|
|
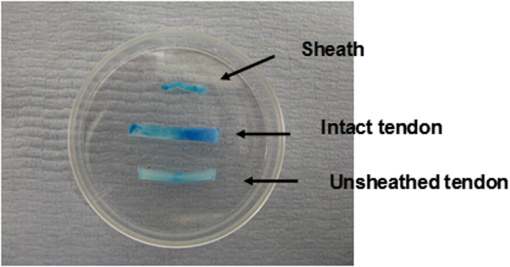
1.IntroductionTendon is a connective tissue, essential to the movement of limbs by transmitting the contractile (tensile) force of the muscle through the tendon to the bone. Tendon comprises of dense collagen bundles enclosed in a sheath referred to as “epitenon.” To fulfill force transmission, tendon exhibits a complex hierarchical arrangement, with collagen fibrils being the smallest structural subunit generated from collagen molecules of aggregated triple helices. A group of fibrils forms collagen fiber bundles, which in turn, are bound together to form fascicles. The relationship between tendon function (i.e., force transmission) and the fiber assembly process requires all these subunits to be packed longitudinally and aligning parallel to tendon’s long axis. It is logically postulated that there should be complex mechanisms to hold the individual fibrils, fibers, and fascicles together since all these constituent units are discontinuous. Two main mechanisms are proposed: first is the cross-linking of collagen fibers during fibrillogenesis,1 while the other important mechanism is the proteoglycan (PG) binding between collagen fiber units.2 In tendon, approximately 65% to 80% of the dry mass is collagen (up to 97% to 98% of which is collagen type I) and 0.2% to 5% is PG. Together with water and other small portions of noncollagenous macromolecules, PG is an integral component of the extracellular matrix (ECM) ground substance for tendon, forming a continuous phase.3 Hence it could be proposed that tendons are composite structures of highly aligned fibers embedded in ground substance in which force can be laterally transferred between neighboring fibrils.4 Over the past years, a few models have been proposed to explain the mechanical integrity of tendons. Dahners et al. conducted experiments that led to the conclusion that decorin, a constituent of PGs, could bind discontinuous collagen fibrils to one another, which provides the mechanical integrity to the structure of tendon.5 A model demonstrating the location and mechanism of PG attachment to collagen fibers was proposed, as shown in Fig. 1.6–8 Fig. 1The model and pictures of interfibrillar connection between proteoglycan and collagen bundles.6–8 (a) The model of collagen fiber bundels with proteoglycan (decorin) cross-linking collagen fiber bundles; (b) SEM picture showing the collagen fiber bundles with the presence of small items between the bundles. Reprinted with permission from the article of Fessel and Snedeker, 2009.6 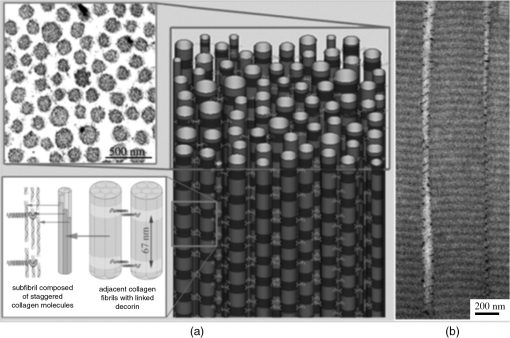 It is well known that the highly aligned collagen fibrils, fibers, or fascicles, possess unique optical properties. Using polarization light microscopy to observe tendon, a color-banded image appears. Furthermore optical coherence tomography (OCT) is a nondestructive, new imaging modality able to reveal three-dimensional (3-D) organized structures if a polarized light is used.9 We have demonstrated previously that healthy tendon exhibits a multiple banded structure when observed with polarization sensitive OCT (PS-OCT).9 Thus we hypothesize that if PGs play a role to form the interfibrillar connections for collagen fibers in tendon, the organization of the collagen bundles will change when PGs are extracted from the tendon. This disorganization could be observed as an alteration of the birefringence images of PS-OCT and polarization light microscope. In this study, we collected time-lapse PS-OCT and polarization light microscopic images during chemical extraction of PGs in chicken tendons, aiming to address 1) the effect of PG content on collagen fibril organization in terms of birefringence; 2) the distribution of PG in tendons; 3) the common region between PS-OCT and polarization light microscope images. 2.Materials and Methods2.1.Dissection of TendonsFresh chicken leg tendons, mainly Achilles tendon, were dissected and stored in phosphate buffered saline (PBS) at 4°C. The muscle and fat were carefully removed; the sheath of tendon was kept intact. Tendons were used within 48 h of dissection. For comparative study, one chicken tendon was cut into two: one half was used for a polarization light microscopic study, and the other half was assigned for PS-OCT imaging. In some tendons, the outer sheath, which was visualized as a viscous membrane-like structure, was carefully dissected by a sharp razor blade. Such treated specimens were denoted as unsheathed specimens. They were used in comparison to sheathed tendons for imaging and histological staining. To evaluate the stability and the heterogeneity of the PGs, tendons specimens from the same batch were kept in PBS at 4°C for five days, and the outer sheath was assessed by histological staining. 2.2.Proteoglycan ExtractionThe total proteoglycan was extracted following the well-established protocol.10 The 4 M extraction solution consisted of 4 M guanidine hydrochloride (GuHCl; Sigma, UK) with 0.05 M sodium acetate, 0.01 M EDTA, and 0.1 M 6-aminohexanoic acid (Fluka Analytical, UK). 0.005 M benzamidine (Aldrich, UK) and 0.01 M N-ethylmaleimide (Sigma, UK) were used as proteinase inhibitors. To study the effect of the concentration of the extraction solution on the rate of removal of proteoglycans, a 2 M GuHCl solution was used as well. 2.3.Time-Lapse Imaging with PS-OCT and Polarization Light MicroscopyA bench-top fibre based time-domain PS-OCT system was used in this study, which employed a 1300-nm superluminescence diode with a bandwidth of 52 nm.9 The beam from the diode passes through a polarizer, a polarizer modulator, and then split by a all-fiber beam splitter into a sample and a reference arm. After recombination, the polarized light is sent through a polarization beam splitter. The interference fringes are detected with two balanced detectors, for horizontal and vertical polarization. The system has an axial resolution of 14 μm in free space and a penetration depth of 1 mm in polarization mode. The PS-OCT images were scanned at 100 Hz. Each PS-OCT B-scan was made of 500 A-scans over a () area. The signal-to-noise ratio of the system was evaluated to be 90 dB for the current investigation. The birefringence , the indicator of fiber alignment, against the PGs extraction time, was calculated over an average of 200 A-scans centred in the picture (i.e., from 150 to 350) following Eq. (1)11 in order to reduce noise: where is the depth; is the source wavelength and is phase retardation, which can be calculated from the stokes vectors from the backscattered light as described by Park et al.12 Typically birefringent materials display a characteristic banding pattern in the phase retardation image arising from phase wrapping. The period of the banding pattern can be directly related to the birefringence, .To stabilize the specimens enabling imaging of the same location during the extraction of PGs, a specialized sample holder was designed. For PS-OCT, the tendons were restrained by fixing a segment of tendon to a thick, transparent plastic sheet, and immersed in approximately 2 ml GuHCl solution while being imaged. To take polarization microscopic images (Brunel, UK), the tendon specimens were slightly compressed and held between two glass slides, with a spacer allowing the specimens to be in contact with the GuHCl solution for the duration of the polarization microscopic imaging. The images were taken at regular intervals up to 60 to 120 min. The outermost region of the tendon specimens was deliberately included in each image. 2.4.Histological AnalysisTo assist the visualization of the content of PG, Alcian blue dye (0.1% ; pH 2.5, dissolved in acetic acid) was used to stain the PGs in the tendon specimens. All specimens were fixed by 70% ethanol for 10 min, stained by Alcian blue for 5 min and then thoroughly washed with distilled water and digital images were taken. 3.ResultsFresh tendons examined by PS-OCT displayed a typical banding pattern characteristic of birefringent material, with an average penetration depth of 700 μm and a mean birefringence of , while polarization light microscopic images exhibited multiple fine colored bands along the long axis of the tendons with less pronounced vertical bands (Fig. 2). Fig. 2PS-OCT (a) and polarization light microscopy (b) images of fresh intact tendon. The PS-OCT image was the scan of () area of the specimen demonstrating banded birefringence feature; while the image of polarization light microscopy exhibits collagen bundle structure. The scale bar is 200 µm. 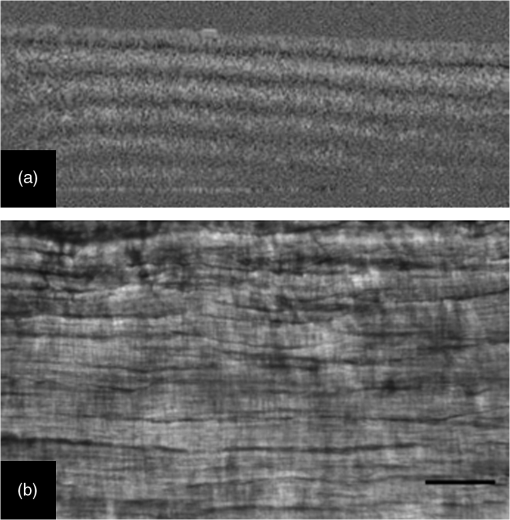 When comparing the birefringence bands in PS-OCT images using different concentrations of extraction solution, it was found that the 4 M GuHCl solution caused rapid changes in the banding pattern over time. The number of bands was reduced, and the width of the bands increased within a short period (as short as 10 min). After 60 min of extraction, the band width had nearly doubled from the initial size, while the changes in band number and width at 2 M GuHCl solution were much less prominent (Fig. 3). Corresponding polarization microscopy images demonstrated a similar pattern with the effect of the concentration of the extraction solutions. The 4 M GuHCl solution proved to be highly effective in the extraction of PGs. Within 10 min, the structure of outermost layer of the tendon became distorted under polarization microscopy (Fig. 4). However, the bands associated with the deeper structure of the tendon persisted for a longer period; by 60 min, the central collagen bundles became considerably disordered, although the bundle boundary was still distinguishable. In contrast, the 2 M GuHCl solution disturbed the fiber bundles at a reduced rate. After 60 min, only a thin layer of the tissue became colorless at the outermost position and the rest of the tissue remained undisturbed. Fig. 3Time-lapse PS-OCT birefringence images showing the effects of GuHCl concentration, extraction time, and unsheathing on tendon structural change during the PG extraction. The PS-OCT image was the scan of () area of the specimen. 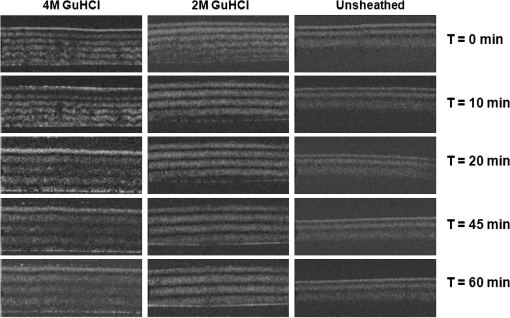 Fig. 4Time-lapse polarization light microscopic images showing the effect of GuHCl concentration and extraction time on tendon structure. The bottom of each image is the outermost region of the tendon specimens. It is demonstrated after 60-min extraction, the collagen bundles of the specimen in 2 M solution remained organized pattern except for the light swollen bundle at the edge; while the bundles of the specimen in 4 M solution became significant distorted (indicated by arrows). The scale bar is 200 µm. 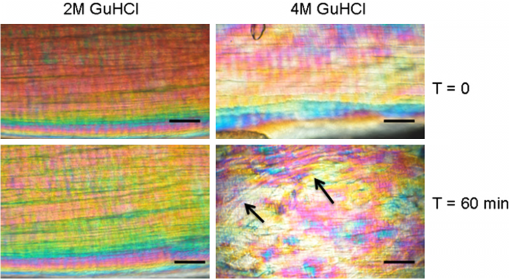 PS-OCT and polarized light microscopy images of unsheathed tendons were taken while PGs were being extracted. Polarization microscopy images of unsheathed specimens demonstrated subtle changes in the band structure as compared to the sheathed ones after around 60 min of extraction (Fig. 5). The corresponding PS-OCT images in Fig. 3 showed that the tendon without a sheath, the rate at which the state of birefringence changed, was reduced in comparison to intact (sheathed) tendon. Fig. 5Time-lapse polarization light microscopic images showing the effect of PG extraction (4 M GuHCl) on tendon’s structure with and without sheath. The bottom of each image is the outermost region of the tendon specimens. It is demonstrated that the specimen without sheath did not change the organization of collagen bundles rapidly within the 60 min extraction; while the sheathed (intact) tendon exhibited rapid distortion of collagen fiber bundles (indicated by arrows). The scale bar is 200 µm. 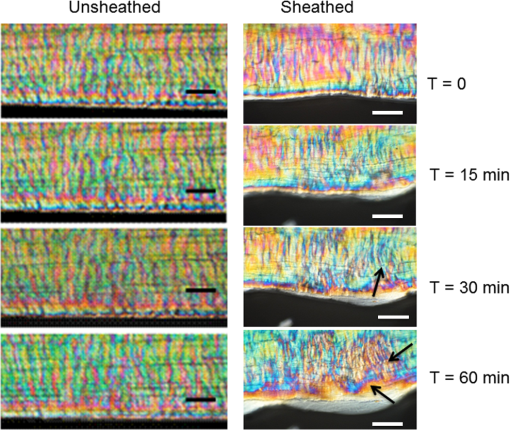 Time-lapse images from PS-OCT under different conditions of PGs extraction were analyzed to quantify the associated birefringence by phase retardation (expressed as normalized phase retardation against the value at ) as shown in Fig. 6. It is clearly exhibited that the intact tendons demonstrated a rapid birefringence loss during extraction of PGs. The rate at which birefringence was lost was greatest between 0 and 20 min. A decrease in the concentration of the extraction solution from 4 M to 2 M considerably reduced the rate at which birefringence was lost. The birefringence of unsheathed tendon remained relatively constant throughout the extraction period (except for a few fluctuations). Fig. 6Phase retardation (expressed as normalized phase retardation against the value at ) of the tendons subjecting to 4 M (for sheathed and unsheathed specimens) and 2 M (for sheathed specimens) GuHCl extraction against extraction time. The birefringence of unsheathed tendon remained relatively constant throughout the extraction period (except for small fluctuation values) in contrast to the rapid reduction of birefringence in sheathed specimens. 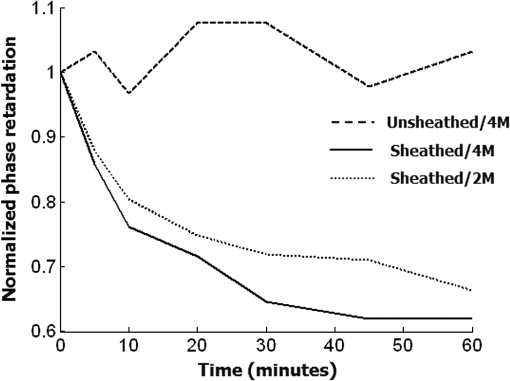 Fresh specimens exhibited more bands, which were well defined by PS-OCT and polarized light microscopy imaging, in comparison with the aged specimens (e.g., stored in PBS at 4 ºC for five days). To clarify the cause of the difference, the sheath of the freshly dissected tendons and tendons stored for five days, was carefully removed by sharp razor blade. The membrane-like sheaths were stained with Alcian blue. Darker, more intense staining was associated with the fresh specimens, but not in the aged ones (Fig. 7). Similarly the differences in the staining pattern were observed for unsheathed and sheathed tendons in freshly dissected tendon specimens. It was demonstrated that the intact tendon (the sheathed tendon) stained homogenously blue, while unsheathed tendon stained less intensely (Fig. 8), indicating that there is likely to be a higher concentration of PGs in the sheath-region of the tendon than in the fascicles. 4.DiscussionIn this study, we have developed a methodology to observe the effect of PG loss on the structural change of the tendon in real-time and in situ. PS-OCT was used to examine the cross-sections of tendon up to 1 mm depth, while polarization light microscopic images, in transmission mode, recorded overlapping structure from the top to bottom layers of the slightly compressed specimens. Although PS-OCT and light microscopy did not image the same part of a tendon specimen, we still can derive compensated information from the two imaging modalities. PS-OCT and polarization light microscopy provided complementary information. It was confirmed that extraction of PG from intact tendon caused the disturbance of the collagen arrangement. The decrease of birefringence, as seen as a decrease in band frequency and increase in band width, was observed after extraction of PGs. This result showed that removal of PGs resulted in a considerable disorganization of the collagen fiber structure. Scott et al. have successfully visualized the glycosaminoglycan (GAG) chains of PG molecules in cornea, sclera, and tendons and the binding site of the PGs along the collagen fibrils by TEM and biochemical staining, which provided evidence for the importance of the PG molecules in collagen fibril organisation, stabilization, and growth.13 Figure 1 demonstrated these organizations, both for real tissue and a model. Other studies also confirm that the collagen fibers in tendons are held together by proteoglycan components, including decorin.14 The proteoglycans are believed to be interwoven with the collagen fibrils; their GAG side chains have multiple interactions with the surface of the fibrils.15 These interconnections were believed to be formed in the fibril assembly process during tendon development. One theory postulated that dermatan sulfate can be responsible for forming associations between fibrils. When decorin molecules are bound to collagen fibrils, their dermatan sulfate chains may extend and associate with other dermatan sulfate chains on decorin that is bound to separate fibrils, therefore creating interfibrillar bridges and eventually causing parallel alignment of the fibrils.14 Our data provided direct evidence to support these theories and visualize the dose-responses (through time-lapse imaging) of PG concentration on collagen fiber organization. When PGs were removed from collagen fibers, the stability of the collagen interfibrillar connections reduced and the local collagen organization was disturbed. It is also noted that after removal of PGs, the tendons became permeable to liquid, which led to an increase in the volume of the specimen (Fig. 3). Both fiber organization and volume affect birefringence. The considerable distortion of the fiber organization (observed from polarization light microscopy images) after PG removal indicates that fiber organization played a predominant role in state of birefringence. It is our intention to see whether the imaging information from the two modalities could be used to complement and support each other, because PS-OCT reveals optical phenomenon (birefringence) caused by structural changes, whilst polarization light microscopy displays real structural organization. At first glance, it appeared that PS-OCT is more sensitive to detect the change in collagen fiber organization than light microscopy because the changes of birefringence bands in PS-OCT occurred within 10 min in some specimens. However, we also noted that the edge of light microscopic images exhibited rapid structural change as well upon PG extraction (Fig. 4). Apparently, the two imaging modalities could reflect similar region in tendon specimens. PS-OCT acquires the cross-section images perpendicular to the surface, denoting as to images in Fig. 9, while the edge of the tendon images in polarization light microscopy, denoting as to images, record the outermost region of the tendon with accumulating volume of the periphery, or sheath. Since the extraction reaction was initiated from the outermost portions, PS-OCT was more sensitive to these dramatic changes (Fig. 3). As the maximum penetration depth in PS-OCT was around 1 mm at the present setting, the deeper structures could not be analysed. Hence the dramatically changed images in Fig. 3 were in fact only the top layer (from the sheath downward) of tendon where the PG content is higher. On the other hand, the transparency of the tendon specimen (slightly compressed) enabled recording the structure through the whole thickness of the tendon when PGs were extracted from the outermost regions by polarization light microscopy. It showed that collagen fiber organization in the deeper layers of the tendon tissue remained intact until the later stages (120 min), providing an opportunity to define and interpret the effects of the extraction process. Combining the data from the PS-OCT and light microscopy images during the PG extraction raised two arguments: there was a difference in the PG distribution (amount and type, requiring further investigation) within tendon tissue; the PG extraction rate was different from the outermost region, sheath, and from the inner collagen fascicles (somewhat understandable as the deeper layers come into less contact with the extraction solution). The Alcian blue staining (Fig. 8) revealed that the PG distribution in tendon was not homogenous and most of the PG appeared to be localized in the outer sheath. Apparently, when this PG was extracted, it led to rapid changes in the PS-OCT images (Fig. 3). The disorganized sheath layer might further interfere with the penetration of light. Furthermore, our data indicated that PGs in the sheath were very vulnerable to decomposition, which may also explain why some tendon specimens that have been stored for a long time showed less birefringence bands by PS-OCT, although the specimens had an intact, organized structure under polarization microscopy. Fig. 9Illustration of the applied observations of a common area in a tendon specimen by PS-OCT and polarization light microscopy. 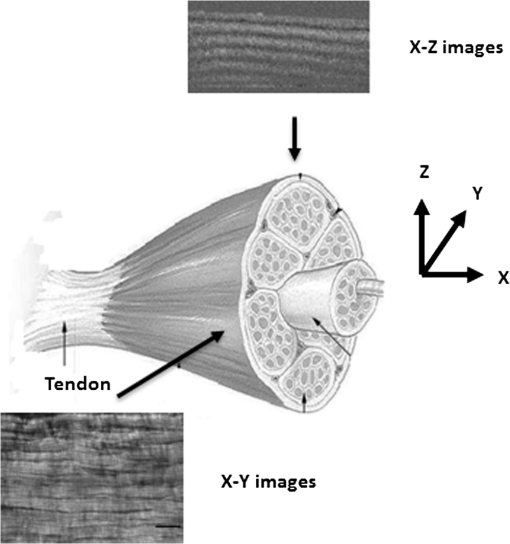 This study illustrated that the PG distribution in tendon is not homogenous; our Alcian blue staining and PG extraction data demonstrate this difference. Most PG is apparently stored in the sheath (epitenon). The PG was easily extracted in GuHCl solution, which led to a rapid distortion of tendon structure, evidenced by PS-OCT images. An increased concentration of GuHCl, led to more rapid extraction of PGs. Fully understanding of the function and location of PGs in tendon and their role in maintaining intrinsic optical properties will help us further interpret the pathology of tendon disease. PS-OCT has been proven as an effective tool for the detection of these changes. The possibility to develop needle probe OCT could allow in vivo detection of PG related diseases in tendons. Furthermore, this research may advance the ability to generate biofunctional models of tendon by understanding the composition of the regions of tendon and epitenon. In conclusion, this study establishes a convenient technique to correlate the relationship between PG content and collagen fiber organization. PG concentration and the degree of binding with collagen fibers directly control the organization of the hierarchal structure. Through the nondestructive imaging technique, PS-OCT, we may develop a reliable and simple diagnostic tool for the diseases of tendons due to abnormalities in PGs. ReferencesP. P. Provenzano JrR. Vanderby,
“Collagen fibril morphology and organization: implications for force transmission in ligament and tendon,”
Matrix Biol., 25
(2), 71
–84
(2006). http://dx.doi.org/10.1016/j.matbio.2005.09.005 MTBOEC 0945-053X Google Scholar
G. Zhanget al.,
“Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development,”
J. Cell. Biochem., 98
(6), 1436
–1449
(2006). http://dx.doi.org/10.1002/(ISSN)1097-4644 JCEBD5 0730-2312 Google Scholar
P. SharmaN. Maffulli,
“Basic biology of tendon injury and healing,”
Surgeon, 3
(5), 309
–316
(2005). http://dx.doi.org/10.1016/S1479-666X(05)80109-X 0035-8835 Google Scholar
R. K. Korhonenet al.,
“Fibril reinforced poroelastic model predicts specifically mechanical behavior of normal, proteoglycan depleted and collagen degraded articular cartilage,”
J. Biomech., 36
(9), 1373
–1379
(2003). http://dx.doi.org/10.1016/S0021-9290(03)00069-1 JBMCB5 0021-9290 Google Scholar
L. E DahnersG. E. LesterP. Caprise,
“The pentapeptide NKISK affects collagen fibril interactions in vertebrate tissue,”
J. Orthop. Res., 18
(4), 532
–536
(2000). http://dx.doi.org/10.1002/(ISSN)1554-527X JOREDR 0736-0266 Google Scholar
G. FesselJ. G. Snedeker,
“Evidence against proteoglycan mediated collagen fibril load transmission and dynamic viscoelasticity in tendon,”
Matrix Biol., 28
(8), 503
–510
(2009). http://dx.doi.org/10.1016/j.matbio.2009.08.002 JBMCB5 0021-9290 Google Scholar
J. LiaoI. Vesely,
“Skewness angle of interfibrillar proteoglycans increases with applied load on mitral valve chordae tendineae,”
J. Biomech., 40
(2), 390
–398
(2007). http://dx.doi.org/10.1016/j.jbiomech.2005.12.011 JBMCB5 0021-9290 Google Scholar
A. Redaelliet al.,
“Possible role of decorin glycosaminoglycans in fibril to fibril force transfer in relative mature tendons—a computational study from molecular to microstructural level,”
J. Biomech., 36
(10), 1555
–1569
(2003). http://dx.doi.org/10.1016/S0021-9290(03)00133-7 Google Scholar
P. O. Bagnaninchiet al.,
“In-depth imaging and quantification of degenerative changes associated with Achilles ruptured tendons by polarization-sensitive optical coherence tomography,”
Phys. Med. Biol., 55
(13), 3777
–3787
(2010). http://dx.doi.org/10.1088/0031-9155/55/13/014 PHMBA7 0031-9155 Google Scholar
S. G. Reeset al.,
“Catabolism of aggrecan, decorin and biglycan in tendon,”
Biochem. J., 350
(1), 181
–188
(2000). http://dx.doi.org/10.1042/0264-6021:3500181 BIJOAK 0264-6021 Google Scholar
J. F. de BoerT. E. Milner,
“Review of polarization sensitive optical coherence tomography and stokes vector determination,”
J. Biomed. Opt., 7
(3), 359
–371
(2002). http://dx.doi.org/10.1117/1.1483879 JBOPFO 1083-3668 Google Scholar
B. Parket al.,
“Real-time multi-functional optical coherence tomography,”
Opt. Express, 11
(7), 782
–793
(2003). OPEXFF 1094-4087 Google Scholar
J. E. Scott,
“Proteoglycan-fibrillar collagen interactions,”
Biochem. J., 252
(2), 313
–323
(1988). BIJOAK 0264-6021 Google Scholar
J. E. Scott,
“Proteodermatan and proteokeratan sulfate (decorin, lumican/fibromodulin) proteins are horseshoe shaped. Implications for their interactions with collagen,”
Biochemistry, 35
(27), 8795
–8799
(1996). http://dx.doi.org/10.1021/bi960773t BICHAW 0006-2960 Google Scholar
E. G. CantyK. E. Kadler,
“Procollagen trafficking, processing and fibrillogenesis,”
J. Cell. Sci., 118
(7), 1341
–1353
(2005). http://dx.doi.org/10.1242/jcs.01731 JNCSAI 0021-9533 Google Scholar
|