|
|
1.IntroductionTraditional biochemical and pharmaceutical antiviral treatments employed today are only partially successful and evoke problems of drug resistance and clinical side effects. New methods that circumvent these limitations are particularly desirable. Microwave/ultrasonic absorption represent techniques toward this effort. Structural changes of brome mosaic virus (BMV) and tomato bushy stunt virus (TBSV) associated with the viral capsids were studied with the ultrasonic absorption technique by Cerf 1 These authors found that the ultrasonic waves in the megahertz range are absorbed much more by capsids than by the dissociated protein. Their findings suggested that the spontaneous motions due to the excitations of ultrasonic waves might play a role in liberating the RNA and as a result might be of functional significance at an early stage of viral infection. Michels 2 investigated the structural fluctuations of tobacco mosaic virus (TMV) with ultrasonic absorption and confirmed that protein assemblies exhibited specific structural fluctuations detected in ultrasonic experiments. Optical excitation of coherent lattice or molecular vibrations through stimulated light scattering can be carried out either by focusing an intense laser into a medium with a Raman-active vibrational mode or by spatially and temporally overlapping two laser outputs of suitable frequency difference in a medium. Impulsive stimulated Raman scattering (ISRS) has been shown to be a viable way of producing large-amplitude vibrational modes in molecules in liquid solution as well as lattice vibrations in solid state systems.3 For example, ISRS has been used to excite coherent acoustic phonons in ethanol, malachite green, and cresyl violet in ethanol,4 and coherent optical phonons in -perylene crystal.5 In this paper, we report the first use of a very low power visible femtosecond laser system to excite coherent acoustic Raman-active vibrational mode (which is associated with the vibrations of viral capsids) in M13 bacteriophages through ISRS to such a high-energy state as to lead to their inactivation. Our work suggests a new method of manipulating, controlling, and inactivating unwanted microorganisms. It also suggests that the basic principles of impulsive coherent excitation using a laser optical system can be a general way to selectively alter function or even inactivate viruses and potentially other microorganisms through the property of their mechanical acoustic excitations. In addition, since damage caused to viral organisms through vibration of their mechanical structures likely would be immune to simple mutation of cell surface receptors, our approach would be applicable to antibiotic-resistant strains of microorganisms as well. 2.Sample and Experimental SetupThe M13 bacteriophage samples used in this work were purchased from Stratagene. The excitation source employed in this work was a diode-pumped cw mode-locked Ti-sapphire laser. 6, 7, 8, 9 The output of the second-harmonic generation system of the Ti-sapphire laser was used to irradiate the samples. The excitation laser, which provided a nearly transform limited pulse train having a pulse width with full width at half maximum (as measured by an autocorrelator6) and spectral width (as determined by a Raman system including a double spectrometer, a photomultiplier tube and its associated photon-counting system8), was chosen to operate at a wavelength of , at a repetition rate of , and with an average power of about , unless otherwise specified. The different excitation pulse widths were obtained by chirping and their pulse widths as well as spectral widths were characterized in a manner similar to that already described. The laser power density was defined as that at the tightest focused region formed by the focusing lens. The various excitation laser power densities ranging from to were achieved by the following method. First, the FWHM of the tightest laser focused spot was measured by using a pinhole with an adjustable opening immersed in the sample and a power meter. For example, a laser spot of and an average power of gave rise to a power density of about . Second, to achieve the desired power density, the average power of the laser was either increased or decreased with the help of a calibrated variable attenuator. In our experiments, the tightest laser-focused volume, which was the most efficient volume for the interaction of laser with M13 bacteriophages through ISRS, was a cylinder approximately in diameter and in height. To facilitate the interaction of the laser with M13 bacteriophages, which were inside a synthetic quartz cuvette and diluted in water, a magnetic stirrer was set up so that M13 bacteriophages would enter the laser-focused volume as already described and interact with the photons. All the laser-irradiated M13 bacteriophage samples contained . The assays were performed on the laser-irradiated samples after proper dilution. The typical exposure time of the sample to laser irradiation was about . A thermocouple was used to monitor the temperature of the sample to ensure that our results were not due to the heating effects. All the experimental results reported here were obtained at and with the single laser beam excitation. The activity of M13 bacteriophages was determined by plaque counts. In brief, M13 bacteriophages with nominally prepared at the specified plague forming unit (pfu) concentration were added into a tube of soft agar at containing of bacteria culture. Here, “nominally prepared” means that we prepare/dilute the M13 bacteriophage samples based on the pfu concentration specified by the manufacturer upon purchasing. The mixture was mixed well by vortexing and then poured onto a Luria-Bertani (LB) agar plate immediately. The plate was swirled well to spread the mixture over the entire plate evenly. The mixture on the agar plate was incubated for 8 to . The plaques formed on the plate were counted. All data were expressed as mean standard deviation (SD). Student’s test was used for comparison of group with 5% as significant level. 3.Experimental Results, Analysis and DiscussionsFrom the measured absorption spectrum of the M13 bacteriophage sample, which contained nominally prepared , we found that there is negligible (less than 2%) absorption for photons with wavelengths between 300 and . This is consistent with our observation that the increase in the temperature (as indicated by the thermocouple) of the sample during and after a laser irradiation at is very minimal (typically, less than ). Figure 1 shows the number of plaques as a function of nominal concentration for five M13 bacteriophage samples having nominally prepared , , , , and , respectively, without the laser irradiation (solid circles) and with the laser irradiation (open circles).The number of plaque counts for the control was determined to be for the sample with . The corresponding number of plaques was [ versus sham treatment, , ] after laser irradiation. For M13 samples with nominally prepared , , , and , the number of counts was for the control, ( versus sham treatment, , ) after laser irradiation; for the control; ( versus sham treatment, , ) after laser irradiation; for the control; ( versus sham treatment, , ) after laser irradiation; for the control; and ( versus sham treatment, , ) after laser irradiation, respectively. The intriguing feature of these experimental results is that there is a very minimal number of plaques for the laser irradiated samples. We attribute the observed inactivation of M13 bacteriophages to laser-driven coherent excitations through the ISRS process. Fig. 1Number of plaques as a function of nominal concentration for M13 bacteriophage samples without laser irradiation (solid circles) and after laser irradiation for about (open circles). The extremely low numbers of plaques observed in the irradiated samples are a manifestation of almost complete inactivation of the M13 bacteriophages in the samples. 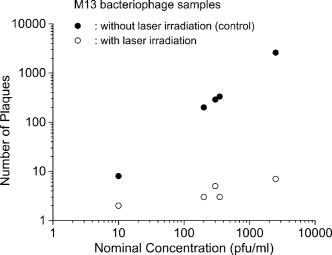 ISRS has been successfully demonstrated in molecular as well as solid state systems. 3, 4, 5, 10, 11, 12 Yan 3 predicted that IERS should occur with no laser intensity threshold even when only one ultrashort laser pulse passed through many types of media. In this case, ISRS is a forward-scattering process that is stimulated because the Stokes frequency is contained within the spectral width of the excitation pulse. Furthermore, they demonstrated that ISRS was a process through which excitation of a coherent lattice or molecular vibrations would take place whenever a sufficiently short laser pulse passed through a Raman-active solid or molecular liquid or gas. Let us assume that the vibrational mode of interest is represented by a normal coordinate labeled . If we ignore dispersion in the index of refraction and assume that the incident electric field from the excitation laser is not depleted by the stimulated scattering, then the equation of motion for can be written as13 where and are the damping constant and the angular frequency of the vibrational mode, respectively; and is the driving force from the excitation laser.However, can be written as andHere, the induced polarization is related to the excitation laser field through the equationwhere is the number density of oscillators, and is the polarizability of the medium.If we make a Taylor series expansion of the polarizability around the equilibrium position of the normal coordinate, i.e., Keeping up to the first order in in Eq. 5, from Eqs. 1, 2, 3, 4, 5 we haveThe fact that the right-hand side of Eq. 6, which corresponds to the driving force for the vibrational mode, is linearly proportional to the derivative of the polarizability with respect to the normal coordinate sheds some light on why the process is called impulsive Raman scattering. In particular, for a single-beam ultrashort laser excitation having a pulse width of , if the damping is ignored, then the amplitude of the displacement away from the equilibrium intermolecular distance caused by ISRS can be shown to be given by3 where is the intensity of the excitation laser, is the displacement away from the equilibrium intermolecular distance, is proportional to the amplitude of Raman scattering cross section, is the molecular mass, is the index of refraction, and is the speed of light.From Eq. 7 it is clear that larger Raman cross sections, higher laser power densities as well as lower vibrational frequencies, would contribute to larger excited vibrational amplitudes. In fact, for a moderate Raman scattering cross section, a sufficiently low vibrational frequency and a reasonable excitation power density, the amplitude of the vibrational displacement in the 0.01 to could be achieved through ISRS. In our previous Raman scattering experiments,14, 15 we reported the observation of the low-frequency vibrational mode of M13 bacteriophages, which was shown to be associated with the axial torsion vibrations of the capsid. One plausible scenario is then that under our current ultrashort pulse laser excitation experiments with M13 bacteriophages, the amplitude of this mode has been coherently excited by ISRS to an extent that leads to their inactivation. To further support this explanation, we carried out similar experiments with M13 bacteriophages by varying the power density as well as pulse width of the excitation laser. Figure 2 shows the number of plaques as a function of the laser power density for M13 bacteriophage samples with nominally prepared after being irradiated with an excitation laser having in pulse width and . It is very interesting to observe an abrupt inactivation of the M13 bacteriophages at an excitation laser power density of . This observation is indicative of the fact that the M13 bacteriophages become inactivated as the amplitude of the vibrations exceeds a certain threshold. The medium lethal laser power density is with a slope of . The reason why the M13 bacteriophages were inactivated at certain threshold amplitude is not clear at this moment. More work related to this area of research is required. Fig. 2Number of plaque counts as a function of the excitation laser power density for a M13 bacteriophage sample with nominally prepared . The sharp cutoff for the number of plaques at around is indicative of the onset of the inactivation of the M13 bacteriophages through ISRS process. The solid circles represent the experimental data. The solid curve was fitted and the medium lethal laser power density was calculated using Prism 3.0 software program (San Diego, California). 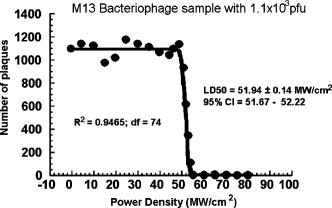 We also found that within the statistical error of the experiments there is no observable inactivation of the M13 bacteriophages if the pulse width of the excitation laser is longer than about , while the intensity of the excitation laser remains constant at (this laser intensity corresponds to an ultrafast laser with 100-fs pulse width and of average power). The experimental results are summarized in Table 1 . According to Eq. 7, if the laser intensity remains constant, the amplitude of vibrational displacement excited by an ultrashort laser decreases with the increasing laser pulse width. As a matter of fact, Eq. 7 predicts that the amplitude of vibrational displacement excited by an ultrashort laser having a pulse width of with laser intensity of approximately equals that by an excitation laser having a 100-fs pulse width but with laser intensity . Therefore, the experimental results in Table 1 are consistent with the predictions by Eq. 7 and with the power density results of Fig. 2. We also notice that because the laser energy per pulse in the case of a 800-fs or longer pulse width is actually the same as that in the case of or shorter, the experimental results in Table 1 rule out the possibility that our observed inactivation of M13 bacteriophages is due to transient, microthermal effects that might develop at the tightest laser focused volume. Figure 3 shows the number of plaques as a function of the exposure time of excitation laser for a M13 bacteriophage sample with nominally prepared , taken with an ultrashort laser having , in pulse width, and an average power of . The exposure time experiments were carried out with M13 bacteriophages of in a 2-ml synthetic quartz cuvette and then later diluted with double distilled water to for assaying. The experimental data have been found to fit very well by an exponential function of the form: , where and . We systematically studied the inactivation of M13 bacteriophages as a function of the exposure time with various stirring rates. We found that the deduced value of is linearly proportional to the stirring rate of the magnetic stirrer. These experimental findings strongly support that the inactivation of M13 bacteriophages depends primarily on the mixing efficiency of the magnetic mixer used in our experiments. In other words, since the power density of excitation laser exceeds the threshold intensity, as indicated in Fig. 2, the most important factor that dictates the exposure time for the complete inactivation of M13 bacteriophages in the sample is how efficiently the M13 bacteriophages in the sample enters the active (the tightest focusing) volume of the excitation laser. In other words, one expects that the number of plaques (or the number of M13 bacteriophages) follows the following rate equation: This gives rise to the exponential form for as already discussed. One would therefore conclude that the exposure time would be significantly shorter for the complete inactivation of M13 bacteriophage sample if a more efficient stirrer and/or a larger laser active volume are available.Fig. 3Number of plaque counts as a function of the exposure time for a M13 bacteriophage sample with nominally prepared . The exponential decay of the data suggests that the inactivation of M13 bacteriophage is primarily dependent on the efficiency of the magnetic stirrer used in our experiments. 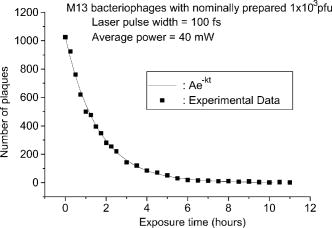 Table 1Dependece of inactivation of M13 phages on laser pulse width.
The peak intensity was kept constant. The number within the parentheses correspond to the spectral widths. We also carried out similar experiments for M13 interference-resistant helper phages. Preliminary results (which are not shown) indicate that, within the experimental uncertainty, the threshold power densities for the inactivation of M13 bacteriophages and M13 interference-resistant helper phages are the same . These experimental results suggest that our method can overcome limitations with current therapeutics that arise due to mutations. We believe that this novel property of our method occurs because the excited coherent acoustic vibrations on the capsids of M13 phages are usually of long wavelength; as a result, they are relatively insensitive to the minor local changes such as those due to mutations. Similar experiments with Jurkat T-cells in serum solution were also performed. Preliminary results with Jurkat T-cells (which are not shown) indicate that the Jurkat T-cells, under similar experimental conditions, become inactivated at a much higher laser power density than that for M13 bacteriophages . These experimental results suggest that there may be a window in excitation laser power density that inactivates viruses while leaving the mammalian cells unharmed. The large differences in threshold power density for inactivation of M13 bacteriophages and mammalian cells most likely result from the nature of the structural compositions in the protein coats or membranes. Other possible factors include differences in the Raman scattering cross section and in the damping constant associated with the coherently excited vibrational modes of viral capsids and mammalian cell plasma membranes. Finally, we notice that viruses have been known to be inactivated by the UV photons with wavelengths around . There is a possibility that our excitation laser might be generating through the nonlinear property of the sample system; as a result, the produced UV photons inactivate the M13 bacteriophages. However, this possibility can be ruled out. Because we have observed that there is no inactivation of M13 bacteriophages if the laser power density is set at while the pulse width is about . If the excitation laser with a pulse width of and power density of inactivated the M13 bacteriophages by the nonlinearly generated UV photons, then a laser with a longer pulse width but the same power density would have produced more UV photons and would have been able to inactivate the M13 bacteriophages as well, which is in contradiction to our observation. Therefore, it is unlikely that the nonlinearly generated UV photons (if there are any) play a significant role in the inactivation of M13 bacteriophages observed in our current experiments. 4.ConclusionThe inactivation of viruses such as M13 bacteriophages has been studied after excitations by a very low power visible femtosecond laser. We showed that microorganisms such as M13 bacteriophages can be inactivated by a very lower power femtosecond laser system through ISRS process with an of . Our work suggests a new method of manipulating, controlling, and inactivating unwanted microorganisms while leaving the sensitive materials such as mammalian cells unharmed. It also suggests that the basic principles of impulsive coherent excitation using a laser optical system can be a general way to selectively alter function or even inactivate viruses and potentially other microorganisms through the property of their mechanical acoustic excitations. In addition, since damage caused to viral organisms through the vibration of their mechanical structures likely would be immune to simple mutation of cell surface receptors, our approach would be applicable to antibiotic-resistant strains of microorganisms as well. AcknowledgmentsThis work is supported by the National Science Foundation under Grant No. DMR-0305147. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Armed Forces Radiobiology Research Institute, Uniformed Services University of the Health Sciences, or the U.S. Department of Defense. ReferencesR. Cerf,
B. Michels,
J. A. Schulz,
J. Witz,
P. Pfeiffer, and
L. Hirth,
“Ultrasonic absorption evidence of structural fluctuations in viral capsids,”
Proc. Natl. Acad. Sci. U.S.A., 76 1780
–1782
(1979). https://doi.org/10.1073/pnas.76.4.1780 0027-8424 Google Scholar
B. Michels,
Y. Dormoy,
R. Cerf, and
J. A. Schulz,
“Ultrasonic absorption in tobacco mosaic virus and its protein aggregates,”
J. Mol. Biol., 181 103
–110
(1985). https://doi.org/10.1016/0022-2836(85)90328-6 0022-2836 Google Scholar
Y.-X. Yan, E. B. Gamble Jr., K. A. Nelson,
“Impulsive stimulated scattering: general importance in femtosecond laser pulse interactions with matter, and spectroscopic applications,”
J. Chem. Phys., 83 5391
–5399
(1985). https://doi.org/10.1063/1.449708 0021-9606 Google Scholar
K. A. Nelson,
R. J. D. Miller,
D. R. Lutz, and
M. D. Fayer,
“Optical generation of tunable ultrasonic waves,”
J. Appl. Phys., 53 1144
–1149
(1982). https://doi.org/10.1063/1.329864 0021-8979 Google Scholar
S. De Silvestri,
J. G. Fugimoto,
E. P. Ippen, E. B. Gamble Jr., L. R. Williams, and
K. A. Nelson,
“Femtosecond time-resolved measurements of optic phonon dephasing by impulsive stimulated raman scattering in -perylene crystal from 20 to ,”
Chem. Phys. Lett., 116 146
–152
(1985). https://doi.org/10.1016/0009-2614(85)80143-3 0009-2614 Google Scholar
K. T. Tsen,
“Ultrafast dynamics in wide bandgap wurtzite ,”
Ultrafast Physical Processes in Semiconductors, 67 109
–149 Academic Press, New York (2001). Google Scholar
K. T. Tsen,
“Electron velocity overshoot, ballistic electron transport and non-equilibrium phonon dynamics in nanostructure semiconductors,”
Ultrafast Phenomena in Semiconductors, 191
–259 Springer, New York (2001). Google Scholar
K. T. Tsen,
“Optical studies of electric-field induced electron and hole transient transports and optical phonon instability in semiconductor nanostructures,”
Ultrafast Dynamical Processes in Semiconductors, 92 193
–258 Springer, Heidelberg (2004). Google Scholar
K. T. Tsen,
“Optical studies of carrier dynamics and non-equilibrium optical phonons in nitride-based semiconductors,”
Non-Equilibrium Dynamics of Semiconductors and Nanostructures, 180
–213 CRC Press, Inc., New York (2005). Google Scholar
R. J. D. Miller,
R. Casalegno,
K. A. Nelson, and
M. D. Fayer,
“Laser-induced ultrasonics: a dynamic holographic approach to the measurement of weak absorptions, optoelastic constants acoustic attenuation,”
Chem. Phys., 72 371
–379
(1982). https://doi.org/10.1016/0301-0104(82)85134-3 0301-0104 Google Scholar
K. A. Nelson,
“Stimulated Brillouin scattering and optical excitation of coherent shear waves,”
J. Appl. Phys., 53 6060
–6063
(1982). https://doi.org/10.1063/1.331556 0021-8979 Google Scholar
M. M. Robinson,
Y. X. Yan, E. B. Gamble Jr., L. R. Williams,
J. S. Meth, and
K. A. Nelson,
“Picosecond impulsive stimulated brillouin scattering: optical excitation of coherent transverse acoustic waves and application to time-domain investigations of structural phase transitions,”
Chem. Phys. Lett., 112 491
–496
(1984). https://doi.org/10.1016/0009-2614(84)85764-4 0009-2614 Google Scholar
Y. R. Shen and
N. Bloembergen,
“Theory of simulated Brillouin and Raman scattering,”
Phys. Rev., 137 A1787
–A1805
(1965). https://doi.org/10.1103/PhysRev.137.A1787 0031-899X Google Scholar
K. T. Tsen,
E. C. Dykeman,
O. F. Sankey,
N.-T. Lin,
S.-W. D. Tsen, and
J. G. Kiang,
“Observation of the low frequency vibrational modes of bacteriophage M13 in water by Raman spectroscopy,”
Virol. J., 3 79
(2006). Google Scholar
K. T. Tsen,
E. C. Dykeman,
O. F. Sankey,
N.-T. Lin,
S.-W. D. Tsen, and
J. G. Kiang,
“Probing the low-frequency vibrational modes of viruses with Raman scattering—bacteriophage M13 in water,”
J. Biomed. Opt., 12 024009
(2007). https://doi.org/10.1117/1.2718935 1083-3668 Google Scholar
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

