|
|
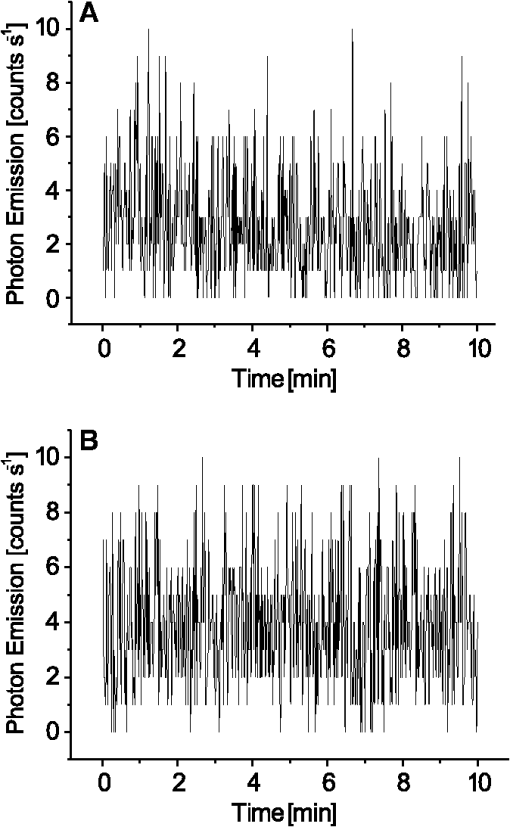
1.IntroductionVarious types of biotic (viral, bacterial, and fungal) and abiotic (chemical and physical) stresses are responsible for the oxidative damage in human skin.1–4 Among the physical stress factors, ultraviolet A (UVA) radiation plays a major role due to its high penetration of Earth’s surface owing to changing environmental conditions during the past decades. The exposure of the epidermal and dermal layers of human skin to UV radiation is accompanied by the formation of reactive oxygen species (ROS).5 To combat the deleterious action of ROS on human skin, nonenzymatic and enzymatic antioxidant defense systems have been developed by the skin.6–8 The nonenzymatic antioxidant defense system consists of low-molecular-weight components such as chromophores (carotenoids, melanins, urocanic acids, porphyrins, bilurubins, flavins, and pterins) and vitamins (A, B, C, D, and E), whereas the enzymatic antioxidant defense system is composed of various types of antioxidant enzymes (superoxide dismutase, peroxidase, catalase, and glutathione reductase).9–13 When the number of ROS exceeds a critical threshold due to the saturation in capacity of the antioxidant defense system, ROS cause oxidative damage to the cells, leading to skin damage in forms such as premature skin aging and even skin cancer,14,15 Under certain circumstances, the endogenous chromophores such as porphyrins (uroporphyrin, coproporphyrin, and protoporphyrin), bilurubins, melanins (eumelanin and pheomelanin), flavins (riboflavin, flavin mononucleotide), pterins (6-carboxypterin, formylpterin, neopterin, and biopterin), and urocanic acid (trans-urocanic acid) act as photosensitizers.16 A photosensitization reaction is initiated by the absorption of visible light and UVA radiation by a photosensitizer, forming the singlet excited state. The singlet excited state is known to form the triplet excited state in a photosensitizer by intersystem crossing. Among the chromophores, melanins and bilurubins, which are the predominant pigment in the human skin, have been demonstrated to absorb both in the UV and visible region of the spectrum at spectral range between 300 and 600 nm.17 On the other hand, other chromophores such as the urocanic acid (250 to 300 nm), riboflavins (355 nm), and pterins (345 to 375 nm) have an absorption maximum in the UV range with almost no absorption in the visible region of the spectrum.18–20 The excited photosensitizer undergoes electron transport and energy transfer, leading to the formation of radical (superoxide anion radical, ; hydroxyl radical, ) and nonradical (hydrogen peroxide, ; singlet oxygen, ) ROS. Radical and non-radical ROS are known to oxidize lipids and proteins via hydrogen abstraction and oxygen addition, respectively.21–24 It is well established that hydrogen abstraction from a biomolecule by leads to the formation of an alkyl radical (), which in the presence of molecular oxygen forms a peroxyl radical ().25 The reactive can further extract an electron from a biomolecule forming another and hydroperoxide (ROOH), which is reduced by transition metals to an alkoxyl radical ().25,26 Electron paramagnetic resonance study has revealed that lipid and can be produced in skin biopsies following exposure to UV radiation.17 Self-reaction of two or forms acyclic intermediate tetroxide, known to decompose either to triplet excited carbonyls [] and molecular oxygen or and ground-state carbonyls ().27,28 The oxidation of biomolecules mediated by through oxygen addition results in the formation of cyclic intermediate dioxetane.1,2 The decomposition of dioxetane forms , known to further transfer the excitation energy to molecular oxygen forming . The electronic transition from the excited state to the ground state of a photosensitizer is accompanied by photon emission at low intensity, which is referred to as ultraweak photon emission.29 It is well established that emits photons in the blue region of the spectrum, which ranges from 400 to 500 nm, while the dimol emission from has been demonstrated in the red region of the spectrum at 634 nm and 703 nm.23,25,27 The spectral analysis of spontaneous ultraweak photon emission from human skin has indicated that photons are spontaneously emitted mainly in the red region of the spectrum, revealing that predominantly contributes to the photon emission.28 In contrast, it has previously been demonstrated that spontaneous ultraweak photon emission is in the blue-green region of the spectrum.30–33 Employing immunoblotting techniques, it has recently been demonstrated that exposure of human skin to UV radiation results in the formation of in the human stratum corneum.7,25 It was observed that UV-radiation-induced ultraweak photon emission in the human skin showed a maximum of photon emission in the spectral range 400 to 580 nm, supporting the assumption that is a main source of ultraweak photon emission.7,28 In contrast, it has previously been demonstrated that UVA-induced ultraweak photon emission is in the red region of the spectrum.30–33 In our present study, we investigated the effect of visible light and UVA radiation on ultraweak photon emission by employing a highly sensitive charge coupled device (CCD) camera and a low-noise photomultiplier tube (PMT). The experimental results show that exposing human skin to UVA radiation enhances ultraweak photon emission, revealing oxidative stress in the human skin. 2.Materials and Methods2.1.SubjectThe following study was conducted on the author’s hand, and no other subject was involved in the study. To prevent the intervention of delayed luminescence during the measurement, the subject was dark-adapted for 30 min prior to performing measurements in a darkroom restricted from any light. To avoid any kind of diurnal fluctuation in ultraweak photon emission,34 the measurements were performed during a fixed period ranging from 11:00 and 14:00 h. Use of any types of cosmetics was prohibited during the course of the study. The current study was performed in agreement with the ethical principles stated in the declaration of Helsinki and its subsequent revisions. 2.2.Light Exposure2.2.1.Visible light exposureThe dorsal and palmar sides of the hand were exposed to visible light using a Philips light source (Philips Electronics Ltd, Guildford, UK) with a spectral range of 400 to 700 nm measured by employing a LI-COR LI-1800 spectral radiometer (LI-COR Biosciences, St. John’s Innovation, Cambridge, UK). The central area of the hand was chosen for PMT measurements, while two-dimensional (2-D) imaging of the complete surface of the dorsal and palmar sides of the hand was measured. The light source was located outside the experimental darkroom. The exposure time was 5 min, and the time between the end of irradiation and the start of measurement was kept at 20 s in each measurement. The hand was distanced 6 cm from the visible light source. The power density on the surface of the skin was . 2.2.2.UVA radiation exposureThe dorsal and palmar sides of the hand were exposed to UVA radiation using a Philips UVA CLEO SWIFT lamp commonly used for tanning purposes with a spectral range of 320 to 400 nm as measured by employing a LI-COR LI-1800 spectral radiometer (LI-COR Biosciences, St. John’s Innovation, Cambridge, UK). The entire dorsal side of the hand was exposed with UVA radiation and the central area of the hand was chosen for PMT measurements, while 2-D imaging of the complete surface of the dorsal side of the hand was measured. The UVA source was located outside the experimental darkroom. The exposure time was kept at 5 min, and the hand was positioned 6 cm from the UVA source. The time between the end of irradiation and the start of measurement was kept at 20 s in each measurement. The power density on the surface of the skin was . 2.3.Ultraweak Photon EmissionUltraweak photon emission measurements were accomplished employing the CCD camera [Fig. 1(a)] and the PMT system [Fig. 1(b)] installed in a black painted inner darkroom with a dimension of . The measurement systems inside the inner darkroom were controlled and data were recorded with the computer located in the outer darkroom. The door in the inner darkroom was protected with a black curtain to restrict any photon entrance from the outer darkroom. For quantitative analysis, all measurements were done in three replicates. Fig. 1Schematic illustration of experimental setup for measurements of (a) 2-D and (b) 1-D ultraweak photon emission. The 2-D ultraweak photon emission was measured using a CCD camera, whereas 1-D ultraweak photon emission was measured using PMT. 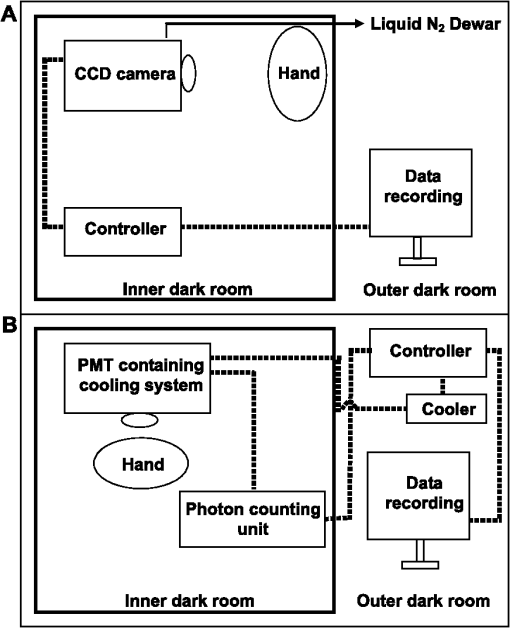 2.3.1.Two-dimensional photon emission imagingThe highly sensitive CCD camera VersArray 1300B (Princeton instruments, Trenton, NJ, USA), with spectral sensitivity in the range 200 to 1000 nm and almost 90% quantum efficiency in the visible range of the spectrum, was employed for the 2-D photon imaging. The spectral sensitivity was limited to 350 to 1000 nm by the lenses. An objective lens of 50 mm focal distance (F mount Nikkor 50-mm, f:1.2, Nikon) was used to enhance the light collecting efficiency. The CCD unit contained a liquid-nitrogen dewar to cool the CCD element down to to reduce the dark count. The following parameters were used during the measurements: scan rate, 100 kHz; gain, 2; image format, ; distance between detector and the hand, 37 cm; and accumulation time, 30 min. Data correction was made by subtracting the background signal prior to each measurement. Improvement of signal-to-noise ratio was accomplished using the binning mode with a binning factor of 4, which resulted in an image format of . 2.3.2.One-dimensional ultraweak photon emissionA low-noise PMT R7518P, sensitive in the spectral range 185 to 730 nm, and a photon counting unit C9744 (Hamamatsu Photonics K.K., Iwata City, Japan) were employed to measure one-dimensional (1-D) photon emission. To reduce the thermal electrons, the PMT was cooled down to using thermoelectric cooler C9143 (Hamamatsu Photonics, K.K., Iwata City, Japan). The overall noise comprising the dark count and background light was 2 counts . The dark count was adjusted to approximately 1.5 counts at . To minimize the background light noise to 0.5 counts , PMT was kept in a vertical position. During measurements of the hand, a distance of 2 cm was kept between the hand and the PMT window. 3.Results3.1.Two-Dimensional Imaging of Ultraweak Photon Emission from the Dorsal Side of the HandTwo-dimensional imaging of spontaneous, visible-light- and UVA-radiation-induced ultraweak photon emission was measured on the dorsal side of the hand using a highly sensitive CCD camera (Fig. 2). The photograph and the corresponding image of spontaneous ultraweak photon emission measured on the dorsal side of the hand are shown in the Fig. 2(a) and 2(b), respectively. To test the effect of visible light and UVA radiation on ultraweak photon emission, 2-D ultraweak photon emission was monitored on the dorsal side of the hand previously exposed to visible light and UVA radiation. It is clearly evident that the photon emission from the visible-light- [Fig. 2(c)] and UVA-irradiated [Fig. 2(d)] dorsal side is higher compared to the spontaneous ultraweak photon emission [Fig. 2(b)] from the dorsal side. The photon emission from the UVA-irradiated dorsal side is higher than the visible-light-irradiated dorsal side, depicting the long-term effect of UVA radiation compared to visible light. The 2-D ultraweak photon emission imaging reveals high oxidative stress on the dorsal side of the hand upon visible light and UVA radiation compared to spontaneous ultraweak photon emission image. The observation also indicates a higher degree of oxidative stress on the dorsal side of the hand upon exposure to UVA radiation compared to visible light. Fig. 2Two-dimensional imaging of the ultraweak photon emission from the dorsal side of the hand measured by a highly sensitive CCD camera. The photographs (a) and the corresponding 2-D images of spontaneous (b), visible-light-induced (c), and UVA-radiation-induced (d) ultraweak photon emission were measured on the dorsal side of the hand. In A, the photograph was obtained under weak light illumination. In B, prior to the measurements, the hand was kept in complete darkness for 30 min. In c and d, the photon emission was recorded after subsequent exposure of the hand to visible light (400 to 700 nm) and UVA radiation (320 to 400 nm) for 5 min, and the measurements were performed after a fixed interval of 20 s. Ultraweak photon emission imaging was measured with an integration time of 30 min. 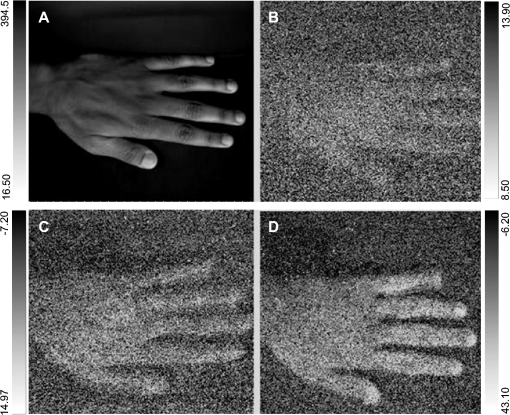 3.2.1-D Spontaneous Ultraweak Photon Emission from the Dorsal and the Palmar Sides of the Hand1-D spontaneous ultraweak photon emission was measured on the dorsal and the palmar sides of the hand using low-noise PMT (Fig. 3). When the dorsal side was put below the PMT window, a count rate of 4 counts was observed, whereas the palmar side showed a count rate of 6 counts . After subtraction of the dark count of the PMT, the spontaneous ultraweak photon emission from the dorsal and the palmar sides of the hand was determined to be 2 and 4 counts , respectively [Fig. 3(a) and 3(b)]. The photon emission persists for several hours with no drop in the count rate, confirming that spontaneous ultraweak photon emission is an inherent property of the cells. The observation that the photon emission on the palmar side of the hand has a higher count rate than the dorsal side of the hand indicates that oxidative metabolic processes on the palmar side are higher than on the dorsal side of the hand. 3.3.Effect of Visible Light on 1-D Ultraweak Photon Emission from the Dorsal and the Palmar Sides of the HandTo study the effect of visible light on ultraweak photon emission, the ultraweak photon emission was studied on the dorsal and the palmar sides of the hand previously exposed to visible light. When the dorsal [Fig. 4(a)] and the palmar [Fig. 4(b)] sides of the hand were exposed to visible light prior to the measurement, an increase in photon emission followed by a decrease in the steady state value was observed. The exposure of the dorsal side of the hand to visible light resulted in the enhancement to 40 counts and the decay in the steady state value of 5 counts . The exposure of the palmar side of the hand to visible light brought about the increase to 120 counts and the decrease in the steady state value of 7 counts . The observation that the ultraweak photon emission from the dorsal and the palmar sides of the hand exposed to visible light remains slightly higher than the spontaneous ultraweak photon emission reflects the oxidative stress generated on the dorsal and the palmar sides of the hand. The observation that the photon emission on the palmar side of the hand is higher than on the dorsal side of the hand indicates that the oxidative stress generated on the palmar side is higher compared to the dorsal side upon visible light exposure. Fig. 4One-dimensional visible-light-induced ultraweak photon emission from the dorsal (a) and the palmar (b) sides of the hand. In A and B, 1-D ultraweak photon emission was measured after exposure of the dorsal and the palmar sides of the hand to visible light, respectively. The radiation was accomplished using a visible light source of wavelength 400 to 700 nm. The hand was exposed to the visible light just prior to measurement for 5 min, and the measurements were performed after a fixed interval of 20 s. The decay curve was measured for 30 min followed by an interruption of 20 min and further measurement of 5 min. 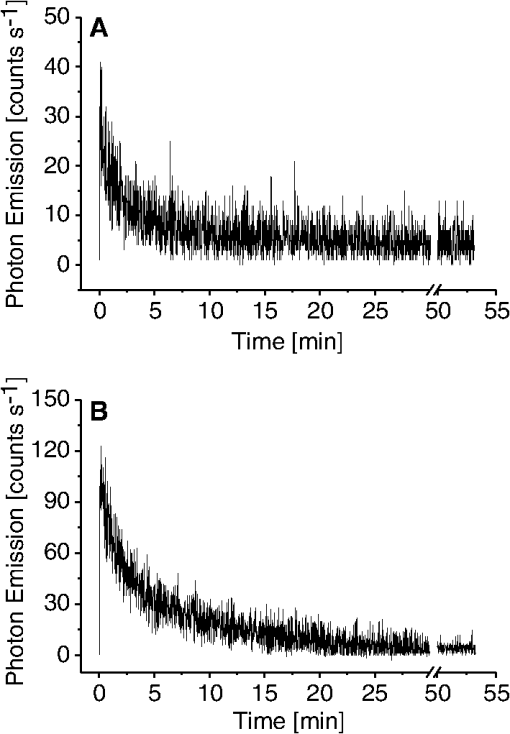 3.4.Effect of UVA Radiation on 1-D Ultraweak Photon Emission from the Dorsal and the Palmar Sides of the HandTo study the effect of UVA radiation on ultraweak photon emission, ultraweak photon emission was studied on the dorsal and the palmar sides of the hand previously exposed to UV radiation (Fig. 5). The increase and subsequent decrease in the steady state value were observed when the dorsal [Fig. 5(a)] and the palmar [Fig. 5(b)] sides of the hand were exposed to UVA radiation prior to measurement. The photon emission upon the exposure of the dorsal side of the hand to UVA radiation resulted in the enhancement to 60 counts and the decay in the steady state value of 8 counts . The exposure of the palmar side of the hand to UVA radiation brought about the increase to 160 counts and the decrease in the steady state value of 10 counts . The observation that the ultraweak photon emission from the dorsal and the palmar sides of the hand exposed to UVA radiation is significantly higher than the spontaneous photon emission indicates that the oxidative stress is generated on the dorsal and the palmar sides of the hand. The observation that the photon emission on the palmar side of the hand is considerably higher than on the dorsal side of the hand indicates that the oxidative stress generated on the palmar side is higher compared to the dorsal side upon UVA exposure. Fig. 5One-dimensional UVA-induced ultraweak photon emission from the dorsal (a) and the palmar (b) sides of the hand. In A and B, 1-D ultraweak photon emission was measured after exposure of the dorsal and the palmar sides of the hand to UVA radiation, respectively. The radiation was accomplished using a UVA source of wavelength 320 to 400 nm. The hand was exposed to the UVA radiation just prior to the measurement for 5 min, and the measurements were performed after a fixed interval of 20 s. The decay curve was measured for 30 min followed by an interruption of 20 min and further measurement of 5 min. 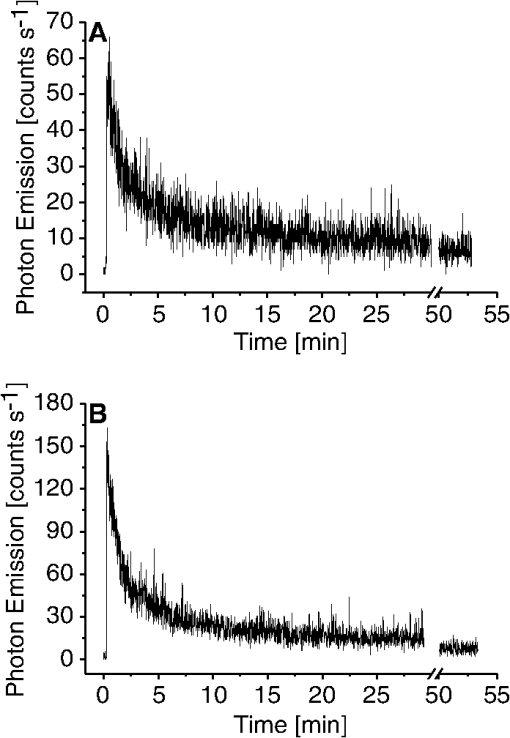 3.5.Efficacy of Ultraweak Photon Emission from the Dorsal and the Palmar Sides of the Hand upon Visible Light and UVA IrradiationTo quantify the effect of visible light and UVA radiation on the ultraweak photon emission, the area under the curve was calculated (Fig. 6). The area under the curve was increased by the exposure of the hand to visible light and UVA radiation. After the visible light exposure, the area under the curve from the dorsal and the palmar sides of the hand was recorded to be two and three times higher, respectively, compared to the area under the curve of spontaneous ultraweak photon emission [Fig. 6(a)]. The exposure of the dorsal and the palmar sides of the hand to UVA radiation resulted in the area under the curve being about four times and six times higher, respectively, than the area under the curve of spontaneous ultraweak photon emission. These observations indicate that UVA radiation generates high oxidative stress on the hand compared to visible light and that both visible light and UVA radiation generate oxidative stress on the hand. Fig. 6Efficacy of the visible light (a) and UVA radiation (b) on ultraweak photon emission from the dorsal and the palmar sides of the hand. The -axis represents the area under the curve (counts in thousands) obtained on the visible-light- and UVA-irradiated dorsal side of the hand, measured for 30 min. The noise represents the area under the curve, composed of the dark count and background light. In a and b, the values corresponding to the area under the curve from the dorsal and the palmar sides of the hand include the noise. The presented data are expressed as the mean value and the standard deviation of at least three measurements (, ). Other experimental conditions are as in Fig. 3. 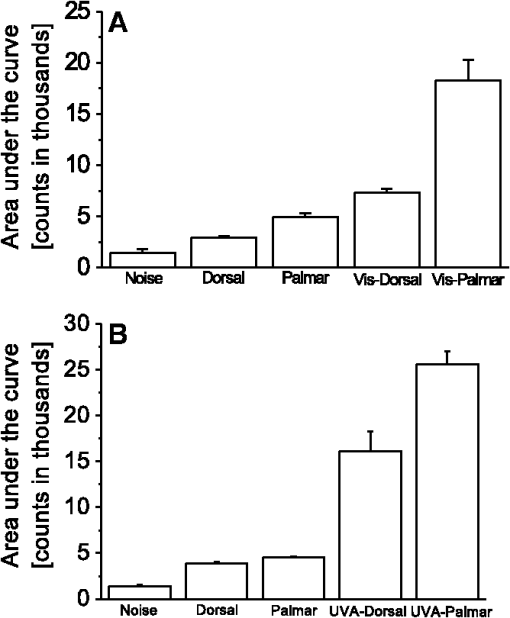 4.DiscussionChromophores play a crucial role in the formation of ROS via photosensitization composed of either electron transfer (type I) or energy transfer (type II) reactions16,35 (Fig. 7). In the photosensitization reaction, the absorption of excitation energy by a photosensitizer causes a transition from the ground state to the singlet excited state of the photosensitizer, which is later converted to the triplet excited states via intersystem crossing. The ROS formed by both type I (, , ) and type II () photosensitization reactions have a capability to oxidize lipids and proteins (Fig. 7). As a by-product of oxidation of lipids and proteins, reactive intermediates such as dioxetane and tetroxide are formed, which further decompose either to and molecular oxygen or and (Fig. 7). The triplet excited carbonyls can emit in the broad range from 400 to 500 nm, while can undergo dimerization resulting in the dimol emission at 634 and 703 nm. In the present study, 2-D ultraweak photon emission was employed as a noninvasive tool to monitor the visible-light- and UVA-radiation-induced oxidative stress of the hand through photosensitization reactions (Figs. 2, 4, and 5). Fig. 7Mechanism of ultraweak photon emission via a photosensitization reaction of skin chromophores: The chromophores in the skin absorb in the visible and the UVA regions of the spectrum. Melanin and bilirubin are known to absorb in the visible and the UVA regions of the spectrum, whereas the other chromophores absorb predominantly in the UVA region. The absorption of visible light and UVA radiation by the chromophores leads to the formation of an excited state of photosensitizer (Sen*), which can either undergo type I or type II reactions. The type I reaction consists of electron transport leading to the formation of a superoxide anion radical () via formation of a photosensitizer anion radical () and a substrate cation radical () or vice versa.36,37,38 The dismutation of can further lead to the formation of hydrogen peroxide (), which in turn can form hydroxyl radical () via the Fenton reaction in the presence of transition metals.39–41 The type II reaction proceeds via energy transfer from the excited photosensitizer to molecular oxygen, forming singlet oxygen (). The ROS (, , ) formed as a result of type I and type II reactions are involved in the oxidation of biomolecules including lipids and proteins. The oxidation of biomolecules results in the formation of dioxetane and tetroxide, known to decompose to electronically excited species such as and ,23,42 which emit photons at the wavelength range of 400 to 500 nm and 634 to 703 nm, respectively. 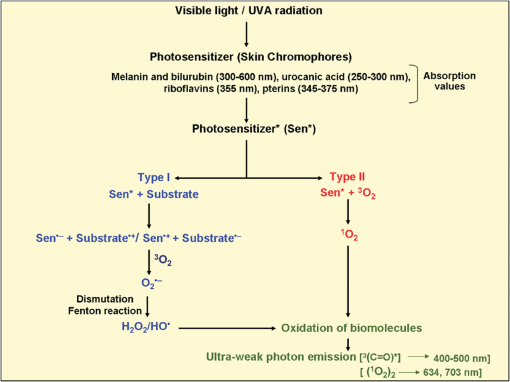 4.1.Visible-Light-Induced Oxidative Stress on the Dorsal Side of the HandThe observation that photon emission from the visible-light-irradiated dorsal side of the hand is higher than from the unexposed dorsal side of the hand reveals that the visible light caused oxidative stress in the human skin [Figs. 2(b), 2(c), 3(a), and 4(a)]. Melanins and bilurubins are known to absorb in the wavelength range of 300 to 600 nm. It has previously been reported that the absorption of visible light by melanins and bilurubins initiates photosensitization, leading to the formation of ROS either by type I or type II reaction.16 Similarly to light-induced enhancement of ultraweak photon emission, it has recently been demonstrated that the topical application of ROS (, , and ) on the dorsal side of the hand resulted in the enhancement in ultraweak photon emission.43 Supporting the role of ROS in ultraweak photon emission, it was demonstrated that various ROS scavengers (ascorbate, glutathione, CoQ10, and -tocopherol) considerably suppressed the ultraweak photon emission.44 The damage to the healthy cells occurs by photosensitization, which proceeds as a consequence of the photodynamic therapy. It has been suggested that the use of blue-light therapy should be done under controlled conditions, thereby preventing photodamage to healthy cells.45 4.2.UVA-Radiation-Induced Oxidative Stress on the Dorsal Side of the HandThe finding that the photon emission observed from the UVA-irradiated dorsal side of the hand is higher compared to the unexposed dorsal side indicates that UVA radiation induced oxidative stress in human skin [Figs. 2(b), 2(d), 3(a), and 5(a)]. Besides melanins and bilurubins, which absorb in a broad range of the spectrum from UV to the visible region (300 to 600 nm), the chromophores such as urocanic acids (250 to 300 nm), porphyrin (protoporphyrins) (320 to 400 nm), flavins (345 to 375 nm), and pterins (345 to 375 nm) absorb particularly in the UV region of the spectrum.18–20 The absorption of UV radiation by these chromophores initiates photosensitization, which leads to the formation of ROS either by type I or type II reaction.36,37 As a consequence, there is a development in human skin of both photoaging, characterized by deep wrinkles, and cancer.24,46 Our observation that UVA-radiation-induced ultraweak photon emission is higher compared to visible-light-induced ultraweak photon emission shows a higher degree of oxidative stress on the dorsal side of the hand. It is proposed here that it is due to the large amount of photosensitizers absorbing in the UV region of the spectrum. Visible light has a higher capability to penetrate to the dermis layer of the skin, whereas UV light penetrates to the epidermal layer of the skin.47 4.3.Comparison of Visible-Light- and UVA-Radiation-Induced Oxidative Stress on the Dorsal and Palmar Sides of the HandThe result that ultraweak photon emission from the palmar side of the hand is higher compared to the dorsal side of the hand upon the visible light radiation reflects the higher oxidative stress on the palmar side [Fig. 5(a) and 5(b)]. It has previously been reported that the palmar side of the hand has low melanin content than the dorsal side,48 whereas the content of other photosensitizers on the dorsal side is more likely to be comparable to the palmar side of the hand. The dorsal side of the hand mainly contains the black and brown pigment, the eumelanin that acts as an antioxidant, whereas the palmar side of the hand contains a low amount of eumelanin and is more prone to oxidative damage. Because the dorsal side contains a high amount of eumelanin, the elimination of ROS formed by type I and type II reactions is highly efficient, and thus the ultraweak photon emission from the dorsal side is comparatively lower than from the palmar side of the hand previously exposed to the visible [Fig. 3(a) and 3(b)] and UV [Fig. 4(a) and 4(b)] radiation. As the palmar side of the hand has low content of eumelanin, the scavenging of ROS formed by type I and type II reactions is less efficient and leads to high ultraweak photon emission from the palmar side compared to the dorsal side of the hand previously exposed to the visible [Fig. 3(a) and 3(b)] and UV [Fig. 4(a) and 4(b)] radiation. In agreement with these considerations, it has previously been claimed that the higher spontaneous ultraweak photon emission from the facial skin might be caused by high melanin content.34 5.ConclusionThe detailed mechanism of ultraweak photon emission has not been clarified completely although it is adequately understood. It is proposed here that ultraweak photon emission can act as a noninvasive method for analyzing the physiological and pathological state of the human skin. The quantitative analysis provides information on the degree of oxidative stress in the human skin, including the viable epidermis and dermis. The application of ultraweak photon emission for monitoring the effect of UVA stress on human skin is highly important for the development of effective photoprotective agents in human skin against UVA radiation. AcknowledgmentsThis work was supported by Grants ED0007/01/01 from the Centre of the Region Haná for Biotechnological and Agricultural Research and CZ.1.07/2.3.00/20.0057 from the Operational Programme Education for Competitiveness from the Ministry of Education Youth and Sports, Czech Republic. We thank Prof. Jan Nauš for measurements with the spectral radiometer and the late Dr. Pavel Krchňák for technical assistance. Thanks also go to Anshu Rastogi, Deepak Kumar Yadav, and Marek Rác for their involvement during the measurements and during the tenure of the study. ReferencesD. R. BickersM. Athar,
“Oxidative stress in the pathogenesis of skin diseases,”
J. Invest. Dermatol., 126
(12), 2565
–2575
(2006). http://dx.doi.org/10.1038/sj.jid.5700340 JIDEAE 0022-202X Google Scholar
F. Laggaret al.,
“Effects of exercises on biophoton emission of the wrist,”
Eur. J. Appl. Physiol., 102
(4), 463
–469
(2008). http://dx.doi.org/10.1007/s00421-007-0607-4 EJAPFN 1439-6319 Google Scholar
E. P. A. Van WijkR. Van WijkS. Bosman,
“Using ultraweak photon emission to determine the effect of oligomeric proanthocyanidins on oxidative stress of human skin,”
J. Photoch. Photobiol. B, 98
(3), 199
–206
(2010). http://dx.doi.org/10.1016/j.jphotobiol.2010.01.003 JPPBEG 1011-1344 Google Scholar
S. OuypornkochagornJ. Feldmann,
“Dermal uptake of arsenic through human skin depends strongly on its speciation,”
Env. Sci. Technol., 44
(10), 3972
–3978
(2010). http://dx.doi.org/10.1021/es903667y ESTHAG 0013-936X Google Scholar
Skin Aging, 144
–146 Springer-Verlag, Heidelberg, Berlin
(2006). Google Scholar
C. TremanD. V. BlakeC. J. Morris,
“Skin inflammation: reactive oxygen species and the role of iron,”
J. Invest. Dermatol., 99
(6), 675
–682
(1992). http://dx.doi.org/10.1111/jid.1992.99.issue-6 JIDEAE 0022-202X Google Scholar
J. J. ThieleM. G. TraberL. Packer,
“Depletion of human stratum corneum vitamin E: an early and sensitive in-vivo marker of UV induced photo-oxidation,”
J. Invest. Dermatol., 110
(5), 756
–761
(1998). http://dx.doi.org/10.1046/j.1523-1747.1998.00169.x JIDEAE 0022-202X Google Scholar
M. E. Darvinet al.,
“The role of carotenoids in human skin,”
Molecules, 16
(12), 10491
–10506
(2011). http://dx.doi.org/10.3390/molecules161210491 MOLEFW 1420-3049 Google Scholar
E. KuamJ. Dahle,
“Pigmented melanocytes are protected against ultraviolet-A-induced membrane damage,”
J. Invest. Dermatol., 121
(3), 564
–569
(2003). http://dx.doi.org/10.1046/j.1523-1747.2003.12418.x JIDEAE 0022-202X Google Scholar
L. Hellemanset al.,
“Antioxidant enzyme activity in human stratum corneum shows seasonal variation with an age-dependent recovery,”
J. Invest. Dermatol., 120
(3), 434
–439
(2003). http://dx.doi.org/10.1046/j.1523-1747.2003.12056.x JIDEAE 0022-202X Google Scholar
A. KellerS. U auf dem Kümin BraunS. Werner,
“Reactive oxygen species and their detoxification in healing skin wounds,”
J. Invest. Dermatol. Symp. Proc., 11
(1), 106
–111
(2006). http://dx.doi.org/10.1038/sj.jidsymp.5650001 JDSPFO 1087-0024 Google Scholar
M. E. Darvinet al.,
“One-year study on the variation of carotenoids antioxidant substances in living human skin: influence of dietary supplementation and stress factors,”
J. Biomed. Opt., 13
(4), 044028
(2008). http://dx.doi.org/10.1117/1.2952076 JBOPFO 1083-3668 Google Scholar
S. TiwariP. C. Mishra,
“Urocanic acid as an efficient hydroxyl radical scavenger: a quantum theoretical study,”
J. Mol. Model., 17
(1), 59
–72
(2011). http://dx.doi.org/10.1007/s00894-010-0699-3 JMMOFK 0948-5023 Google Scholar
S. PillaiC. OresajoJ. Hayward,
“Ultraviolet radiation and skin aging: roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degeneration—a review,”
Int. J. Cosmet. Sci., 27
(1), 17
–34
(2005). http://dx.doi.org/10.1111/ics.2005.27.issue-1 IJCMDW 0142-5463 Google Scholar
M. F. Bennettet al.,
“Skin immune systems and inflammation: protector of the skin or promoter of aging?,”
J. Invest. Dermatol., 13
(1), 15
–19
(2008). http://dx.doi.org/10.1038/jidsymp.2008.3 JIDEAE 0022-202X Google Scholar
G. T. WondrakM. K. JacobsonE. L. Jacobson,
“Endogenous UVA-photosensitizers: mediators of skin photodamage and novel targets for skin photoprotection,”
Photochem. Photobiol. Sci., 5
(2), 215
–237
(2006). http://dx.doi.org/10.1039/b504573h PPSHCB 1474-905X Google Scholar
H. Ou-Yanget al.,
“A chemiluminescence study of UVA-induced oxidative stress in human skin in-vivo,”
J. Invest. Dermatol., 122
(4), 1020
–1029
(2004). http://dx.doi.org/10.1111/jid.2004.122.issue-4 JIDEAE 0022-202X Google Scholar
R. T. Parkeret al.,
“Room temperature phosphorescence of selected pteridines,”
Anal. Chem., 51
(12), 1921
–1926
(1979). http://dx.doi.org/10.1021/ac50048a007 ANCHAM 0003-2700 Google Scholar
K. M. HansonJ. D. Simon,
“Epidermal trans-urocanic acid and the UV-A-induced photoaging of the skin,”
Proc. Natl. Acad. Sci., 95
(18), 10576
–10578
(1998). http://dx.doi.org/10.1073/pnas.95.18.10576 0369-3236 Google Scholar
J. Baieret al.,
“Singlet oxygen generation by UVA light exposure of endogenous photosensitizers,”
Biophys. J., 91
(4), 1452
–1459
(2006). http://dx.doi.org/10.1529/biophysj.106.082388 BIOJAU 0006-3495 Google Scholar
B. HalliwellJ. M. C. Gutteridge, Free Radical Bio Med, Oxford University Press, New York
(2007). Google Scholar
B. Halliwell,
“Free radicals and antioxidants—quo vadis,”
Trends Pharmacol. Sci., 32
(3), 125
–130
(2011). http://dx.doi.org/10.1016/j.tips.2010.12.002 0165-6147 Google Scholar
G. F. Federovaet al.,
“Peroxy-radical-mediated chemiluminescence: mechanistic diversity and fundamentals for antioxidant assay,”
ARKIVOC, 38
(9), 163
–215
(2007). 1424-6376 Google Scholar
E. Kohlet al.,
“Skin ageing,”
J. Eur. Acad. Dermatol. Venereol., 25
(8), 873
–884
(2011). http://dx.doi.org/10.1111/jdv.2011.25.issue-8 JEAVEQ 0926-9959 Google Scholar
C. S. Sanderet al.,
“Photoaging is associated with protein oxidation in human skin in vivo,”
J. Invest. Dermatol., 118
(4), 618
–625
(2002). JIDEAE 0022-202X Google Scholar
Y. FuA. A. Krasnovsky Jr.C. S. Foote,
“Singlet oxygen dimol-sensitized luminescence from thermally generated singlet oxygen,”
J. Am. Chem. Soc., 115
(22), 10282
–10285
(1993). http://dx.doi.org/10.1021/ja00075a050 JACSAT 0002-7863 Google Scholar
Y. Haradaet al.,
“Chemiluminescence from singlet oxygen that was detected at two wavelength and effect of biomolecules on it,”
Talanta, 77
(3), 1223
–1227
(2009). http://dx.doi.org/10.1016/j.talanta.2008.08.025 TLNTA2 0039-9140 Google Scholar
F. Khabiriet al.,
“Non-invasive monitoring of oxidative skin stress by ultraweak photon emisiion (UPE) measurement, I: Mechanism of UPE of biological materials,”
Skin Res. Technol., 14
(1), 103
–111
(2008). 0909-752X Google Scholar
R. H. Friendet al.,
“Electroluminescence in conjugated polymers,”
Nature, 397 121
–128
(1999). http://dx.doi.org/10.1038/16393 NATUAS 0028-0836 Google Scholar
R. Van WijkE. P. A. Van Wijk, Biophotonics-Optical Science and Engineering for the 21st Century, Springer, New York
(2006). Google Scholar
F. Khabiraet al.,
“Photoaging and DNA repair,”
J. Dermatol. Sci., 50
(3), 169
–176
(2008). http://dx.doi.org/10.1016/j.jdermsci.2007.08.011 JDSCEI 0923-1811 Google Scholar
R. Hagenset al.,
“Non-invasive monitoring of oxidative skin stress by ultraweak photon emission measurement, II: biological validation on ultraviolet A-stressed skin,”
Skin Res. Technol., 14
(1), 112
–120
(2008). 0909-752X Google Scholar
H. J. Niggliet al.,
“Laser-ultraviolet-A induced ultra weak photon emission in human skin cells: a biophotonic comparison between keratinocytes and fibroblasts,”
Indian J. Exp. Biol., 46
(5), 358
–363
(2008). IJEBA6 Google Scholar
M. KobayashiD. KikuchiH. Okamura,
“Imaging of ultraweak spontaneous photon emission from human body displaying diurnal rhythm,”
PLoS One, 4
(7), e6256
(2009). http://dx.doi.org/10.1371/journal.pone.0006256 1932-6203 Google Scholar
B. I. KruftA. Greer,
“Photosensitization reactions in vitro and in vivo,”
Photochem. Photobiol., 87 1204
–1213
(2011). http://dx.doi.org/10.1111/php.2011.87.issue-6 PHCBAP 0031-8655 Google Scholar
G. R. Buettner,
“Molecular targets of photosensitization—some biological chemistry of singlet oxygen (),”
http://www.photobiology.info/Buettner.html Google Scholar
J. R. Kanofsky,
“Determining the mechanism for photosensitized oxidations,”
http://www.photobiology.info/Kanofsky.html Google Scholar
J. R. Kanofsky,
“Measurement of singlet oxygen in-vivo: progress and pitfalls,”
Photochem. Photobiol., 87
(1), 14
–17
(2011). http://dx.doi.org/10.1111/php.2010.87.issue-1 PHCBAP 0031-8655 Google Scholar
C. C. Winterbourn,
“Toxicity of iron and hydrogen peroxide: the Fenton reaction,”
Toxicol. Lett., 82 969
–974
(1995). http://dx.doi.org/10.1016/0378-4274(95)03532-X TOLED5 0378-4274 Google Scholar
C. CoudrayS. RachidiA. Favier,
“Effect of zinc on superoxide-dependent hydroxyl radical production,”
Biol. Trace Elem. Res., 38
(3), 273
–287
(1993). http://dx.doi.org/10.1007/BF02785311 BTERDG 0163-4984 Google Scholar
D. R. LloydD. H. Phillips,
“Oxidative DNA damage mediated by copper(II), iron(II) and nickel(II) Fenton reactions: evidence for site specific mechanism in the formation of double-strand breaks, 8-hydroxydeoxyguanosine and putative intrastrand cross-links,”
Mutat. Res., 424
(1–2), 23
–36
(1999). http://dx.doi.org/10.1016/S0027-5107(99)00005-6 MUREAV 0027-5107 Google Scholar
R. T. Deanet al.,
“Biochemistry and pathology of radical-mediated protein oxidation,”
Biochem. J., 324
(Pt. 1), 1
–18
(1997). BIJOAK 0264-6021 Google Scholar
A. PrasadP. Pospíšil,
“Two-dimensional imaging of spontaneous ultraweak photon emisiion from the human skin: role of reactive oxygen species,”
J. Biophotonics, 4
(11–12), 840
–849
(2011). http://dx.doi.org/10.1002/jbio.v4.11/12 JBOIBX 1864-063X Google Scholar
A. RastogiP. Pospíšil,
“Spontaneous ultraweak photon emission imaging of oxidative metabolic processes in human skin: effect of molecular oxygen and antioxidant defense systems,”
J. Biomed. Opt., 16
(9), 096005
(2011). http://dx.doi.org/10.1117/1.3616135 JBOPFO 1083-3668 Google Scholar
E. M. Gasynaet al.,
“Blue light induces apoptosis in human fetal retinal pigment epithelium,”
Invest. Ophtalmol. Vis. Sci., 46 248
(2005). http://dx.doi.org/10.1167/iovs.04-0340 IOVSDA 0146-0404 Google Scholar
S. MoriwakiY. Takahashi,
“Photoaging and DNA repair,”
J. Dermatol. Sci., 50 169
–176
(2008). http://dx.doi.org/10.1016/j.jdermsci.2007.08.011 JDSCEI 0923-1811 Google Scholar
D. J. Goldberg, Laser Dermatology: Pearls and Problems, Blackwell Publishing, Massachusetts
(2008). Google Scholar
S. Alalufet al.,
“Dietary carotenoids contribute to normal human skin color and UV photosensitivity,”
J. Nutr., 132
(3), 399
–403
(2002). JONUAI 0022-3166 Google Scholar
|