|
|
1.IntroductionLasers are common in everyday life and can be found in a variety of devices, including range finders, scanners, printers, welding tools, fiber optics, and medical instruments. While science, medicine, and society in general have enjoyed the benefits of lasers, there are also inherent ocular safety issues that should not be overlooked. Awareness of these dangers has fostered the use of lasers operating in the 1400- to 2200-nm region,1, 2 since these wavelengths are absorbed by the cornea and anterior chamber before they can cause permanent damage to the retina. However, this approach is not without risk. The cornea has a high density of pain receptors, so even small amounts of damage can cause severe pain.3, 4 The cornea provides approximately 85% of the focusing power in the eye,5 so laser-induced damage that alters corneal shape or transparency can have a pronounced effect on vision. The majority of studies reported to evaluate laser eye injuries and ocular safety issues rely on in vivo laser exposures of experimental animals.6 The maximum permissible exposure (MPE) recommendation currently listed in the ANSI standards for is specifically based on median effective dose data derived from in vivo exposures of nonhuman primate corneas to erbium lasers.7 However, the literature 7, 8, 9, 10 provides several different values for the 1540-nm laser that range from 4.7 to . This difference has been suggested to correlate with pulse duration and the dependence on the spot size of the laser beam.11 Rabbits are a well-established, nonprimate, in vivo model for human eye research, and a robust database exists for laser-induced corneal injury in rabbits. 10, 12, 13, 14, 15 However, in order to reduce the number of experimental animals used in potentially painful studies, researchers continue to look for alternatives to traditional in vivo animal models.16, 17, 18 Alternative testing methods such as those using ex vivo or in vitro tissues support the concept of reducing painful experimental procedures while allowing researchers to rapidly screen treatment techniques and develop safety standards for ocular laser exposure. The purpose of the present study was to determine the of 1540-nm laser-induced injuries in ex vivo and in vitro rabbit corneal models and compare the immediate and post-exposure tissue responses. Because of the correlation between , pulse duration, and laser beam spot size, the present study used analogous laser parameters to those reported by Clarke for in vivo exposure of rabbit eyes to 1540-nm laser light.10 2.Methods2.1.Laser SystemAn erbium-glass laser (Laser Sight Technologies, Winter Park, Florida) producing 0.8-ms pulses at was used for all in vitro and ex vivo exposures. The laser is a free-running, optically pumped system, with pulse shapes that are roughly . The laser beam was focused with a 50-mm lens (BK-7 lens material, LA1708, Newport, Irvine, California), and the spot size was determined using a knife-edge technique.19 Laser energy was measured with a Molectron EPM-2000 meter and a J25 energy detector (Coherent, Santa Clara, California). Pulse duration was measured using a germanium photon detector (PDA 255, Thor Labs, Newton, New Jersey) connected to a Tektronix TDS 644B digitizing oscilloscope (Beaverton, Oregon). The epithelial surfaces for both in vitro corneal tissues and ex vivo eyeballs were positioned at a distance of from the lens. 2.2.Ex Vivo Rabbit Corneal ModelRabbit eyes for the ex vivo experiments were provided in a single shipment from a commercial source (Pel-Freeze, Rogers, Arizona). All eyes were obtained from rabbits approximately 6 months of age and shipped overnight, on wet ice, in bottles containing 0.1-M phosphate buffer, pH 7.2 supplemented with 1% fresh rabbit serum, penicillin, streptomycin, and amphotericin. Eyeballs that showed corneal opacity after shipment were excluded from the study. A total of 31 eyes were used for this study. There were four control eyes, which were handled in the same way as the exposed eyes except for laser exposure. The corneas from 27 eyes were exposed in four locations (1 marking lesion and 3 experimental; see laser exposure parameters below) for a total of 108 exposures. The ex vivo corneal models were maintained in an incubator at, and 5% immediately prior to exposure and post-exposure. This approach was taken to mimic the natural environment of the in vivo cornea, where the optical absorption characteristics of the tissue are temperature dependent.20 The maximum exposure duration of the ex vivo corneal model to the room temperature environment was prior to the first exposure, during the exposure sequence, and post-exposure. After laser exposure, each eye was placed in a separate well of a 6-well plate and incubated with F12/DMEM media (MediaTech) supplemented with 10% NuSerum (Collaborative Biomedical), L-glutamine, penicillin, streptomycin, and amphotericin for at and 5% to evaluate the tissue response to laser injury. The eyes were moved by grasping the optic nerve with forceps to ensure that the cornea was not injured during handling. 2.3.In Vitro Rabbit Corneal ModelPrimary corneal epithelial cells and stromal keratocytes isolated from New Zealand White rabbits served as the seed cultures for the in vitro corneal models. All cell culture procedures were conducted using F12/DMEM media. Corneal equivalents were produced in two steps.21 First, a liquid collagen/corneal keratocyte seed culture suspension was added to a Transwell (Costar) tissue culture insert, with a porous polycarbonate supporting membrane, contained within a 12-well tissue culture plate. The collagen/keratocyte suspension formed a gel during incubation ( , 5% ) that was supported by the polycarbonate membrane, and the keratocytes were grown in culture for 5 to 7 days. Second, a seed culture suspension of corneal epithelial cells was plated upon the collagen/keratocyte gel and grown in culture for an additional 14 to 21 days. The tissue culture fluid levels were slowly lowered over the incubation period until an epithelial cell-air interface was established that allowed stratification of the epithelial layers into basal, wing, and superficial cells. A total of 24 in vitro rabbit corneal models were used for this study. Four corneal models were used as controls, and twenty corneal models were exposed in four locations (1 position marker and 3 experimental; see laser exposure parameters below) accounting for 80 exposures. The in vitro corneal models were maintained in an incubator at and 5% immediately prior to exposure and post-exposure in a manner analogous to the ex vivo corneal models. The maximum exposure duration of the in vitro corneal model to the room temperature environment was prior to the first exposure, during the exposure sequence, and post-exposure. The shorter time period during the in vitro exposure sequence, compared to the analogous ex vivo exposures, related to the flat surface of the in vitro tissues. After laser exposure, each corneal model was placed in a separate well of a 12-well plate and incubated with F12/DMEM media for at and 5% to evaluate the tissue response to laser injury. 2.4.Laser Exposure ParametersThe spot size and calibration factor were calculated for each input energy voltage. Ten pulses were used to create a positional marking lesion at the edge of the in vitro and ex vivo corneal models. This marking lesion provided a reference point for histological analyses. The first experimental exposure was made using a single pulse, exactly from the marking lesion. Two successive single pulse exposures were spaced exactly and from the marking lesion (Fig. 1 ). Because of the curvature of the cornea in the ex vivo model, the placement of the lens relative to the corneal surface was adjusted for each exposure to maintain a constant distance. The in vitro corneal model was flat and received a consistent spot size and shape for all experimental exposures without continuous adjustment. 2.5.Evaluation for InjuryAll experimental laser exposure sites were independently evaluated within post-exposure and post-exposure by two graders. Exposure site examinations were conducted with and without the aid of a handheld ophthalmoscope (Welch Allyn, Skaneateles Falls, New York). Lesions were graded on a nominal scale of present or absent, based on agreement by both graders. Fluorescein staining (Fluor-I-Strip-AT, strips, Bausch & Lomb, Rochester, New York) of ex vivo exposure sites was evaluated with a Woods lamp and used to highlight laser lesions. Fluorescein staining was not necessary and not used to highlight laser lesions with in vitro exposure sites. The ex vivo corneal models were rinsed with sterile water after fluorescein application to reduce background staining. All corneal models were photographed before laser exposure, within post-exposure, and post-exposure using a Nikon D1H digital camera with an F-50 Nikon macroscopic lens (El Segundo, California). After grading and photography, each corneal model was placed into an individual well of a 12-well culture plate containing F12/DMEM media and incubated for at and 5% . After the post-exposure incubation, the corneal models were removed, graded, and photographed for presence or absence of a visible lesion. 2.6.Embedding and Freezing of Corneal Tissues for Histological EvaluationThe corneas were recovered from the ex vivo models by sharp, circumferential dissection at the limbal margins. The in vitro corneal models were entirely removed from the transwell by sharp dissection of the underlying polycarbonate membrane. The marking lesion on each corneal tissue was highlighted using a surgical dye to facilitate positioning within the embedding matrix. Each corneal tissue was placed into a 5-ml disposable beaker filled with cryotome embedding medium (OCT, Sakura FineTech, Torrance, California) and oriented with the marking lesion at the 12 o’clock position and subsequent lesions along the center line. The corneal tissues were then placed in a freezing bath of hexane surrounded by liquid nitrogen. Once frozen, the 5-ml disposable beaker was removed, covered with aluminum foil, placed into a labeled cryostorage bag, and stored at until processed for histology. 2.7.HistologyTissue sections were made using a motorized cryomicrotome (Bright Instruments, Huntingdon, England). The tissues were rapidly trimmed in the cryomicrotome until the highlighted marking lesion was revealed. The micrometer on the cryomicrotome was then set and the tissues trimmed to reveal the exposure sites that were exactly apart. The marking lesion and the exact spacing of the exposure sites allowed sectioning of the lesions with micrometer precision and accurately resolved the inside edge, middle, and outside edge of the laser lesion. Sections to be evaluated for histopathology were stained using Gill’s #3 hematoxylin for , rinsed in tap water for , and counterstained using eosin/phloxine for . Stained sections were then dehydrated in a series of alcohols, dipped in xylene, and mounted using Eukitt (Electron Microscopy Services, Hatfield, Pennsylvania). Images for histopathological analyses were captured using a Leitz Orthoplan microscope equipped with a SpotRT digital camera (Diagnostic Instruments, Sterling Heights, Michigan). Criteria used to evaluate the tissue responses to laser exposure included alterations in the epithelial parameters (e.g., area of hyaline coagulative change versus area of granular coagulative change) and stromal parameters (e.g., area of coagulative necrosis and number and distribution of keratocytes). Comparisons were made between treatment groups and control groups to evaluate the post-exposure healing response. 2.8.Statistical Analysis of Laser LesionsA probit statistical model was used to determine the for all stochastic injury results.22, 23 The SAS 9.1 Probit Procedure (SAS, Inc., Cary, North Carolina) was used to determine the with 95% fiducial intervals, as well as a value. The goodness-of-fit test for the predicted data was determined using the Pearson’s chi-square and likelihood ratio chi-square. If the Pearson goodness-of-fit chi-square test was applied and the -value for the test was too small, a heterogeneity factor and a critical value from the distribution were used to compute the fiducial limits. If the injuries followed a deterministic pattern, the technique described by McCally and Bargeron was utilized to determine the threshold of injury.24 3.Results3.1.Laser ParametersThe laser produced an exposure spot size range of to at a distance of above the epithelial surface of the rabbit corneal models. The spot size was oval in shape, with diameters ranging from to , respectively. The pulse duration was . The ex vivo corneal models had radiant exposures ranging between 17.61 and . The in vitro corneal models had radiant exposures ranging between 14.87 and (Table 1 ). Table 1Median effective dose and fiducial limits of tissue response by experimental model. 3.2.Ex Vivo Rabbit Corneal ModelsAnalysis of the data using the probit statistical method yielded an of for the immediate post-exposure observations and an of for the post-exposure observations (Table 1). The Pearson chi-square and the log-likelihood ratio chi-square tests were not significant at the 0.1 level for the immediate ex vivo corneal model data. The immediate post-exposure ex vivo corneal model Type III analysis of effects for the dose gave a Wald chi-square of 11.95 and a -value of 0.0005. The ex vivo corneal models had 42 lesions out of 75 exposures apparent immediately after exposure. All lesions were clearly identified immediately after exposure except for one exposure at , which had a visible lesion only at the 24-h examination. The lesions produced immediately after laser exposures were clearly visible, with no disagreement between graders. The lesions appeared as circular, discrete, raised, opaque, white areas on the cornea (Fig. 2 ). Use of the ophthalmoscope and fluorescein staining did not reveal any lesions that were not visible with careful gross examination, although these aids did provide better detail of the lesion border. After of incubation, the gross appearance of the ex vivo lesions remained as circular, discrete, raised, opaque, white areas on the cornea. Histological analyses of tissues post-exposure revealed both tissue necrosis and a proliferative response (Figs. 3 and 4 ). Fig. 2Digital photographs of corneal lesions immediately after laser exposures to in vitro (a) and ex vivo (b) corneal tissues. In vitro: Marking lesion and experimental exposures appear as discrete, round holes in the surface. Ex vivo: Marking lesion and experimental exposures appear as discrete, raised, opaque areas on the surface. 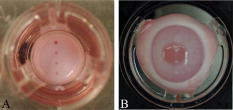 Fig. 3Representative image of damage to an ex vivo rabbit cornea after laser exposure at . The epithelium within the beam path was markedly damaged. The stroma directly underlying the epithelial layer within the beam path showed an increased basophilia typical of thermal denaturation. The epithelial cells outside the beam path and adjacent to the damaged epithelium demonstrated a marked proliferation. Minimal cellular response was observed in the adjacent stroma. Original . 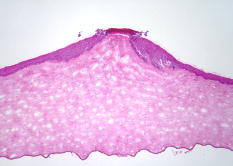 Fig. 4Higher magnification image of the ex vivo rabbit cornea shown in Fig. 3. The epithelial cells within the beam path demonstrated a distinct coagulative necrosis. There was a decreased number of stromal keratocytes within the area of increased stromal basophilia. The marked epithelial proliferation is primarily restricted to the cells of the stratum basale located outside the periphery of the beam path. Original . 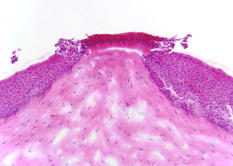 The ex vivo corneal model’s immediate fell within the 95% fiducial limits of the 24-h , indicating that the two were not significantly different and that the could be taken immediately post-exposure instead of waiting to determine the threshold (Table 1). 3.3.In Vitro Rabbit Corneal ModelProbit analysis was not used to evaluate the in vitro corneal models because the data was deterministic. McCally and Bargeron’s technique to determine the threshold was applied.24 The threshold exposure was defined as the midpoint energy between where the laser produced a minimal lesion and had no observable effect. Radiant exposures below did not produce a visible lesion, even when viewed using the ophthalmoscope, while all exposures over produced a visible lesion. The data did not change for the immediate versus post-exposure; therefore, the threshold exposure was . All lesions appeared immediately after laser exposure and were clearly visible (without fluorescein staining), with no disagreement between graders (Fig. 2). Fluorescent staining was not useful in the in vitro model, as the entire tissue was dyed pale yellow. The lesions appeared as discrete holes in the tissues. There was no color change in the tissue. Lesions did not appear to change grossly after the 24-h post-exposure incubation. Histological analyses revealed both tissue necrosis and a proliferative response (Figs. 5 and 6 ). Fig. 5Representative image of damage to an in vitro rabbit corneal model after laser exposure at . The most superficial layer of the epithelium within the beam path was markedly damaged. However, the underlying epithelial cells showed a proliferative response that was analogous to the ex vivo rabbit cornea. The stromal matrix in the model tissue was affected more severely than the ex vivo rabbit cornea with a distinct tissue ablation within the beam path. Original . 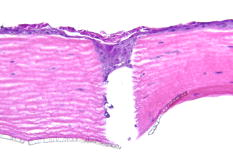 Fig. 6Higher magnification image of the in vitro corneal model shown in Fig. 5. (a) Superficial layer of epithelial cells damaged by laser and not able to repair. (b) Proliferating cells arising from the basal layer of the corneal epithelium. (c) Proliferating cells seen filling the ablated tissue space produced by the laser energy. Original . 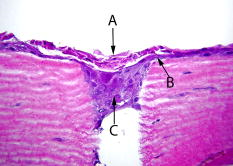 Table 2Fixed factor correlation relative to in vivo data applied to median effective dose and fiducial limits of tissue response by experimental model.
The in vitro corneal model of was not within the ex vivo corneal model’s 95% fiducial limits of 29.49 to , indicating that the two were different from each other (Table 1). However, the two models showed similarity in their histopathological responses with tissue necrosis and epithelial cell proliferation. 3.4.DiscussionThe ex vivo and in vitro corneal models allow for alternatives to the gold standard in vivo model when exploring damage thresholds for the infrared wavelengths. The ex vivo and in vitro corneal models exposed to the 1540-nm wavelength also provide new data to aid in the determination of the infrared laser safety MPE. These models have certain benefits and disadvantages. Both the in vitro and ex vivo model’s median effective dose thresholds are different from the thresholds reported for in vivo laser exposures in rabbits using analogous exposure conditions.10 The in vitro model uses far fewer animals to produce the corneal models for experimentation, but the model takes time to produce and requires personnel and equipment for cell culturing. The ex vivo tissue model uses the same number of animals, but the globes may be obtained through tissue sharing or from approved tissue sources, and the number of animals experiencing pain during experimental procedures is reduced. The in vitro corneal model allows for exact spot size duplication while moving the exposures along a horizontal axis due to the flatness of the tissue. This is important, as it reduces the time to expose the tissue, compared to the ex vivo tissue, and ensures reproducibility for each exposure point. Both models allow researchers without lab animal facilities to conduct laser tissue experiments more readily. The in vitro and ex vivo corneal models were incubated for to encourage healing, but only the ex vivo corneal model showed a change in the number of grossly visible lesions after and subsequently a change in the . This change in the ex vivo was not significant. While the grossly visible changes in the ex vivo model were not seen in the in vitro model, histology revealed a different picture, with both models showing a proliferation of the epithelial cells, indicating that a healing process was initiated. The of both the ex vivo and the in vitro models were much higher than the that was reported by Lund, Avdeev, and Stuck for the 1540-nm laser.7, 8, 9 We propose that this difference is related to the spot size and pulse duration of the laser beam. The spot size reported by Lund, Avdeev, and Stuck ranged from 1 to in diameter. The beam spot size determined in the present study was oval in shape and ranged from 0.582 to in diameter, similar to Clarke’s work,10 where the diameter of the beam was . McCally reported that both spot size and pulse duration affect the median effective dose for the 1540-nm wavelength. They also reported that at shorter pulse durations, the 1540-nm laser does not follow a modified critical temperature model.11 Both the in vitro and the ex vivo determined in the present study were well below the of reported for analogous in vivo infrared laser exposures (Table 1).10 This increased sensitivity of the model systems may be useful in rapid screening of laser-tissue interactions but should be taken into account when extrapolating experimental data. Since the biological effects of laser exposure depend on tissue chromophores and the absorption properties of the exposed tissue, the higher in vivo most likely indicates differences in the structural and chemical characteristics of the corneal models compared to the living native tissue. Factors that could contribute to the elevated threshold for laser injury following in vivo exposures would include: the thickness and composition of the tear film layer, the relative deturgescence of the cornea, and the homoiothermic temperature of the tissue.25 The tear film layer provides nutrients to the cornea, and the different nutrients may contain chromophores that alter the laser energy before being absorbed by the corneal tissue. The inherent light-absorbing characteristics of the corneal models and the native tissue may also differ. An obvious difference is the coloring of the three tissues. The in vivo exposure data were reported using Dutch Belted rabbits,10 which have a dark brown iris, while the ex vivo models in the present study used a New Zealand White rabbit with a pink eye, and the in vitro model was light pink throughout. The different coloring of the three models may affect the laser absorption and influence the laser damage threshold. Last, in vivo, ex vivo, and in vitro ocular systems have inherently different levels of tissue complexity that may be reflected in the complexity of the tissue chromophores. The in vivo tissue would have the most complex system, as it gains nutrients from both the tear film layer and the aqueous humor, which are dynamic systems. The ex vivo would be less complex, and the in vitro the least complex. The in vitro and ex vivo ocular tissues appear not to have an overlap of fiducial limits, indicating a significant statistical difference in the . This statistical difference cannot be explained by spot size or pulse duration, because these laser parameters were held constant during the study, and most likely is related to inherent differences between the model tissues. However, there is a relationship between the data from the in vitro and ex vivo models used in the present study and the in vivo data reported by Clarke that should provide a method to extrapolate in vitro and ex vivo data to predict the response of in vivo exposures. We determined that the in vivo reported by Clarke is approximately 2.64 times that for the in vitro corneal model and 1.81 times that for the ex vivo corneal model (Table 2 ).10 The tissue reaction in the first post-exposure appeared to primarily involve the epithelial cells. The histopathological response of the in vitro corneal tissue was analogous to the response of the ex vivo rabbit corneas with essentially identical nuclear and cytoplasmic patterns of coagulative necrosis. The marked epithelial proliferation observed with the in vitro model was primarily restricted to the cells of the stratum basale in a similar manner as observed with the ex vivo tissue. These results indicate that from a histopathological perspective, the in vitro rabbit corneal tissues developed in the present study are appropriate models for the acute post-exposure response of native corneal tissue following 1540-nm laser exposure. In addition, we believe that this is the first report of acute, post-exposure corneal epithelial cell proliferation induced by single-pulse, 1540-nm laser light. The histopathological responses of the ex vivo and in vitro corneal models appear to bracket the histological observations reported by Clarke in a manner that may reflect the radiant exposure deposition and highlight an increased sensitivity of the in vitro model to radiant exposure. The representative histological image shown in Clarke’s publication revealed a full-thickness stromal response following a single, 1540-nm and 0.8-ms pulse with a radiant exposure of . The ex vivo model used in the present study showed only a small stromal effect with a radiant exposure of (Fig. 3). The in vitro model used in the present study showed a total ablation of the stromal matrix within the beam field with a radiant exposure of (Fig. 5). The combination of a fixed factor that can be applied to in vitro and ex vivo experimental data and the similarity of the histopathological response between in vitro and ex vivo corneal models will improve the extrapolation of experimental data to in vivo results and help fill the gap of research data appropriate for infrared laser safety standards. In addition, the apparent increased sensitivity of the in vitro model to laser effects (supported by and histopathological observations) may help detect subtle laser-tissue interactions. AcknowledgmentsExcellent technical support for the histopathology was provided by Ms. Sharon Meachum. Additionally, the technical laser support of Ms. Tridaugh Winston, Ms. Golda Winston, and Mr. Don Randolph is gratefully acknowledged. The authors would also like to recognize the excellent editorial support of Ms. Aimee Buelow. This work was supported by U.S. Army Medical Research and Material Command CDMRP Grant No. DAMD17-03-2-0032. ReferencesS. J. Hamlin,
A. D. Hays,
C. W. Trussell, and
V. King,
“Eye safe erbium glass microlaser,”
97
–102
(2004). Google Scholar
S. D. Mayor,
S. M. Spuler, and
B. M. Morley,
“Scanning eye-safe depolarization lidar at 1.54 microns and potential usefulness in bioaerosol plume detection,”
137
–148
(2005). Google Scholar
D. H. Sliney,
J. Mellerio,
V. Gabel, and
K. Schulmeister,
“What is the meaning of threshold in laser injury experiments? Implications for human exposure limits,”
Health Phys., 82 335
–347
(2002). https://doi.org/10.1097/00004032-200203000-00006 0017-9078 Google Scholar
E. Zander and
G. Weddell,
“Observations on the innervation of the cornea,”
J. Anat., 85 68
–99
(1951). 0021-8782 Google Scholar
D. G. Cogan and
V. E. Kinsey,
“The cornea V. Physioloci aspects,”
Arch. Ophthalmol. (Chicago), 28 661
(1942). 0003-9950 Google Scholar
M. M. Zaret,
G. M. Breinin,
H. Schmidt,
H. Ripps, and
I. M. Siegel,
“Ocular lesions produced by an optical maser (laser),”
Science, 134 1525
–1526
(1961). https://doi.org/10.1126/science.134.3489.1525 0036-8075 Google Scholar
D. J. Lund,
M. B. Landers,
G. H. Bresnick,
J. O. Powell,
J. E. Chester, and
C. Carver,
“Ocular hazards of the Q-switched erbium laser,”
Invest. Ophthalmol., 9 463
–470
(1970). 0020-9988 Google Scholar
P. S. Avdeev,
Y. D. Berezin,
Y. P. Gudakovskiĭ,
V. R. Muratov,
A. G. Murzin, and
V. A. Fromzel,
“Experimental determination of maximum permissible exposure to laser of wavelength,”
Sov. J. Quantum Electron., 8 137
–139
(1978). https://doi.org/10.1070/QE1978v008n01ABEH008460 0049-1748 Google Scholar
B. E. Stuck,
D. J. Lund, and
E. S. Beatrice,
“Ocular effects of holmium and erbium laser radiation,”
Health Phys., 40 835
–846
(1981). https://doi.org/10.1097/00004032-198106000-00006 0017-9078 Google Scholar
T. F. Clarke,
T. E. Johnson,
M. B. Burton,
B. Ketzenberger, and
W. P. Roach,
“Corneal injury threshold in rabbits for the infrared laser,”
Aviat Space Environ. Med., 73
(8), 787
–790
(2002). 0095-6562 Google Scholar
R. L. McCally,
J. Bonney-Ray, and
C. B. Bargeron,
“Corneal injury thresholds for exposures to radiation—dependence on beam diameter,”
Health Phys., 87 16
–24
(2004). https://doi.org/10.1097/01.HP.0000137181.53428.04 0017-9078 Google Scholar
W. T. Ham and
H. A. Mueller,
“Ocular effects of laser infrared radiation,”
J. Laser Appl., 3
(3), 19
–21
(1991). 1042-346X Google Scholar
A. Heisterkamp,
T. Mamom,
O. Kermani,
W. Drommer,
H. Welling,
W. Ertmer, and
H. Lubatschowski,
“Intrastromal refractive surgery with ultrashort laser pulses: in vivo study on the rabbit eye,”
Graefe's Arch. Clin. Exp. Ophthalmol., 241
(6), 511
–517
(2003). 0721-832X Google Scholar
B. Ketzenberger,
T. E. Johnson,
Y. A. Van Gessel,
S. P. Wild, and
W. P. Roach,
“Study of corneal lesions induced by 1,318-nm laser radiation pulses in Dutch belted rabbits (Oryctolagus cuniculus),”
Comp. Med., 52
(6), 513
–517
(2002). Google Scholar
T. Miyamoto,
S. Saika,
A. Yamanaka,
Y. Kawashima,
Y. Suzuki, and
Y. Ohnishi,
“Wound healing in rabbit corneas after photorefractive keratectomy and laser in situ keratomileusis,”
J. Cataract Refractive Surg., 29
(1), 153
–158
(2003). https://doi.org/10.1016/S0886-3350(02)01450-5 0886-3350 Google Scholar
W. M. S. Russell and
R. L. Burch, The Principles of Humane Experimental Technique, Hyperion Books, Inc., New York (1959). Google Scholar
A. Guaitani,
L. De Francesco, and
S. Garattini,
“Reduced use of laboratory animals in research institute,”
Lancet, 349
(1064), 1557
–1558
(1997). https://doi.org/10.1016/S0140-6736(05)62145-9 0140-6736 Google Scholar
J. Zurlo,
D. Rudacille, and
A. M. Goldberg, Animals and Alternatives to Animal Testing: History, Science and Ethics, Mary Ann Liebert, Inc., Larchmont, NY (1994). Google Scholar
A. E. Siegman,
M. W. Sasnett, and
J. T. F. Johnston,
“Choice of clip levels for beam width measurements using knife-edge techniques,”
IEEE J. Quantum Electron., 27 1098
–1104
(1991). https://doi.org/10.1109/3.83346 0018-9197 Google Scholar
J. T. Walsh,
“Pulsed laser angioplasty: a paradigm for tissue ablation,”
Optical-Thermal Response of Laser Irradiated Tissue, Plenum Press, New York (1995). Google Scholar
T. E. Eurell,
T. E. Johnson, and
W. P. Roach,
“Histomorphometric and proteomic analysis of the acute corneal tissue response following in vitro exposure to laser light,”
113
–116
(2003). Google Scholar
C. I. Bliss,
“The calculation of the dosage mortality curve from small numbers,”
J. Pharmacol., 11 192
–216
(1935). 0021-793X Google Scholar
D. J. Finney, Probit Analysis, 3rd ed.Cambridge University Press, Cambridge, England (1971). Google Scholar
R. L. McCally and
C. B. Bargeron,
“Epithelial damage thresholds for multiple-pulse exposures to pulses of CO2 laser radiation,”
Health Phys., 80
(1), 41
–46
(2001). https://doi.org/10.1097/00004032-200101000-00008 0017-9078 Google Scholar
G. W. Mikesell,
“Corneal temperature—a study of normal and laser injured corneas in the Dutch belted rabbit,”
Am. J. Optom. Physiol. Opt., 55 108
–115
(1978). 0093-7002 Google Scholar
|
||||||||||||||||||||||||||||||