|
|
1.IntroductionBlood-oxygen-level–dependent functional magnetic resonance imaging (BOLD-fMRI) has been used to perform functional imaging in brain disorders such as stroke1, 2, 3 and brain tumors,4, 5, 6 under the assumption that such patients exhibit normal evoked cerebral blood oxygenation (CBO) changes in the activation areas. Recent studies have revealed, however, that BOLD-fMRI does not image activation areas correctly in these patients. 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Nevertheless, BOLD-fMRI cannot elucidate the precise mechanisms underlying the failure of BOLD imaging, because BOLD-fMRI provides information mainly about concentration changes of deoxyhemoglobin (deoxy-Hb), which is paramagnetic.19, 20 In contrast, near infrared spectroscopy (NIRS) can measure the concentration of oxyhemoglobin (oxy-Hb) as well as that of deoxy-Hb: changes in total hemoglobin (the sum of oxy-Hb and deoxy-Hb; t-Hb) indicate cerebral blood volume (CBV) changes.21 NIRS thus provides more information about the evoked CBO changes than does BOLD-fMRI. To clarify the mechanisms underlying the failure of BOLD imaging in stroke and brain tumors, we have been comparing NIRS and BOLD-fMRI recording during functional brain activation in these patients.22, 23, 24, 25 This paper reviews the results of functional imaging studies on the brain disorders including our recent studies. In our functional studies, we measured the evoked CBO responses in the primary sensorimotor cortex (PSMC) contralateral to the motor task performance using a NIRO-300 (Hamamatsu Photonics K.K., Hamamatsu, Japan). The BOLD-fMRI signals were measured with a MRI (Symphony, Siemens, Munich, Germany) employing an echoplanar technique. The task paradigm consisted of 40 sec of rest and 40 sec of self-paced hand grasping; this task-rest cycle was repeated six times. The patients studied could perform the motor task similarly to the control subjects at the time of the examination. 2.Technical Differences between NIRS and BOLD-fMRINIRS is an optical method for measuring concentration changes of both oxy-Hb and deoxy-Hb in the cerebral vessels by means of the characteristic absorption spectra of hemoglobin in the near infrared range.21 In contrast, BOLD-fMRI detects neuronal activity by measuring changes in BOLD signal (i.e., the signal), which is caused by concentration changes of paramagnetic deoxy-Hb in the cerebral vessels.19, 20 NIRS studies on normal adults have revealed that neuronal activation generally induce a decrease of deoxy-Hb with increases of oxy-Hb and t-Hb in the activated cortical area, 26, 27, 28, 29, 30, 31, 32 and this is consistent with the physiological basis of BOLD imaging.19, 20 Simultaneous measurements of NIRS and BOLD-fMRI have demonstrated correlations between NIRS parameters and the BOLD signal;33, 34, 35, 36 however it has not yet been established which NIRS parameter correlates best with the BOLD signal in population with altered vasculature. When comparing the data obtained by NIRS and BOLD-fMRI, the following technical differences between NIRS and BOLD-fMRI need to be taken into account. First, there is a large difference in spatial resolution between NIRS and BOLD-fMRI. NIRS measures the averaged blood oxygenation changes within the illuminated area, including the intracranial and extracranial tissues. Therefore, NIRS not only underestimates the CBO changes occurring in the activation area, but also mixes brain-related changes with changes occurring in overlaying tissue.37 In contrast, BOLD-fMRI can selectively detect the blood oxygenation changes in the brain with high spatial resolution. Second, NIRS may not permit measurements of CBO changes in the deep structure of the brain; recent simulation studies have suggested that NIRS measures the CBO changes only at the surface of the cortex due to light reflection at the cerebrospinal fluid layer.38, 39 Third, NIRS is sensitive to all compartments of the cerebral vessels (i.e., arterial, capillary, and venous compartments) within the illuminated area; for deoxy-Hb changes, NIRS is sensitive to the venous and capillary compartments, because changes of deoxy-Hb occur in these compartments. In contrast, BOLD-fMRI is thought to be sensitive mainly to the venous compartment.40 3.Comparison of NIRS and BOLD-fMRI in Stroke PatientsNIRS studies on chronic stroke patients have provided conflicting data concerning the evoked CBO response patterns.3, 22, 23, 30, 41 Kato 3 and Miyai 41 found that chronic stroke patients exhibited a normal evoked CBO response pattern in the activated areas. In contrast, Sakatani revealed that a number of chronic stroke patients exhibited an atypical evoked CBO response pattern (i.e., an increase of deoxy-Hb associated with increases of oxy-Hb and t-Hb) in the left prefrontal cortex during language tasks.30 In addition, we have observed similar atypical evoked CBO changes in the PSMC of stroke patients during contralateral motor tasks.22 Interestingly, despite normal motor function, BOLD-fMRI showed small activation areas in the PSMC on the lesion side, suggesting that altered evoked CBO responses induced by cerebral ischemia cause failure of BOLD imaging in stroke patients. Recently, we have evaluated the quantitative relationships among cerebral ischemic levels, evoked CBO responses, and BOLD imaging in chronic stroke patients displaying different cerebral circulatory conditions, that is, moderate cerebral ischemia [slight reduction of regional CBF (rCBF) and cerebrovascular reserve capacity (CVRC)] and severe cerebral ischemia (marked reduction of rCBF and CVRC), which corresponds to “misery perfusion.”23 The evoked CBO responses in the PSMC were measured by NIRS during contralateral motor tasks and compared with the activation volumes and BOLD signal changes of BOLD-fMRI in the PSMC. In the PSMC on the nonlesion side, the motor task consistently caused a decrease of deoxy-Hb with increases of oxy-Hb and t-Hb, which is consistent with the evoked CBO response observed in normal adults [Fig. 1a ]. In addition, BOLD-fMRI demonstrated robust activation areas in the PSMC on the nonlesion side [Fig. 1b]. In the moderate cerebral ischemia group, the evoked CBO response pattern on the lesion side was similar to that in the control subjects [Fig. 2a ]; however, in the severe cerebral ischemia group, the deoxy-Hb concentrations increased during the entire course of the task concomitantly with increases of oxy-Hb and t-Hb [Fig. 2b]. Fig. 1Evoked CBO responses in the PSMC on the nonlesion side (a) and activation maps of BOLD-fMRI (b) during contralateral motor tasks in a severe cerebral ischemia patient (Ref. 23). The ordinate indicates the concentration changes of the NIRS parameters in arbitrary units. Horizontal thick bars denote the task period . 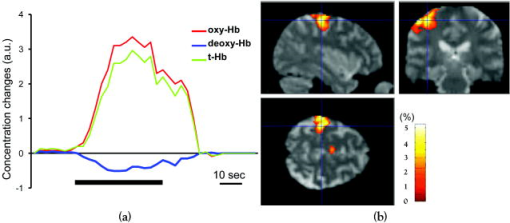 Fig. 2Comparison of evoked CBO responses in the PSMC on the lesion side and activation maps of BOLD-fMRI between patients with moderate cerebral ischemia and severe cerebral ischemia (Ref. 23). (a), (b) Evoked CBO responses in moderate cerebral ischemia (a) and severe cerebral ischemia (b). (c), (d) Activation maps in the moderate cerebral ischemia (c) and severe cerebral ischemia (d). The ordinates indicate the changes of the NIRS parameters in arbitrary units. Horizontal thick bars denote the task period . 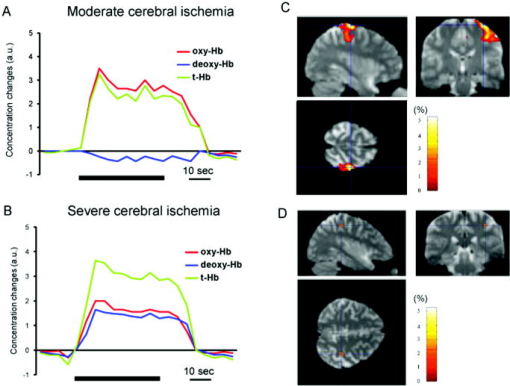 BOLD-fMRI clearly demonstrated activation areas on the lesion side in the moderate cerebral ischemia group [Fig. 2c]; however, the activation volume on the lesion side was slightly smaller than that on the nonlesion side. In the severe cerebral ischemia group, BOLD-fMRI revealed only small activation areas [Fig. 2d]. (It should be noted that none of the patients had motor paresis at the time of examination.) We found that significant correlations existed between the normalized activation volume (activation volume on the lesion side divided by that on the nonlesion side), and the baseline rCBF and % CVR ( ; Fig. 3 ). The BOLD signal increased consistently during the tasks in the PSMC on the nonlesion side in severe cerebral ischemia, where NIRS demonstrated a decrease of deoxy-Hb. In contrast, the BOLD signal did not increase consistently during the tasks in the PSMC on the lesion side, where BOLD-fMRI did not identify neuronal activation, but NIRS revealed increases of deoxy-Hb (Fig. 4 ). The BOLD signal did not change in some areas of the PSMC, while it tended to decrease in other areas during the tasks. Fig. 3Correlations between the normalized activation volume of BOLD-fMRI and the rCBF at rest (a) and %CVR (b) (Ref. 23). Significant positive correlations were observed for both the rCBF at rest and %CVR . 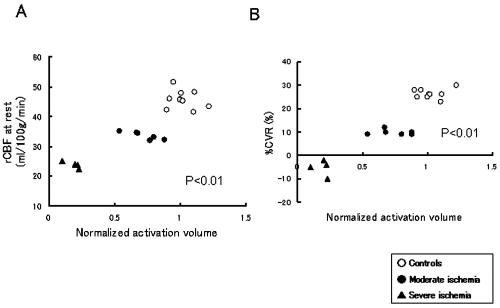 Fig. 4Comparison of BOLD signal changes in the PSMC on the nonlesion side and lesion side in severe cerebral ischemia (Ref. 23). (a), (b) Activation maps for the left grasping task [nonlesion side (a)] and time courses of the BOLD signal changes in the PSMC on the nonlesion side [(b) corresponds to the white circle in (a)]. (c), (d) Activation maps for the right grasping task [lesion side (c)] and time courses of the BOLD signal changes in the PSMC on the lesion side [(d) corresponds to the red circle in (c)]. Thick bars indicate the task period . 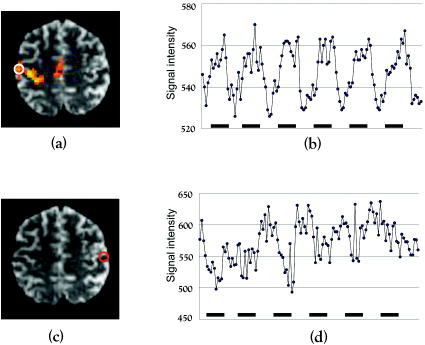 It should be emphasized that cerebral ischemia, particularly misery perfusion, affects the evoked CBO response pattern and impairs BOLD imaging in stroke patients. BOLD-fMRI should therefore be performed on stroke patients while giving consideration to the baseline cerebral circulatory status at the time of examination. Otherwise, the results of the BOLD-fMRI in stroke patients could be open to misinterpretation. 4.Comparison of NIRS and BOLD-fMRI in Brain TumorsAlthough BOLD-fMRI has, in the past, been employed for the preoperative brain function mapping of brain tumors, doubts have recently been expressed concerning its accuracy.7, 8, 9, 10, 11 For example, BOLD-fMRI indicated that patients with brain tumors in or adjacent to the PSMC displayed significantly less activation of the PSMC on the lesion side than on the nonlesion side, although these patients had only mild sensorimotor deficits.8, 9 In addition, the fMRI-defined central sulcus did not coincide with the central sulcus as defined by magnetoencephalography.7 To clarify the underlying mechanisms of the false-negative activation of BOLD-fMRI, we compared the NIRS measured evoked CBO response in the PSMC and the activation maps of BOLD-fMRI in patients with brain tumors.24 NIRS demonstrated two different patterns of evoked CBO changes in the PSMC on the lesion side according to the changes of deoxy-Hb occurring during activation: namely, deoxy-decrease and deoxy-increase patterns, where the concentration of deoxy-Hb decreased and increased during activation, respectively. Figures 5a and 5b compare the evoked CBO changes in the deoxy-decrease and deoxy-increase patterns. It should be noted that the oxy-Hb and t-Hb were increased during the task in both patterns, indicating the occurrence of rCBF increases in response to neuronal activation. Fig. 5Comparison of evoked CBO changes in the PSMC on the lesion side and activation maps of BOLD-fMRI between the deoxy-decrease and deoxy-increase patterns (Ref. 24). (a), (b) Evoked CBO changes in the PSMC on the lesion side in the deoxy-decrease (a) and deoxy-increase (b) patterns. (c), (d) Activation maps of BOLD-fMRI in the deoxy-decrease (c) and deoxy-increase (d) patterns. Asterisks indicate the tumors.  BOLD-fMRI clearly demonstrated activation areas in the PSMC on the lesion side in the deoxy-decrease group [Fig. 5c]. However, in the deoxy-increase group, BOLD-fMRI revealed only small or no activation areas [Fig. 5d]. The normalized activated volumes in the deoxy-increase group were significantly smaller than those in the deoxy-decrease group . Intraoperative brain mapping, which was performed in the patients with glioma, identified the primary motor cortex that could not be detected by BOLD-fMRI (Fig. 6 ), indicating that BOLD-fMRI failed to detect the activation areas in this patient. Fig. 6Activation maps of BOLD-fMRI for the right grasping task (a) and cortical mapping (b) of the left motor cortex in glioma (Ref. 24). The activation maps of BOLD-fMRI demonstrated only limited areas, but the cortical mapping detected the motor cortex (arrows) during surgery. 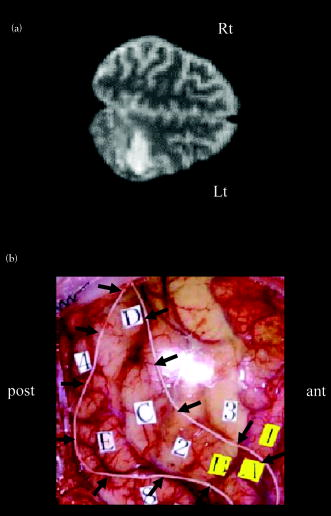 These findings indicate that the atypical evoked CBO changes in activation areas of the brain that have been observed with cerebral ischemia also occur with brain tumors, and that, using BOLD-fMRI, the activation areas in such cases are imaged as being much smaller than they really are. 5.Relationship between NIRS Parameters and BOLD Signal During ActivationBOLD-fMRI disclosed significantly small activation volumes in the PSMC on the lesion side in stroke and brain tumors. We suggest that a failure of BOLD imaging might be caused by the atypical evoked CBO response (i.e., increases of both deoxy-Hb and t-Hb), which would have opposite effects on the BOLD signal change. That is to say, an increase of t-Hb increases the water fraction around the deoxy-Hb molecules in a given voxel leading to an increase of BOLD signal, while an increase of the paramagnetic deoxy-Hb concentration tends to decrease the BOLD signal.19, 20, 42, 43 Such opposite effects of deoxy-Hb and t-Hb on the BOLD signal could lead to a marked reduction of activation volume. The relation between the BOLD signal and evoked CBO responses in the pathological brain may differ from that in the normal brain. Hess observed positive BOLD signals in the gerbil barrel cortex, where optical imaging demonstrated increases of deoxy-Hb and t-Hb during activation.43 However, the BOLD signal did not increase consistently during the motor tasks in the PSMC on the lesion side in severe ischemia, where NIRS revealed increases of deoxy-Hb and t-Hb; the BOLD signal did not change in some areas, while it tended to decrease in other areas during the tasks (Fig. 4). Such a decrease of BOLD signal might be caused by an increase of paramagnetic deoxy-Hb in the activated cortical areas. We have observed a similar decrease of BOLD signals in the PSMC of a glioma patient after resection of the tumor; NIRS indicated increases of deoxy-Hb and t-Hb during activation (Fig. 7 ).25 Such a paradoxical decrease of BOLD signal has also been noted in a stroke patient.13 In addition, a fMRI study on rat stroke models has demonstrated that, compared to the normal cortex, the affected cortex revealed a lower covariance between activated voxels by BOLD and CBV-weighted fMRI during stroke recovery.44 Fig. 7(a) Activation maps of BOLD-fMRI for the right grasping task (lesion side) and the left grasping task (nonlesion side) before and after resection of glioma, which was located in the right frontal lobe (Ref. 25). Postoperative fMRI demonstrated negative BOLD signal changes in the motor cortex on the lesion side. (b) Time course of the BOLD signal changes. White arrows in (a) indicate the region of interest: (a) positive signal changes and (b) negative signal changes. 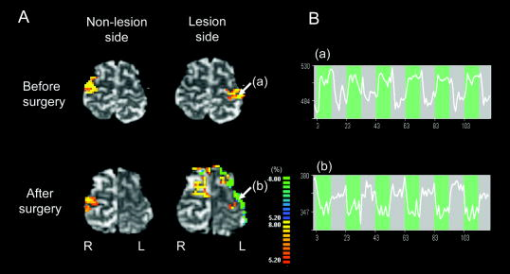 6.Physiological Mechanism of Deoxy-Hb Increase During ActivationAlthough the physiological mechanism of the deoxy-Hb increase occurring during neuronal activity remains unclear, either a decrease of oxygen supply or an increase of oxygen consumption can cause a deoxy-Hb increase. In stroke patients, the reduced rCBF and impaired CVRC under resting conditions could elicit a lesser degree of rCBF rise during activation, resulting in a decrease in the driving force to wash out deoxy-Hb in the capillaries and veins. When such an impairment of the hemodynamic response is advanced, the oxygen extraction could increase to compensate for the decrease in oxygen delivery during activation. Quantitative models of the oxygen delivery during activation predict that disproportionately large increases of rCBF are required for small increases of the oxygen consumption.45 Thus, a small decrease in the evoked rCBF response can give rise to oxygen deficiency during activation, leading to a lesser decrease or elevation of the deoxy-Hb concentrations in the vessels. Such alterations in the hemodynamic effects and oxygen metabolism might occur in patients with brain tumors, because compression by brain tumors or steeling of blood flow by neovascularization of tumors may cause cerebral ischemia around the tumors. A large increase of oxygen consumption during activation can also cause a deoxy-Hb rise. However, the oxygen metabolism during neuronal activity in brain disorders, including stroke and brain tumors, remains unclear because most examinations have been made on normal adults.46, 47, 48 Further studies are needed to clarify in detail the oxygen metabolism and hemodynamics during neuronal activity in brain disorders. 7.SummaryRecent functional imaging studies have demonstrated that BOLD-fMRI does not image activation areas correctly in patients with stroke and brain tumors. We compared NIRS and BOLD-fMRI recording in the brain disorders and revealed that the false-negative activations in the BOLD-fMRI were associated an increase of deoxy-Hb during activation. Under such evoked CBO changes, BOLD signal may not increase consistently because an increase of the paramagnetic deoxy-Hb concentration tends to decrease the BOLD signal. The combined use of NIRS and BOLD-fMRI could facilitate the attainment of a higher level of accuracy in the brain functional imaging of diseased brains. AcknowledgmentsThis work was supported by a grant-in-aid from the Ministry of Education, Culture, Sports, Sciences, and Technology of Japan (Grant No. A12307029) and a grant from Hamamatsu Photonics K.K. (Hamamatsu, Japan). ReferencesS. C. Cramer,
G. Nelles,
R. R. Benson,
J. D. Kaplan,
R. A. Parker,
K. K. Kwong,
D. N. Kennedy,
S. P. Finklestein, and
B. R. Rosen,
“A functional MRI study of subjects recovered from hemiparetic stroke,”
Stroke, 28 2518
–2527
(1997). 0039-2499 Google Scholar
Y. Cao,
L. D’Olhaberriague,
E. M. Vikingstad,
S. R. Levine, and
K. M. A. Welch,
“Pilot study of functional MRI to assess cerebral activation of motor function after poststroke hemiparesis,”
Stroke, 29 112
–122
(1998). 0039-2499 Google Scholar
H. Kato,
M. Izumiyama,
H. Koizumi,
A. Takahashi, and
Y. Itoyama,
“Near-infrared spectroscopic topography as a tool to monitor motor reorganization after hemiparetic stroke: A comparison with functional MRI,”
Stroke, 33 2032
–2036
(2002). https://doi.org/10.1161/01.STR.0000021903.52901.97 0039-2499 Google Scholar
S. W. Atlas,
R. S. Howard,
J. Maldjian,
D. Alsop,
J. A. Detre,
J. Listerud,
M. D’Esposito,
K. D. Judy,
E. Zager, and
M. Stecker,
“Functional magnetic resonance imaging of regional brain activity in patients with intracerebral gliomas: Findings and implications for clinical management,”
Neurosurgery, 38 329
–338
(1996). 0148-396X Google Scholar
W. M. Mueller,
F. Z. Yetkin,
T. A. Hammeke, G. L. Morris III, S. J. Swanson,
K. Reichert,
R. Cox, and
V. M. Haughton,
“Functional magnetic resonance imaging mapping of the motor cortex in patients with cerebral tumors,”
Neurosurgery, 39 515
–520
(1996). 0148-396X Google Scholar
L. Nelson,
S. Lapsiwala,
V. M. Haughton,
J. Noyes,
A. H. Sadrzadeh,
C. H. Moritz,
M. E. Meyerand, and
B. Badie,
“Preoperative mapping of the supplementary motor area in patients harboring tumors in the medial frontal lobe,”
Neurosurgery, 97 1108
–1114
(2002). 0148-396X Google Scholar
T. Inoue,
H. Shimizu,
N. Nakasato,
T. Kumabe, and
T. Yoshimoto,
“Accuracy and limitation of functional magnetic resonance imaging for identification of central sulcus: Comparison with magnetoencephalography in patients with brain tumor,”
Neuroimage, 10 738
–748
(1999). 1053-8119 Google Scholar
A. I. Holodny,
M. Schulder,
W. C. Liu,
J. A. Maldjian, and
A. J. Kalnin,
“Decreased BOLD functional MR activation of the motor and sensory cortices adjacent to a glioblastoma multiforme: Implications for image-guided neurosurgery,”
AJNR Am. J. Neuroradiol., 20 609
–612
(1999). 0195-6108 Google Scholar
A. I. Holodny,
M. Schulder,
W. C. Liu,
J. Wolko,
J. A. Maldjian, and
A. J. Kalnin,
“The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: Implications for image-guided neurosurgery,”
AJNR Am. J. Neuroradiol., 21 1415
–1422
(2000). 0195-6108 Google Scholar
J. T. Lurito,
M. J. Lowe,
C. Sartorius, and
V. P. Mathews,
“Comparison of fMRI and intraoperative direct cortical stimulation in localization of receptive language areas,”
J. Comput. Assist. Tomogr., 24 99
–105
(2000). https://doi.org/10.1097/00004728-200001000-00021 0363-8715 Google Scholar
A. Schreiber,
U. Hubbe,
S. Ziyeh, and
J. Hennig,
“The influence of gliomas and nonglial space-occupying lesions on blood-oxygen-level-dependent contrast enhancement,”
AJNR Am. J. Neuroradiol., 21 1055
–1063
(2000). 0195-6108 Google Scholar
R. Pineiro,
S. Pendlebury,
H. Johansen-Berg, and
P. M. Matthews,
“Altered hemodynamic responses in patients after subcortical stroke measured by functional MRI,”
Stroke, 33 103
–109
(2002). 0039-2499 Google Scholar
J. Röther,
R. Knab,
F. Hamzei,
J. Fiehler,
J. R. Reichenbach,
C. Buchel, and
C. Weiller,
“Negative dip in BOLD fMRI is caused by blood flow-oxygen consumption uncoupling in humans,”
Neuroimage, 15 98
–102
(2002). 1053-8119 Google Scholar
M. D’Esposito,
L. Y. Deouell, and
A. Gazzaley,
“Alterations in the BOLD fMRI signal with aging and disease: A challenge for neuroimaging,”
Nat. Rev. Neurosci., 4 863
–872
(2003). 1471-003X Google Scholar
F. Hamzei,
R. Knab,
C. Weiller, and
J. Rother,
“The influence of extra- and intracranial artery disease on the BOLD signal in FMRI,”
Neuroimage, 20 1393
–1399
(2003). 1053-8119 Google Scholar
P. M. Rossini,
C. Altamura,
A. Ferretti,
F. Vernieri,
F. Zappasodi,
M. Caulo,
V. Pizzella,
C. Del Gratta,
G. L. Romani, and
F. Tecchio,
“Does cerebrovascular disease affect the coupling between neuronal activity and local haemodynamics?,”
Brain, 127 99
–110
(2004). 0006-8950 Google Scholar
F. Binkofski and
R. J. Seitz,
“Modulation of the BOLD-response in early recovery from sensorimotor stroke,”
Neurology, 63 1223
–1229
(2004). 0028-3878 Google Scholar
A. Krainik,
M. Hund-Georgiadis,
S. Zysset, and
D. Y. von Cramon,
“Regional impairment of cerebrovascular reactivity and BOLD signal in adults after stroke,”
Stroke, 36 1146
–1152
(2005). 0039-2499 Google Scholar
S. Ogawa,
T. M. Lee,
A. R. Kay, and
D. W. Tank,
“Brain magnetic resonace imaging with contrast dependent on blood oxygenation,”
Proc. Natl. Acad. Sci. U.S.A., 87 9868
–9872
(1990). https://doi.org/10.1073/pnas.87.24.9868 0027-8424 Google Scholar
S. Ogawa,
D. W. Tank,
R. Menon,
J. M. Ellermann,
S. G. Kim,
H. Merkle, and
K. Ugurbil,
“Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping with magnetic resonance imaging,”
Proc. Natl. Acad. Sci. U.S.A., 89 5951
–5955
(1992). https://doi.org/10.1073/pnas.89.13.5951 0027-8424 Google Scholar
F. F. Jöbsis,
“Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters,”
Science, 198 1264
–1267
(1977). https://doi.org/10.1126/science.929199 0036-8075 Google Scholar
Y. Murata,
K. Sakatani,
Y. Katayama, and
C. Fukaya,
“Increase in focal concentration of deoxyhemoglobin during neuronal activity in cerebral ischemic patients,”
J. Neurol., Neurosurg. Psychiatry, 73 182
–184
(2002). 0022-3050 Google Scholar
Y. Murata,
K. Sakatani,
T. Hoshino,
N. Fujiwara,
T. Kano,
S. Nakamura, and
Y. Katayama,
“Effects of cerebral ischemia on evoked cerebral blood oxygenation responses and BOLD contrast functional MRI in stroke patients,”
Stroke, 37 2514
–2520
(2006). 0039-2499 Google Scholar
N. Fujiwara,
K. Sakatani,
Y. Katayama,
Y. Murata,
C. Fukaya, and
T. Yamamoto,
“Evoked-cerebral blood oxygenation changes in false-negative activations in BOLD contrast functional MRI of patients with brain tumors,”
Neuroimage, 21 1464
–1471
(2004). 1053-8119 Google Scholar
Y. Murata,
K. Sakatani,
Y. Katayama,
N. Fujiwara,
C. Fukaya, and
T. Yamamoto,
“Decreases of blood oxygenation level-dependent signal in the activated motor cortex during functional recovery after resection of a glioma,”
AJNR Am. J. Neuroradiol., 25 1242
–1246
(2004). 0195-6108 Google Scholar
Y. Hoshi and
M. Tamura,
“Detection of dynamic changes in cerebral oxygenation coupled to neuronal function during mental work in man,”
Neurosci. Lett., 150 5
–8
(1993). https://doi.org/10.1016/0304-3940(93)90094-2 0304-3940 Google Scholar
A. Villringer,
J. Planck,
C. Hock,
L. Schleinkofer, and
U. Dirnagl,
“Near infrared spectroscopy (NIRS): A new tool to study hemodynamic changes during activation of brain function in human adults,”
Neurosci. Lett., 14 101
–104
(1993). 0304-3940 Google Scholar
A. Kleinschmidt,
H. Obrig,
M. Requardt,
K. D. Merboldt,
U. Dirnagl,
A. Villringer, and
J. Frahm,
“Simultaneous recording of cerebral blood oxygenation changes during human brain activation by magnetic resonance imaging and near-infrared spectroscopy,”
J. Cereb. Blood Flow Metab., 16 817
–826
(1996). https://doi.org/10.1097/00004647-199609000-00006 0271-678X Google Scholar
A. Villringer and
B. Chance,
“Non-invasive optical spectroscopy and imaging of human brain function,”
TINS, 20 435
–442
(1997). https://doi.org/10.1016/S0166-2236(97)01132-6 0166-2236 Google Scholar
K. Sakatani,
Y. Xie,
W. Lichty,
S. Li, and
H. Zuo,
“Language-activated cerebral blood oxygenation and hemodynamic changes of the left prefrontal cortex in poststroke aphasic patients: A near infrared spectroscopy study,”
Stroke, 29 1299
–1304
(1998). 0039-2499 Google Scholar
K. Sakatani,
W. Lichty,
Y. Xie,
S. Li, and
H. Zuo,
“Effects of aging on language-activated cerebral blood oxygenation changes of the left prefrontal cortex: Near infrared spectroscopy study,”
J. Stroke Cerebrovasc. Dis., 8 398
–403
(1999). Google Scholar
M. Tanida,
K. Sakatani,
R. Takano, and
K. Tagai,
“Relation between asymmetry of prefrontal cortex activities and the autonomic nervous system during a mental arithmetic task: Near infrared spectroscopy study,”
Neurosci. Lett., 369 69
–74
(2004). 0304-3940 Google Scholar
V. A. W. Toronov,
J. H. Choi,
M. Wolf,
A. Michalos,
E. Gratton, and
D. Hueber,
“Investigation of human brain hemodynamics by simultaneous near-infrared spectroscopy and functional magnetic resonance imaging,”
Med. Phys., 28 521
–527
(2001). https://doi.org/10.1118/1.1354627 0094-2405 Google Scholar
G. Strangman,
J. P. Culver,
J. H. Thompson, and
D. A. Boas,
“A quantitative comparison of simultaneous BOLD fMRI and NIRS recording during functional brain activation,”
Neuroimage, 17 719
–731
(2002). 1053-8119 Google Scholar
T. Yamamoto, and
T. Kato,
“Paradoxical correlation between signal in functional magnetic resonance imaging and deoxyhemoglobin content in capillaries: A new theoretical explanation,”
Phys. Med. Biol., 47 1121
–1141
(2002). https://doi.org/10.1088/0031-9155/47/7/309 0031-9155 Google Scholar
T. J. Huppert,
R. D. Hoge,
S. G. Diamond,
M. A. Franceschini, and
D. A. Boas,
“A temporal comparison of BOLD, ASL, and NIRS hemodynamic responses to motor stimuli in adult humans,”
Neuroimage, 29 368
–382
(2006). 1053-8119 Google Scholar
D. A. Boas,
T. Gaudette,
G. Strangman,
X. Cheb,
J. J. A. Marota, and
J. B. Mandeville,
“The accuracy of near infrared spectroscopy and imaging during focal changes in cerebral hemodynamics,”
Neuroimage, 13 76
–90
(2001). 1053-8119 Google Scholar
E. Okada and
D. T. Delpy,
“Near-infrared light propagation in an adult head model. I. Modeling of low-level scattering in the cerebrospinal fluid layer,”
Appl. Opt., 42 2906
–2914
(2003). https://doi.org/10.1364/AO.42.002906 0003-6935 Google Scholar
E. Okada and
D. T. Delpy,
“Near-infrared light propagation in an adult head model. II. Effect of superficial tissue thickness on the sensitivity of the near-infrared spectroscopy signal,”
Appl. Opt., 42 2915
–2922
(2003). https://doi.org/10.1364/AO.42.002915 0003-6935 Google Scholar
S. P. Lee,
T. Q. Duong,
G. Yang,
C. Iadecola, and
S. G. Kim,
“Relative changes of cerebral arterial and venous blood volume during increased cerebral blood flow: Implications for BOLD fMRI,”
Magn. Reson. Med., 45 791
–800
(2001). 0740-3194 Google Scholar
I. Miyai,
H. Yagura,
M. Hatakenaka,
I. Oda,
I. Konishi, and
K. Kubota,
“Longitudinal optical imaging study for locomotor recovery after stroke,”
Stroke, 34 2866
–2870
(2003). 0039-2499 Google Scholar
J. I. Boxerman,
P. A. Bandettini,
K. K. Kwong,
J. R. Baker,
T. L. Davis,
B. R. Rosen, and
R. M. Weisskopf,
“The intravascular contribution to fMRI signal change: Monte Carlo modeling and diffusion-weighted studies in vivo,”
Magn. Reson. Med., 34 4
–10
(1995). 0740-3194 Google Scholar
A. Hess,
D. Stiller,
T. Kaulisch,
P. Heil, and
H. Scheich,
“New insights into the hemodynamic blood oxygenation level-dependent response through combination of functional magnetic resonance imaging and optical recording in gerbil barrel cortex,”
J. Neurosci., 20 3328
–3338
(2000). 0270-6474 Google Scholar
Y. R. Kim,
I. J. Huang,
S. R. Lee,
E. Tejima,
J. B. Mandeville,
M. P. van Meer,
G. Dai,
Y. W. Choi,
R. M. Dijkhuizen,
E. H. Lo, and
B. R. Rosen,
“Measurements of BOLD/CBV ratio show altered fMRI hemodynamics during stroke recovery in rats,”
J. Cereb. Blood Flow Metab., 25 820
–829
(2005). 0271-678X Google Scholar
R. B. Buxton and
L. R. Frank,
“A model for the coupling between cerebral blood flow and oxygen metabolism during neural stimulation,”
J. Cereb. Blood Flow Metab., 17 64
–72
(1997). 0271-678X Google Scholar
P. Fox and
M. E. Raichle,
“Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects,”
Proc. Natl. Acad. Sci. U.S.A., 83 1140
–1144
(1986). https://doi.org/10.1073/pnas.83.4.1140 0027-8424 Google Scholar
P. T. Fox,
M. E. Raichle,
M. A. Mintun, and
C. Dence,
“Nonoxidative glucose consumption during focal physiologic neural activity,”
Science, 241 462
–464
(1988). 0036-8075 Google Scholar
R. D. Hoge,
J. Atkinson,
B. Gill,
G. R. Crelier,
S. Marrett, and
G. B. Pike,
“Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex,”
Proc. Natl. Acad. Sci. U.S.A., 96 9403
–9408
(1999). 0027-8424 Google Scholar
|

