n=24).
3.2.Microvascular Function ReproducibilityTable 2 describes mean NIRS parameters measured during both PORH and shows that mean NIRS parameters did not differ significantly. Table 3 describes intra-subject reproducibility of NIRS parameters measured during both PORH. Most parameters had good (, , Hmax , TtHb, AUC , and AUC tHb, ICC ranging from 0.62 to 0.73) to excellent (, ICC of 0.84) intra-subject reproducibility. The less reproducible NIRS parameters classified as poor to satisfactory were: Hmax tHb, increase rates to max. tHb and and AUC HHb; ICC: 0.31 to 0.59. Table 4 describes inter-observer reproducibility of NIRS parameters measured during both PORH. Inter-observer reproducibility of all NIRS parameters ranged from excellent to perfect (ICC ranging from 0.85 to 1.00). Table 2NIRS parameters measured during postocclusive reactive hyperemia.
Table 3Intra-subjects reproducibility of NIRS parameters during postocclusive reactive hyperemia.
Table 4Inter-observer reproducibility of NIRS parameters during postocclusive reactive hyperemia.
4.DiscussionThe main findings of the present study were that 1. intra-subject relative reproducibility of microvascular function assessed by NIRS during PORH ranged from good to excellent for most parameters and that 2. excellent inter-observer relative reproducibility of microvascular function assessed by NIRS during PORH was obtained for all parameters. This study is the first to document intra-subject relative and absolute reproducibility of such a variety of NIRS parameters during brachial PORH and is the first to document their inter-observer relative and absolute reproducibility. 4.1.Relative Intra-Subject ReproducibilityRelative intra-subject reproducibility (ICC) of , , , Hmax , TtHb, AUC , and AUC tHb ranged from good to excellent when assessed by NIRS during PORH in young healthy males. Among these parameters, is the most often studied since it is the ultimate measure of resting muscle metabolic rate and reflects resting muscle oxygen consumption.6 The average obtained from our study () agreed with previously published data measured in the forearm of healthy subjects ( to ).13 The assessment of forearm is of particular interest since it was shown to be significantly reduced in patients with CHF,6,9 metabolic syndrome,11 and with chronic or acute smoking.10 Lowered reflects lower muscular ability to extract oxygen and impaired mitochondrial function.6 Other NIRS parameters that showed good relative intra-subject reproducibility reflect the ability of muscle to recruit arterioles and capillaries during reperfusion. Of these parameters, Hmax , Hmax tHb, and AUC were recently shown by our group to be impaired in patients with metabolic syndrome and/or coronary heart disease.11 Additionally, impairment of these parameters was related to the number of cardiovascular risk factor (ranging from 0 to 4–5 risk factors) independently of cardiovascular status (healthy, metabolic syndrome, or coronary artery disease patients).11 Time to maximal , TtHb, and Hmax are also significantly affected by peripheral arterial disease (longer and TtHb and lower Hmax values).7,19 A parameter similar to increased rate (named tissue oxygen saturation rate) was impaired in CHF and is predictive of reduced peak and inefficient ventilatory capacity.9,20 Microvascular function can also be improved by lifestyle interventions21 (such as exercise training) and NIRS can be useful in assessing the impact of such interventions.22 In fact, a three-month exercise-training program in patients with CHF increased the AUC O2Hb and increased rate O2Hb during PORH.21,22 This demonstrates that increased physical activity improved muscular arteriolar and capillary recruitment and increased the speed of reperfusion9 and adds to the interest of having a simple and reliable method to evaluate such microvascular improvements in the clinical setting. 4.2.Absolute Intra-Subject ReproducibilityAbsolute intra-subject reproducibility assessed with CV and SEM is important to determine the usefulness of NIRS parameters in evaluating the impact of therapeutic interventions (lifestyle or treatment) on microvascular function. We obtained CVs ranging from 6.51% () to 21.71%. To our knowledge, only Van Beekvelt et al. calculated CVs for forearm PORH parameters and obtained a CV of 17.6% for .13 Absolute reproducibility was also studied for other NIRS parameters by Kragelj et al. who obtained higher CV for , , , Hmax , and TtHb following lower limb ischemia in healthy subjects.8 Our study therefore shows improved CV, which could be attributed to our larger sample size and to methodological differences (i.e., ease of NIRS measurement at the forearm, longer occlusion period and use of an automated pressure cuff inflator). In fact, a rapid cuff pneumatic inflator eliminating the short venous occlusion inevitable when inflating by hand could have lowered variation of measurement.7,8 The SEM allows for identification of the minimal detectable change needed to identify statistically important variations between two evaluations of a single subject that cannot be attributed to measurement error. Standard error of measure has already been calculated for some NIRS parameters upon leg artery occlusion7,8 but has never been assessed during forearm PORH. 4.3.Inter-Observer ReproducibilityInter-observer relative reproducibility of all NIRS parameters was found to be excellent, meaning that evaluation of the same NIRS signal by two observers is expected to yield very similar results. Absolute inter-observer reproducibility (CV and SEM) of most NIRS parameters was very small and was lower than that found for intra-subject reproducibility. These important findings indicate that NIRS testing can be performed by different trained-observers and add to the ease of use of this method by eliminating the need for analysis to be done centrally in multicenter studies. 4.4.LimitationsFirstly, this sub-study was part of a principal study only including young male subjects, free of cardiovascular risk factors. Females were excluded from the main study to eliminate potential variations in endothelial function related to the menstrual cycle. There is, however, no reason to believe that reproducibility of microvascular function using NIRS during PORH would be affected by gender. Secondly, there was a broad variation in the length of time between evaluations (from 7 to 30 days). Shorter and constant length between visits, reducing the potential impact of changes in dietary habits, sleep patterns, and stress level variations, might have yielded more reproducible results. On the other hand, the present study demonstrated NIRS parameters to be reproducible over a 7 to 30-day period, which enables their use in studies spanning such periods of time. In conclusion, intra-subject and inter-observer reproducibility of NIRS parameters during PORH was assessed and showed that a majority of parameters had good to excellent intra-subject reproducibility and that every parameters had excellent inter-observer reproducibility.23 Further studies are now needed in patients with cardiovascular risk factors or diseases and in elderly subjects. However, reproducibility of repeated measurement of fasting NIRS parameters in patients with stable conditions and medications should be similar to what was described previously. The impact of lifestyle interventions such as dietary habit modifications and physical activity and therapeutic interventions on NIRS parameters should also be evaluated. There is also a need for standardization of NIRS parameter nomenclature and occlusion protocols to enhance the comparability of future studies. AcknowledgmentsAnil Nigam is funded by “Fonds de Recherche en Santé du Québec.” Mathieu Gayda is funded by the ÉPIC Centre and Montreal Heart Institute Foundations. ReferencesA. M. Jonket al.,
“Microvascular dysfunction in obesity: a potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension,”
Physiology, 22 252
–260
(2007). http://dx.doi.org/10.1152/physiol.00012.2007 PHYSCI 1548-9213 Google Scholar
T. J. Andersonet al.,
“Microvascular function predicts cardiovascular events in primary prevention: long-term results from the firefighters and their endothelium (FATE) study,”
Circulation, 123
(2), 163
–169
(2011). http://dx.doi.org/10.1161/CIRCULATIONAHA.110.953653 CIRCAZ 0009-7322 Google Scholar
N. Ahmadiet al.,
“Low fingertip temperature rebound measured by digital thermal monitoring strongly correlates with the presence and extent of coronary artery disease diagnosed by 64-slice multi-detector computed tomography,”
Int. J. Cardiovasc. Imag., 25
(7), 725
–738
(2009). http://dx.doi.org/10.1007/s10554-009-9476-8 1569-5794 Google Scholar
N. Ahmadiet al.,
“Concomitant insulin resistance and impaired vascular function is associated with increased coronary artery calcification,”
Int. J. Cardiol., 144
(1), 163
–165
(2010). http://dx.doi.org/10.1016/j.ijcard.2008.12.200 IJCDD5 0167-5273 Google Scholar
N. Ahmadiet al.,
“Vascular dysfunction measured by fingertip thermal monitoring is associated with the extent of myocardial perfusion defect,”
J. Nucl. Cardiol., 16
(3), 431
–439
(2009). http://dx.doi.org/10.1007/s12350-008-9044-y JNCAE2 1071-3581 Google Scholar
K. Abozguiaet al.,
“Reduced in vivo skeletal muscle oxygen consumption in patients with chronic heart failure—a study using near infrared spectrophotometry (NIRS),”
Eur. J. Heart Fail., 10
(7), 652
–657
(2008). http://dx.doi.org/10.1016/j.ejheart.2008.05.009 EJHFFS 1388-9842 Google Scholar
R. Krageljet al.,
“Parameters of postocclusive reactive hyperemia measured by near infrared spectroscopy in patients with peripheral vascular disease and in healthy volunteers,”
Ann. Biomed. Eng., 29
(4), 311
–320
(2001). http://dx.doi.org/10.1114/1.1359451 ABMECF 0090-6964 Google Scholar
R. KrageljT. JarmD. Miklavcic,
“Reproducibility of parameters of postocclusive reactive hyperemia measured by near infrared spectroscopy and transcutaneous oximetry,”
Ann. Biomed. Eng., 28
(2), 168
–173
(2000). http://dx.doi.org/10.1114/1.241 ABMECF 0090-6964 Google Scholar
C. Manetoset al.,
“Skeletal muscle microcirculatory abnormalities are associated with exercise intolerance, ventilatory inefficiency, and impaired autonomic control in heart failure,”
J. Heart Lung Transplant., 30
(12), 1403
–1408
(2011). http://dx.doi.org/10.1016/j.healun.2011.08.020 JHLTES 1053-2498 Google Scholar
A. Siafakaet al.,
“Acute effects of smoking on skeletal muscle microcirculation monitored by near-infrared spectroscopy,”
Chest, 131
(5), 1479
–1485
(2007). http://dx.doi.org/10.1378/chest.06-2017 CHETBF 0012-3692 Google Scholar
M. Gaydaet al.,
“Effects of cardiovascular risk factors on macrovascular endothelial function and microvascular reactivity assessed with near-infrared spectroscopy in adults with different cardiovascular status,”
Eur. Heart J., 32
(Suppl. 1), 857
(2011). EHJODF 0195-668X Google Scholar
M. C. Van Beekveltet al.,
“Performance of near-infrared spectroscopy in measuring local O(2) consumption and blood flow in skeletal muscle,”
J. Appl. Physiol., 90
(2), 511
–519
(2001). JAPYAA 0021-8987 Google Scholar
M. C. Van Beekveltet al.,
“In vivo quantitative near-infrared spectroscopy in skeletal muscle during incremental isometric handgrip exercise,”
Clin. Physiol. Funct. Imag., 22
(3), 210
–217
(2002). http://dx.doi.org/10.1046/j.1475-097X.2002.00420.x CPFICA 1475-0961 Google Scholar
L. N. Liveraet al.,
“Effects of hypoxaemia and bradycardia on neonatal cerebral haemodynamics,”
Arch. Dis. Child., 66
(4 Spec No), 376
–380
(1991). http://dx.doi.org/10.1136/adc.66.4_Spec_No.376 ADCHAK 0003-9888 Google Scholar
M. C. Van Beekveltet al.,
“Adipose tissue thickness affects in vivo quantitative near-IR spectroscopy in human skeletal muscle,”
Clin. Sci., 101
(1), 21
–28
(2001). http://dx.doi.org/10.1042/CS20000247 CSCIAE 0143-5221 Google Scholar
W. G. Hopkins,
“Measures of reliability in sports medicine and science,”
Sports Med., 30
(1), 1
–15
(2000). http://dx.doi.org/10.2165/00007256-200030010-00001 SPMEE7 0112-1642 Google Scholar
G. AtkinsonA. M. Nevill,
“Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine,”
Sports Med., 26
(4), 217
–238
(1998). http://dx.doi.org/10.2165/00007256-199826040-00002 SPMEE7 0112-1642 Google Scholar
D. Altman, Practical Statistics for Medical Research, Chapman & Hall/CRC, London
(1991). Google Scholar
H. M. Kooijmanet al.,
“Near infrared spectroscopy for noninvasive assessment of claudication,”
J. Surg. Res., 72
(1), 1
–7
(1997). http://dx.doi.org/10.1006/jsre.1997.5164 JSGRA2 0022-4804 Google Scholar
S. Nanaset al.,
“Inotropic agents improve the peripheral microcirculation of patients with end-stage chronic heart failure,”
J. Card. Fail., 14
(5), 400
–406
(2008). http://dx.doi.org/10.1016/j.cardfail.2008.02.001 JCFAF9 1071-9164 Google Scholar
L. Pasqualiniet al.,
“Lifestyle intervention improves microvascular reactivity and increases serum adiponectin in overweight hypertensive patients,”
Nutr. Metabol. Cardiovasc. Dis., 20
(2), 87
–92
(2010). http://dx.doi.org/10.1016/j.numecd.2009.03.002 NMCDEE 0939-4753 Google Scholar
V. Gerovasiliet al.,
“Physical exercise improves the peripheral microcirculation of patients with chronic heart failure,”
J. Cardiopulm. Rehabil. Prev., 29
(6), 385
–391
(2009). http://dx.doi.org/10.1097/HCR.0b013e3181b4ca4e 1932-7501 Google Scholar
D. H. Thijssenet al.,
“Assessment of flow-mediated dilation in humans: a methodological and physiological guideline,”
Am. J. Physiol. Heart Circ. Physiol., 300
(1), H2
–H12
(2011). http://dx.doi.org/10.1152/ajpheart.00471.2010 0363-6135 Google Scholar
|