|
|
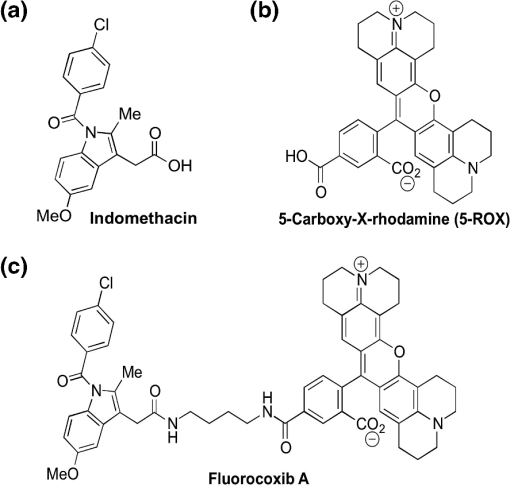
1.IntroductionThere are two isoforms of the cyclooxygenase (COX) enzyme—COX-1 and COX-2—which catalyze the biosynthesis of prostaglandins from arachidonic acid.1,2 In contrast to COX-1, COX-2 is not expressed in most normal tissues, but rather is induced by many growth factors and cytokines in neoplastic and inflamed tissues.3–12 Because it is present at high levels in various cancers,13–16 the COX-2 enzyme is an attractive target for selective detection of tumors with fluorescent and radioactively labeled COX-2 selective inhibitors (COXIBs). Thus syntheses of radioactively labeled COXIBs, as potential imaging agents have been reported.17,18 Promising results were obtained using a derivative of celecoxib as a SPECT radiotracer to identify carcinogen-induced lung lesions expressing COX-2 in hamsters.18 Better resolution and specificity in recognizing COX-2-expressing tumors were achieved with PET and SPECT imaging tracers that were synthesized and evaluated in rodent tumor models.19,20 In addition, novel derivatives of nonsteroidal anti-inflammatory drugs (NSAIDs) fluorescently labeled with 5-carboxy-X-rhodamine dyes, fluorocoxibs have been synthesized and evaluated as optical imaging agents in rodent models of inflammation and cancer.21,22 Fluorocoxib A, N-[(5-carboxy-X-rhodaminyl)but-4-yl]-2-[1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl]acetamide, is a fluorescent indomethacin derivative ( and ) that selectively binds to COX-2.21 Fluorocoxib’s potential for optical in vivo imaging was evaluated using carrageenan-induced acute inflammation in the mouse footpad, COX-2-expressing human tumor xenografts in nude mice and in mice with spontaneous tumors. In each case, the fluorocoxib signal was detected by whole-body imaging using a Xenogen IVIS 200 equipped with a DsRed filter (excitation 500 to 550 nm and emission 575 to 650 nm).21 Human colorectal cancer HCT-116 xenografts that do not express COX-2 emitted a minimal signal, whereas human squamous cell carcinoma (SCC) 1483 xenografts with high levels of COX-2 expression, exhibited bright fluorescence.21 Despite the listing of approximately 1170 contrast agents in the Molecular Imaging and Contrast Agents Database (as of March 2012) for in vitro and in vivo studies, there are only 113 contrast agents, including two optical agents, that are currently approved by the United States Food and Drug Administration for clinical use in human medicine (as of October 2011).23,24 That means that many contrast imaging agents fail in the translation from bench to bedside. Consistently, no selective COX-2-targeted imaging agents have been translated into clinical use to date. Unfortunately, chemically or genetically induced tumor models in rodents cannot accurately simulate the in vivo behavior of naturally occurring heterogeneous human tumors. The artificial subcutaneous location of the majority of xenograft tumors may also influence the uptake of imaging agents.25 Thus to further evaluate this promising new optical imaging fluorophore, fluorocoxib A, we investigated its safety and efficacy in a large animal model—dogs—with naturally occurring tumors. As published before, indomethacin is highly toxic to the canine gastro-intestinal (GI) tract and may result in ulceration, hematemesis, and melena at therapeutic multiple-dose and long-term exposure in dogs.26 Ester and amide derivatives of indomethacin, such as fluorocoxib A, show selective COX-2 inhibition, resulting in substantial anti-inflammatory activity with markedly reduced GI toxicity in rodents.27 It is generally accepted that efficacy of NSAIDs is mainly dependent on COX-2 inhibition, whereas adverse events related to the GI tract (irritation and ulceration of stomach) and inhibition of hemostatic mechanisms are mainly related to COX-1 inhibition.28 Thus there is good reason to expect that the COX-2-selective fluorocoxib A would show significantly reduced toxicity as compared with its parent, indomethacin, in dogs. Since the toxicity of NSAIDs is a major concern, we performed a pilot study in healthy dogs in order to validate the safety of single-dose administration of fluorocoxib A for client-owned dogs diagnosed with tumors. Fluorocoxib A has been shown to have a 30-fold higher binding affinity to COX-2 as compared with indomethacin (Table 1).21,22 We investigated the safety of the single-dose administration of fluorocoxib A in healthy research dogs by physical examination in addition to blood and urine evaluations. Furthermore, we evaluated pharmacokinetic properties of fluorocoxib A in plasma of healthy research dogs by HPLC analysis. To test fluorocoxib A uptake by COX-2-expressing canine cancer in vivo, we performed an endoscopic evaluation after i.v. administration of fluorocoxib A to a dog diagnosed with colorectal carcinomas. Further toxicity studies using long-term multidose administration and determination of the are needed for a complete safety assessment of fluorocoxib A, but this was beyond the scope of the current study. Table 1Indomethacin and fluorocoxib A inhibit COX-1 and COX-2 activities. 2.Material and Methods2.1.Antibodies and Other ReagentsAn antibody for human COX-2 was obtained from Cayman Chemical Corporation (Ann Arbor, MI), and an antibody for cytokeratin was purchased from Dako (Carpinteria, CA). All other chemicals and reagents were purchased the Sigma-Aldrich Chemical Company (Milwaukee, WI) and Fisher Scientific Inc. (Pittsburgh, PA) and used without additional purification. Fluorocoxib A was synthesized according to a previously described method.21,22 Briefly, conjugation of indomethacin with -Boc-1,4-diaminobutane was performed using 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, 1-hydroxybenzotriazole hydrate, and N, N-diisopropylethylamine, followed by treatment with HCl (gas) to give N-(4-aminobutyl)-2-{1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl}acetamide hydrochloride. Succinimidyl esters of 5-carboxy-rhodamines were generated in situ using N, N, N, N-tetramethyl-O-(N-succinimidyl)uronium tetrafluoroborate. The resulting ester was then reacted with N-(4-aminobutyl)-2-{1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl}acetamide hydrochloride in the presence of triethylamine to give the target N-[(5-carboxy-X-rhodaminyl)but-4-yl]-2-[1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl]acetamide (fluorocoxib A). The chemical structures of indomethacin, 5-carboxy-rhodamine, and fluorocoxib A, along with their potencies as COXIBs are shown in Fig. 1 and Table 1. HPLC analysis with ultraviolet detection was performed on a Waters 2695 Separation Module in-line with a Waters 2487 Dual Wavelength Absorbance detector to confirm the identity and purity of the synthesized fluorocoxib A product.26,27 We used a fluorescence detector set at 581 nm in our HPLC system. The free dye and fluorocoxib A are distinguishable by their retention times (2.5 and 5.1 min, respectively) under the HPLC conditions used. We identified the parental fluorocoxib A as the only detectable analyte in all biological samples. In our study, fluorocoxib A was dissolved in dimethyl sulfoxide/ethanol/propylene glycol/saline to a final concentration of for i.v. injection under sterile conditions and filtered using a 0.2-μm pore size filter. 2.2.AnimalsAll animal studies were performed in accordance with University of Tennessee Institutional Animal Care and Use Committee (UTIACUC) approved protocols and in accordance with NIH guidelines. Individually housed male and female beagle dogs (Covance Research Product) with weights of 10 to 20 kg were randomly divided into two treatment groups for single-dose safety assessment (; and fluorocoxib A) and additional dogs () were used to assess pharmacokinetic parameters of fluorocoxib A administrated in doses of and .30–33 2.3.Pilot Safety Study of Single-dose Fluorocoxib A in DogsFor single-dose safety evaluation studies, we administrated two doses of fluorocoxib A, 0.1 and , i.v. over 20 min to normal beagle research dogs. We used three dogs per treatment group, as this is a common number employed in dose-escalation studies for chemotherapy toxicity in this species.33 The medical history of all research dogs was carefully evaluated before enrollment in the study. Physical examinations for signs of potential drug toxicity were performed before, during, and daily for three days after fluorocoxib A administration. Physical assessment included capillary refill time, auscultation of heart and lungs, abdominal palpation, appetite, attitude, activity levels, and other health-related events. Hypersensitivity was evaluated by direct observation of each dog during treatment for clinical signs of an allergic reaction (facial swelling, flushing, urticaria, dyspnea, fever, scratching, and changes of heart rates). Signs of adverse events after fluorocoxib A administration were monitored on a daily basis including vomiting, diarrhea, depression, nausea, and increased salivation. Laboratory evaluations of blood and urine were carried out before and three days after compound administration to monitor signs of fluorocoxib A toxicity. Blood (3 ml) was collected into ethylenediaminetetraacetic acid (EDTA)- and heparin- treated tubes, and urine (up to 5 ml) was collected by cystocentesis or from the floor of the runs. Note that research dogs urinate ad libitum, and urine samples collected from the floor of the runs were used, when necessary, for analysis. Renal toxicity effects, such as casts, presence of urine glucose, and proteins, are not likely to be affected by contact with the floor for a short time period. The laboratory evaluation consisted of a complete blood count (CBC), serum chemistry profile, and complete urinalysis. The complete blood count evaluated total white blood cells (WBC) and hematocrit (HCT) in addition to absolute numbers of neutrophils, lymphocytes, monocytes, eosinophils, and platelets. The plasma chemistry profile evaluated blood urea nitrogen (BUN), creatinine, proteins (albumin, globulins, and total proteins), alanine aminotransferase (ALT), total bilirubin, glucose, and electrolytes. The complete urinalysis evaluated urine proteins, glucose, and ketones as well as a sediment examination and urine specific gravity. All laboratory tests were carried out at the Veterinary Medical Center of the University of Tennessee in Knoxville. Because our study evaluated the safety of a single-dose administration rather than a long-term multidose exposure to fluorocoxib A, euthanasia of the exposed dogs was not a part of the protocol. 2.4.Pharmacokinetics of Fluorocoxib A in DogsTo determine the pharmacokinetic parameters of fluorocoxib A in dogs, we treated six additional dogs with a dose of fluorocoxib A administrated over 20 min using a preplaced jugular catheter. Blood (2 to 3 ml) was collected from the jugular vein into EDTA-treated tubes at 0, 0.5, 1, 2, 4, 8, and 24 h after compound administration. The blood was centrifuged within 20 min of sample collection. Plasma was removed and stored at until HPLC analysis. This protocol was repeated with the same animals, using a dose of fluorocoxib A after a 14-day washout period. This study design was developed to minimize the number of dogs required. 2.5.HPLC Analysis of PlasmaLevels of fluorocoxib A were determined in collected plasma using HPLC. The frozen samples were thawed and extracted with acetonitrile. The organic layer was collected, dried, and reconstituted in methanol and water. The unknown samples were quantitated against a five-point standard curve, which was prepared by mixing known quantities of fluorocoxib A with commercial rat plasma and subjecting the resulting samples to liquid-liquid extraction and HPLC. Samples were analyzed using a reverse-phase column (C18, , Phenomenex) with gradient elution. The mobile phase component A was water, and B was acetonitrile, each containing 0.1% acetic acid. The gradient was 50% B to 90% B over 5 min, followed by a brief hold and return to initial conditions. The flow rate was . 2.6.Pharmacokinetic ParametersThe pharmacokinetic parameters for fluorocoxib A were estimated from the plasma concentration-time data by a nonlinear mixed effects approach, as implemented with Monolix 4.1.2 (Lixoft S.A.S., Orsay, France). This approach allowed analyzing data from all animals at the same time and was ideal for relatively sparse datasets like the one used in this study. The plasma clearance (Cl), representing the overall ability of the body to eliminate fluorocoxib A, was determined by scaling its elimination rate (amount per time) by the corresponding plasma concentration level. The volume of distribution (Vd) was defined as the ratio of the total amount of fluorocoxib A in the body to the blood plasma concentration. The Cl and Vd were estimated directly from the study population data. The peak concentration in plasma () and the following pharmacokinetic parameters were obtained by averaging the corresponding post-hoc Bayesian estimates. The individual partial areas under the curve between times 0 and 8 h () and the with extrapolation to infinity were calculated using the log-linear trapezoidal rule, as implemented with WinNonlin 5.1 (Pharsight, Mountain View, CA). The mean residence time (MRT) value was determined as the ratio of the area under the first moment curve over The elimination rate constant was determined as the slope obtained by linear regression of the terminal log-linear portion of the concentration versus time curve, and the elimination half-life () was then calculated. The plasma concentration graphs were generated using calculated averaged plasma concentrations of fluorocoxib A from six dogs after the i.v. dose of fluorocoxib A 2.7.In Vivo Imaging of a Dog Diagnosed with Colorectal Cancer Using Fluorocoxib A During EndoscopyFor endoscopy and optical imaging, a client-owned dog diagnosed with colorectal carcinoma was enrolled in the study through the Oncology Service of the Veterinary Medical Center. All endoscopic and imaging procedures in the dog were performed with the owner’s signed consent by a board-certified internal medicine specialist (JWB) in accordance with standard veterinary care and the UTIACUC-approved protocol. Fluorocoxib A () was administered i.v. over 20 min through a catheter. Endoscopy was done 24 h later to allow adequate time for fluorocoxib A uptake by colorectal tumor cells expressing COX-2. The dose of fluorocoxib A was based on our safety and pharmacokinetic studies. Endoscopy was used to evaluate the uptake of fluorocoxib A by colorectal cancer lesions and to obtain biopsy samples. The dog was premedicated with acepromazine () and butorphanol (), and general anesthesia was induced with propofol i.v. and maintained with inhaled isoflurane/oxygen gas. Endoscopy was performed using a 2.7-mm, 30-deg, 18-cm rigid cystoscope (Karl Storz Veterinary Endoscopy) attached to a Tricam® Photodynamic Diagnostic (Tricam® PDD), a three-chip camera head using the NTSC color system with an integrated parfocal zoom lens (2×) and two freely programmable camera head buttons (Karl Storz Veterinary Endoscopy America), allowing an easy switch between reduced white light, high-powered white light, and fluorescent modes. The cystoscope with TRICAM PDD was connected to a D-Light AF light 300-watt xenon source (Karl Storz Veterinary Endoscopy America). In this PDD or fluorescence system there were two filters. The light source allowed both white and fluorescence imaging. Excitation light was obtained by switching an excitation filter into the optical path of the light source. This filter allowed only the transmission of light from 380 to 450 nm in the AF mode (blue light). The emission filter was built into an adapter, which was placed between the endoscope and camera to allow transmission of the visible light spectrum, but block the excitation light. Images (still photographs and video) were captured on an Aida DVD-M (Karl Storz Veterinary Endoscopy America) while projecting endoscopic images on a TV monitor. After complete recovery from the endoscopic examination and anesthesia in a quiet room, the dog was returned to its owners. 2.8.ImmunohistochemistryBiopsy samples obtained from the dog diagnosed with colorectal carcinoma were formalin-fixed, paraffin-embedded, and sectioned at 7 μm. After de-paraffinization and rehydration, the sections were heated in 10-mM sodium citrate buffer (pH 6) for 20 min to unmask the COX-2 antigen. Proteinase K antigen retrieval was used for the cytokeratin antibody. Endogenous peroxidase activity was blocked by 3% hydrogen peroxide, and nonspecific binding of secondary antibody was eliminated by incubation with protein block buffer (Biogenex USA, San Ramon, CA) for 30 min at room temperature. Slides were then incubated with primary antibody for COX-2 ( overnight at 4°C) or cytokeratin ( overnight at 4°C) followed by incubation with specific secondary antibodies. The samples were then incubated with a streptavidin/biotin horseradish peroxidase complex (Biogenex), which was visualized by 3,3′-diaminobenzidine staining. Sections were lightly stained with Mayer’s hematoxylin. The tissue specimens were evaluated, and representative images of tissue samples were taken by a DP71 digital camera (HuntOptics and Imaging, Pittsburg, PA) attached to a BX41Olympus microscope (Olympus, Pittsburgh, PA). The CellSens Standard imaging software (HuntOptics and Imaging, Pittsburg, PA) and Adobe Photoshop CS5 (Adobe) were used to analyze images. 3.Results3.1.Safety of Administration of Fluorocoxib A to Research DogsTo assess the safety of a single-dose of fluorocoxib A, we performed a pilot study in a larger animal model: dogs. Six adult research dogs () were administered fluorocoxib A (i.v.) at concentrations of and . A baseline physical examination, CBC (Table 2), plasma chemistry profile (Table 3), and complete urinalysis (Table 4) confirmed the clinically healthy status of the tested dogs. The dogs remained normal on physical examination during and three days after administration of fluorocoxib A. The examination of blood and urine samples did not show clinically relevant abnormal values for the tested parameters three days after fluorocoxib A treatment at either dose. Note that a small number of test values were outside of the normal laboratory reference intervals (indicated in the tables in italics with an asterisk). Two dogs (Dog #5 and #6, fluorocoxib A) had urine proteins prior to and three days after fluorocoxib A treatment as shown in Table 4. One dog (Dog #4, fluorocoxib A) had an increased number of eosinophils before and after treatment as shown in Table 2. One dog (Dog #2, fluorocoxib A) had a borderline low plasma glucose pretreatment of (normal 86 to ) and a post-treatment glucose of as shown in Table 3, but no clinical signs of hypoglycemia. A marginally low pretreatment glucose concentration was seen in one dog (Dog #4, fluorocoxib A). These findings may reflect chronic conditions in these animals, but are not relevant to this acute toxicity study. Table 2CBC analysis of blood before and three days after the single-dose i.v. administration of fluorocoxib A in six research dogs.
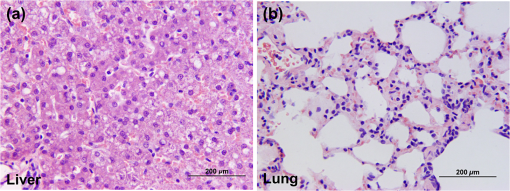
Abbreviations: WBC: white blood cells; HCT: hematocrit; Abs SEG: absolute segmented neutrophils; Abs BAN: absolute band neutrophils; Abs LYM: absolute lymphocytes; Abs MON: absolute monocytes; Abs EOS: absolute eosinophils; PLT EST: platelet estimate; PLT CNT: platelets counts; ADQ: adequate visual examinations of the blood smears identified an adequate number of platelets; Table 3Chemistry panel of plasma before and three days after the single-dose i.v. administration of fluorocoxib A in six research dogs.
Abbreviations: BUN: blood urea nitrogen; CREATI: creatinine; TOT PROT: total proteins; ALBUM: albumin; GLOB: globulin; GLUCO: glucose; CAL: calcium; PHOS: phosphorus; ALT: alanine aminotransferase; SOD: sodium; POTAS: potassium; CHLOR: chlorine; MAG: magnesium; TOT BILI: total bilirubin. Table 4Urinalysis of urine samples before and three days after the single-dose i.v. administration of fluorocoxib A in six research dogs.
Abbreviations: SP. GRAV: urine specific gravity; PH: pH; BLD/Hb: blood/hemoglobin; WBC/HPF: white blood cells per high power field; RBC/HPF: red blood cells per high power field; LPF: low power field; n.a.: not available. Adverse events including diarrhea, vomiting, nausea, or loss of appetite were not observed, although two dogs (one in each treatment group) exhibited increased salivation during the administration of fluorocoxib A over 20 min. After we completed the single-dose safety study, the dogs were transferred to other UTIACUC-approved research and teaching protocols that were not related to our study. The three dogs treated with fluorocoxib A were humanly euthanized after three days, and samples of lung, liver, urine, and blood were collected for biodistribution of fluorocoxib A using HPLC analysis. The HPLC data showed no detectable levels of fluorocoxib A in any of the tissue samples. Microscopic evaluation of lung tissue specimens revealed normal histology (Fig. 2) with the exception of rare small granulomas observed in one section. Some central veins of the liver had acute hemorrhage with dilation of lymphatics, and there was a slight increase in periportal capillaries in the liver from one dog. These changes are common in older dogs and were not considered significant. Overall, the data indicate that single-dose administration of fluorocoxib A at the doses used is safe in dogs. Toxicity of fluorocoxib A after long-term administration needs to be further evaluated, but was not the subject of this pilot study. 3.2.Pharmacokinetic Characteristics of Fluorocoxib A in DogsPharmacokinetic parameters for fluorocoxib A were evaluated following i.v. administration of 0.1 or . We could not generate pharmacokinetic data at the dosage because concentrations in all samples were below the limit of detection by HPLC. The plasma concentrations of fluorocoxib A after i.v. administration of dosage over time are shown at Fig. 3. The calculated pharmacokinetic parameters are shown as in Table 5. These values were used to guide the design of the following study, in which fluorocoxib A was used to endoscopically visualize a COX-2-expressing tumors in vivo. Fig. 3Plasma concentrations of fluorocoxib A after i.v. administration () over 20 min. The data represent the mean plasma concentrations () at specific time points. 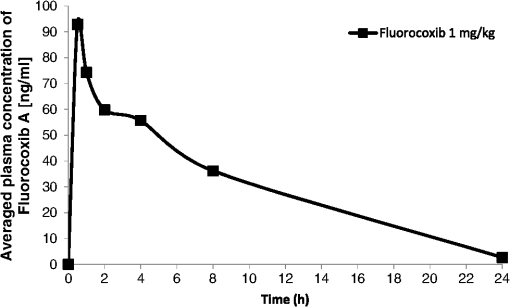 Table 5Pharmacokinetic parameters of fluorocoxib A determined after i.v. 1 mg/kg administration in 6 research dogs.
Note: Mean±S. D.; Abbreviations: #Indicates n=5 dogs were used in the analysis. (Cmax) maximum plasma concentration, (t1/2) terminal half-life, (AUC0–∞) area under the plasma concentration time curve from time 0 to infinity, (AUC0–8) area under the plasma concentration time curve from time 0 to 8 h, (MRT) mean residence time, (Cl) clearance, and (Vd) volume of distribution. 3.3.Detection of COX-2-Expressing Canine Colorectal Carcinoma by Fluorocoxib A During EndoscopyWe evaluated fluorocoxib A in a dog diagnosed with a naturally occurring colorectal carcinoma to determine if this is a suitable preclinical animal model for testing optical imaging agents. The enrolled dog with colorectal carcinoma received fluorocoxib A i.v. over 20 min using a catheter and was imaged after 24 h during endoscopy as described in detail in Sec. 2. In our experiments, we used a Karl Storz imaging system to detect fluorocoxib A in tumors expressing COX-2. As we expected, no uptake of fluorocoxib A was detected after i.v. administration in normal epithelium of the colon or rectum during endoscopic evaluation. Representative images of normal mucosa of canine colon under white light and fluorescence (blue color) acquired during the endoscopy procedure are shown in Fig. 4(a) and 4(b). Specific uptake of fluorocoxib A by COX-2-expressing canine colorectal adenocarcinoma was visualized as a bright pink color, as shown in representative images of canine colorectal adenocarcinoma using white light (left images) and matching fluorescence (right images) acquired during endoscopy [Fig. 4(c) to 4(l)]. Fig. 4Fluorocoxib A in canine colorectal adenocarcinomas during endoscopy. (a) and (b) Representative images of normal mucosa of canine large intestinal mucosa using white (left) and fluorescent (right) light. No uptake of fluorocoxib A in normal epithelium was detected on endoscopic evaluation 24 h after i.v. administration of . (c) to (l) Representative images of canine colorectal adenocarcinomas imaged using bright (left) and fluorescence (right) light during endoscopy. Specific uptake of fluorocoxib A by COX-2-expressing canine colorectal adenocarcinoma is shown by the bright pink color. 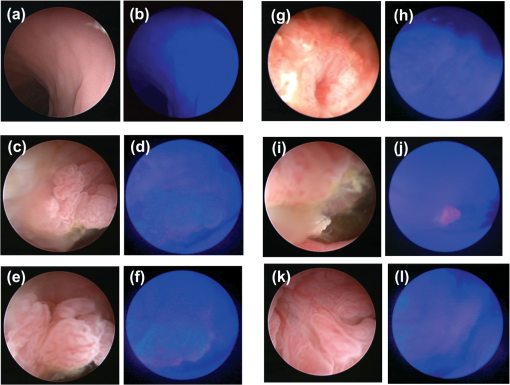 3.4.Confirmed COX-2 Expression in Canine Colorectal Adenocarcinoma Biopsy SamplesTo confirm the specificity of fluorocoxib A uptake by the tumor, we performed immunohistochmical (IHC) staining for COX-2 expression in biopsy samples that were surgically removed by the veterinary surgeon during endoscopy. The two masses identified in the colon and rectum were confirmed as tubulopapillary adenocarcinoma by a veterinary pathologist. As show in Fig. 5(a) and 5(c), IHC analysis revealed diffuse, cytoplasmic expression of COX-2 (brown color, arrows) in the colorectal adenocarcinoma samples. Strong COX-2 expression (brown color, arrows) was also detected in surrounding macrophages in the lamina propria [Fig. 5(c)]. The neoplastic adenocarcinoma cells showed down-regulation of cytokeratin (brown color) expression as compared to surrounding normal epithelial cells [Fig. 5(b) and 5(d)]. Fig. 5Confirmed COX-2 expression in tested colorectal adenocarcinomas. Biopsy samples of canine colorectal adenocarcinomas were surgically removed enodscopically after imaging procedures. The samples were formalin-fixed and paraffin-embedded for histology analysis. (a) and (c) COX-2 (brown color, arrows) and (b) and (d) cytokeratin (brown color) expression in canine colorectal adenocarcinomas by IHC. Magnification objective 20x with scale bar 100 μm. 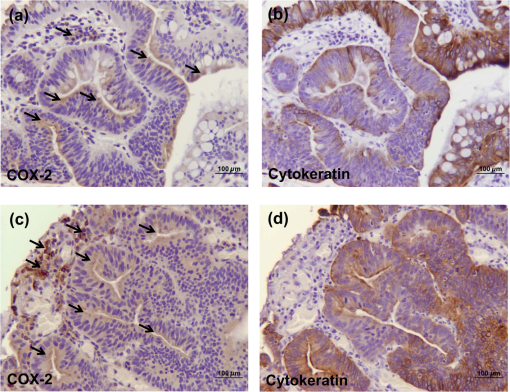 4.DiscussionA large number of prescription and nonprescription NSAIDs has been available for the treatment of musculoskeletal inflammation, pain, and cancer for many years.2,34 In contrast, the potential use of these compounds as imaging agents has only recently come under intense investigation.17–22 As previously published, several COX-2-targeted optical, SPECT, and PET imaging agents have been synthesized and shown to detect COX-2-expression in cultured human tumor cell lines and/or in tumor xenografts in nude mice.18,21 However, none of the current clinically used imaging agents provides information about COX-2 expression in tumor tissue. Thus, to fill this gap, our goal was to evaluate new COX-2-targeted imaging agents in naturally-occurring tumors in a large animal model to promote more effective translation of these agents for human clinical use. Because the use of NSAIDs for imaging purposes does not require chronic exposure, a single i.v. administered dose of fluorocoxib A was evaluated in research dogs to validate the safety of this model. Both tested concentrations of fluorocoxib A, and confirmed safe administration with no clinically relevant adverse events based on physical examination, as well as blood and urine analysis. The doses of fluorocoxib A used in this study were based on prior studies using mouse models and were in the range of other NSAIDs used therapeutically in the veterinary clinic (0.2 to ). The safety of various NSAIDs has been evaluated in several studies, such as a multicenter, prospective, randomized, blinded field trial that was conducted to compare robenacoxib (97 dogs) and meloxicam (43 dogs) for therapeutic applications. Each dog received an initial dose (robenacoxib, ; meloxicam, ) via subcutaneous injection before surgery and this was followed with daily doses (robenacoxib, 1 to ; meloxicam, ) administered orally for up to 15 days after surgery.35 The study revealed that both treatments were associated with only minor adverse events assessed from hematologic and clinical biochemical analyses from blood samples collected at baseline, day 1 and day 12. Even after 12 days of multiple dosages of NSAIDs, the detected adverse events were not necessarily related to the administered treatments.35 In another safety study of meloxicam, the drug was administered i.v. to six beagle dogs at one, three, and five times the recommended dose (0.2, 0.6, and ) for three consecutive days (insert of Metacam® Boehringer Ingelheim Vetmedica, Inc.; St. Joseph, MO). Vomiting occurred in one of six dogs in the 5X group, and fecal occult blood was detected in three out of six dogs in the 5X group. No clinically significant hematologic changes were seen, but serum chemistry changes were observed after administration of meloxicam. Meloxicam (Metacam®, Boehringer Ingelheim Vetmedica, Inc.) used clinically in veterinary medicine has been reported to show acute toxicity () by oral administration in rats at , in mice at , and in rabbits at . The oral of indomethacin in mice and rats (based on a 14 day mortality response) was 50 and , respectively. Rats and dogs apparently tolerate indomethacin less well than does man or monkey.36 To date, the of fluorocoxib A has not been determined in any species. Single-dose i.v. administration of fluorocoxib A did not cause any adverse events within three days including anorexia, vomiting, or diarrhea. The only potentially clinically relevant three-day post-treatment effect of fluorocoxib A was a glucose level of in one dog (normal 86 to ) that otherwise appeared clinically normal. However, the pretreatment glucose was lower than the reference interval in this dog, which was in the group of fluorocoxib A administration. There was a marginally low glucose of 85 (reference range 86 to ) post-treatment in one of three dogs receiving of fluorocoxib A. This finding was not considered clinically relevant. Monitoring of glucose in clinically imaged dogs should be done to determine that low glucose concentrations are not a problem. We observed increased salivation in two dogs, suggesting nausea during administration. Because the salivation appeared in dogs within both groups, this observation indicates that this was not due to fluorocoxib A, but was most likely reaction of the dog to the vehicle containing dimethylsulfoxide, ethanol, and propylene glycol. Once NSAIDs are absorbed, they are highly plasma-protein bound (80% to 97%) and have a relatively long elimination half-life.37–40 Metabolism and elimination of NSAIDs is via the liver followed by renal excretion. The dog converts indomethacin to indomethacin glucuronide, which is excreted in the bile. Terminal elimination half-life after a single-dose of meloxicam is estimated to be approximately 24 h () in dogs regardless of the route of administration. However, there is some evidence of enhanced drug accumulation and terminal elimination half-life prolongation when dogs were dosed for 45 days or longer by meloxicam. Meloxicam bioavailability, volume of distribution, and total systemic clearance remain constant for up to five times of the recommended dose () for use in dogs as provided by the insert of Metacam® Boehringer Ingelheim Vetmedica, Inc. Based on i.v. administration in beagle dogs, the meloxicam Vd is approximately , Cl is , and 97% is bound to canine plasma proteins according to the manufacturer’s instruction for Metacam®. The pharmacokinetics parameters () of of i.v. administered ketorolac (Toradol, Merck&Co., Inc.) were: elimination of 4.55 h, Cl of , and Vd at steady state of .41 Indomethacin (Indocin) bioavailability is 100% when administrated orally, and approximately 99% to 97% of indomethacin is bound to protein in plasma with an approximate of 0.3 to 4.5 h in various species.36 As shown in our study, the elimination of fluorocoxib A was after i.v. administration of in dogs. This indicates higher stability of fluorocoxib A than indomethacin or robenacoxib (1.1 h after oral administration of ).42 Fluorocoxib’s stability is an advantage for use as an optical imaging agent since it provides sufficient time for the compound to reach and specifically bind to COX-2-expressing cancer cells. As compared to meloxicam, indomethacin, or robenacoxib, fluorocoxib A has a large Vd in dogs () that indicates that fluorocoxib A extensively binds to tissue. The tissue distribution of fluorocoxib A three days after i.v. administration of was evaluated by HPLC analysis. No detectable levels of fluorocoxib A were found in any of the tested samples, suggesting that fluorocoxib A was degraded and eliminated from the blood and normal tissue at that time point. Histologic findings of lung and liver tissue samples were consistent with blood and urine analysis with insignificant changes unrelated to the administration of fluorocoxib A. Unfortunately, we were not able to obtain biopsy samples from the three dogs treated with of fluorocoxib A. Companion animals with spontaneous neoplasms are a formidable and underexploited tool for testing and rapidly advancing new compounds and delivery systems that have shown promise in vitro. Spontaneous cancers in companion dogs offer a unique model for human cancer biology and translational cancer therapeutics.43 The relatively high incidence of many cancers, similar biological behavior and response to therapy, comparable responses to cytotoxic agents, shared environment with their owners, and a shorter overall lifespan are contributing factors to the advantages of a companion animal model.43–45 The histologic and biologic characteristics of many cancers in dogs are similar to those in humans.46,47 The average age of the affected dog with spontaneous cancers is 8.4 years, which corresponds to an average age of 50 years for humans, suggesting that, as in humans, spontaneous carcinomas are influenced by age and environmental factors. Colorectal adenocarcinoma is the third most commonly diagnosed cancer in the United States (ACS, Colorectal Cancer Facts and Figures 2011 to 2013) and the third leading cause of cancer death. Early detection using colonoscopy screening prevents colorectal cancer because most of these cancers develop from adenomatous polyps. Furthermore, being screened at the recommended frequency increases the likelihood that, when colorectal cancer is present, it will be detected at an earlier stage when it is more likely to be cured, treatment is less extensive, and recovery is much faster. Detection of malignant or premalignant polyps would be markedly facilitated by a fluorescent signal originating from a dye that specifically targets cancer cells. To this end, as a proof-of principal experiment, we evaluated fluorocoxib A, which specifically targets COX-2-expressing tumors using a dog with naturally occurring colorectal adenocarcinoma. As seen in humans, COX-2-positive tumors in dogs44,47–50 show strong expression of the protein in the perinuclear area of the cells and in macrophages surrounding the tumors. Fluorocoxib A specifically bound to colorectal COX-2-expressing canine tumor cells and allowed better visualization and identification of the canine COX-2-positive colorectal adenocarcinomas as shown in Fig. 4 (pink signal). COX-2 expression was confirmed by IHC analysis from biopsy samples as shown in Fig. 5(a) and 5(c). Several studies, including animal model and epidemiological studies, have provided evidence that inhibition of COX-2 pathways may have significant benefits for cancer treatment and prevention.5,46,48,51–54 On the other hand, NSAID treatment will not be beneficial for patients with COX-2 negative tumors. In fact, such therapy might be even harmful, as shown by a study in which patients with COX-2 negative, nonsmall-cell lung cancer (NSCLC) fared worse when treated with celecoxib as an adjuvant treatment to carboplatin and gemcitabine than patients given chemotherapy alone.55 Thus early endoscopic identification of patients bearing COX-2-positive tumors provides an opportunity to target NSAID- or COXIB-based therapy to those who will most benefit. In conclusion, the study reported here demonstrates for the first time the safety and pharmacokinetic parameters of fluorocoxib A in normal dogs. The specificity of fluorocoxib A was confirmed by its selective uptake in canine colorectal adenocarcinoma in vivo as proof-of-principle. The results indicate that detection of COX-2-positive tumors in intact animals is feasible using fluorocoxib A, providing a starting point for future clinical trials in dogs and ultimate translation to the clinic for use in imaging human tumors. AcknowledgmentsWe thank Dr. Sherry Cox (UTCVM) for her assistance with pharmacokinetic analysis, Dr. Michael F. McEntee (UTCVM) for reviewing the H&E slides and providing a brief histology report, and Dr. Amy L. Holford (UTCVM) for identifying the client-owned dog diagnosed with colorectal carcinoma for our study. We thank Dr. Carol A. Rouzer (VU) for critical revision of the manuscript. This study was supported by The University of Tennessee Center of Excellence in Livestock Diseases and Human Health (COE) Grants R181721223 and R181721257 and research Grants to Vanderbilt University VUIIS (NIH CA128323 and CA136465). AppendicesAppendix A:Collection and Histological Evaluation of Tissues after Fluorocoxib A AdministrationAfter we completed our single-dose safety administration study, the dogs were transferred to other research or teaching UTIACUC approved protocols. Three dogs that were treated with fluorocoxib A with a low dose at were humanely euthanized under an approved UTIACUC protocol. The euthanasia was not related to our study. We were able to obtain small samples of liver, lung, skeletal muscle, blood, and urine for histological and HPLC analysis for tissue distribution of fluorocoxib A. The tissue samples for histological evaluation were formalin-fixed and paraffin-embedded. Seven μm tissues were cut and processed for hematoxylin and eosin staining. The tissue specimens were evaluated by a certified veterinary pathologist, and representative images of tissue samples were taken by a DP71 digital camera (HuntOptics and Imaging, Pittsburg, PA) attached to BX41 Olympus microscope (Olympus, Pittsburgh, PA). The CellSens Standard imaging software (HuntOptics and Imaging, Pittsburg, PA) and Adobe Photoshop CS5 (Adobe) were used to analyze images. Appendix B:HPLC AnalysisThe levels of fluorocoxib A in urine were determined by HPLC analysis. The frozen samples were thawed and extracted with acetonitrile. The sample was dried and reconstituted in methanol and water. The unknown samples were quantitated against a 5-point standard curve, which was constructed by adding known quantities of fluorocoxib A to commercial rat plasma and subjecting the resultant samples to liquid-liquid extraction. Liver, lung, and skeletal muscle samples collected from three dogs treated with of fluorocoxib A, were thawed and homogenized mechanically with phosphate-buffered saline using Kimble Chase Tissue Grind Comp 885450-0023. An aliquot of the homogenization solution was extracted with acetonitrile, dried, and reconstituted in methanol and water. Samples were analyzed using a reverse-phase column (C18, , Phenomenex) with gradient elution. The mobile phase component A was water, and B was acetonitrile, each containing 0.1% acetic acid. The gradient was 50% B to 90% B over 5 min, followed by a brief hold and return to initial conditions. The flow rate was . ReferencesW. L. SmithD. L. DewittR. M. Garavito,
“Cyclooxygenases: structural, cellular, and molecular biology,”
Annu. Rev. Biochem., 69 145
–182
(2000). http://dx.doi.org/10.1146/annurev.biochem.69.1.145 ARBOAW 0066-4154 Google Scholar
L. J. Marnett,
“The COXIB experience: a look in the rearview mirror,”
Annu. Rev. Pharmacol. Toxicol., 49 265
–290
(2009). http://dx.doi.org/10.1146/annurev.pharmtox.011008.145638 ARPTDI 0362-1642 Google Scholar
S. I. Abdallaet al.,
“Gastrin-induced cyclooxygenase-2 expression in Barrett’s carcinogenesis,”
Clin. Cancer Res., 10
(14), 4784
–4792
(2004). http://dx.doi.org/10.1158/1078-0432.CCR-04-0015 CCREF4 1078-0432 Google Scholar
E. G. Cohenet al.,
“Microsomal prostaglandin E synthase-1 is overexpressed in head and neck squamous cell carcinoma,”
Clin. Cancer Res., 9
(9), 3425
–3430
(2003). CCREF4 1078-0432 Google Scholar
A. J. DannenbergK. Subbaramaiah,
“Targeting cyclooxygenase-2 in human neoplasia: rationale and promise,”
Cancer Cell, 4
(6), 431
–436
(2003). http://dx.doi.org/10.1016/S1535-6108(03)00310-6 CCELER 1042-2196 Google Scholar
C. E. Eberhartet al.,
“Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas,”
Gastroenterology, 107
(4), 1183
–1188
(1994). GASTAB 0016-5085 Google Scholar
E. Fosslien,
“Molecular pathology of cyclooxygenase-2 in neoplasia,”
Ann. Clin. Lab. Sci., 30
(1), 3
–21
(2000). ACLSCP 0091-7370 Google Scholar
J. S. GoodwinJ. Ceuppens,
“Regulation of the immune response by prostaglandins,”
J. Clin. Immunol., 3
(4), 295
–315
(1983). http://dx.doi.org/10.1007/BF00915791 JCIMDO 0271-9142 Google Scholar
H. Inoueet al.,
“Transcriptional regulation of human prostaglandin-endoperoxide synthase-2 gene by lipopolysaccharide and phorbol ester in vascular endothelial cells. Involvement of both nuclear factor for interleukin-6 expression site and cAMP response element,”
J. Biol. Chem., 270
(42), 24965
–24971
(1995). http://dx.doi.org/10.1074/jbc.270.42.24818 JBCHA3 0021-9258 Google Scholar
H. Shenget al.,
“Cyclooxygenase-2 induction and transforming growth factor beta growth inhibition in rat intestinal epithelial cells,”
Cell Growth Differ., 8
(4), 463
–470
(1997). CGDIE7 1044-9523 Google Scholar
M. M. Taketo,
“COX-2 and colon cancer,”
Inflamm. Res., 47
(Suppl 2), S112
–116
(1998). http://dx.doi.org/10.1007/s000110050295 INREFB 1023-3830 Google Scholar
M. Tsujiiet al.,
“Cyclooxygenase regulates angiogenesis induced by colon cancer cells,”
Cell, 93
(5), 705
–716
(1998). http://dx.doi.org/10.1016/S0092-8674(00)81433-6 CELLB5 0092-8674 Google Scholar
G. Neufanget al.,
“Abnormal differentiation of epidermis in transgenic mice constitutively expressing cyclooxygenase-2 in skin,”
Proc. Natl. Acad. Sci. U. S. A., 98
(13), 7629
–7634
(2001). http://dx.doi.org/10.1073/pnas.121574098 PNASA6 0027-8424 Google Scholar
C. H. Liuet al.,
“Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice,”
J. Biol. Chem., 276
(21), 18563
–18569
(2001). http://dx.doi.org/10.1074/jbc.M010787200 JBCHA3 0021-9258 Google Scholar
M. Oshimaet al.,
“Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2),”
Cell, 87
(5), 803
–809
(1996). http://dx.doi.org/10.1016/S0092-8674(00)81988-1 CELLB5 0092-8674 Google Scholar
P. C. Chuladaet al.,
“Genetic disruption of Ptgs-1, as well as Ptgs-2, reduces intestinal tumorigenesis in Min mice,”
Cancer Res., 60
(17), 4705
–4708
(2000). CNREA8 0008-5472 Google Scholar
Y. Kugeet al.,
“Synthesis and evaluation of radioiodinated cyclooxygenase-2 inhibitors as potential SPECT tracers for cyclooxygenase-2 expression,”
Nucl. Med. Biol., 33
(1), 21
–27
(2006). http://dx.doi.org/10.1016/j.nucmedbio.2005.10.004 NMBIEO 0969-8051 Google Scholar
H. M. Schulleret al.,
“Detection of overexpressed COX-2 in precancerous lesions of hamster pancreas and lungs by molecular imaging: implications for early diagnosis and prevention,”
Chem. Med. Chem., 1
(6), 603
–610
(2006). http://dx.doi.org/10.1002/(ISSN)1860-7187 CHEMGX 1860-7179 Google Scholar
M. J. Uddinet al.,
“Synthesis and evaluation of [123I]-indomethacin derivatives as COX-2 targeted imaging agents,”
J. Labell. Compds Radiopharmaceut., 52
(9), 387
–393
(2009). http://dx.doi.org/10.1002/jlcr.v52:9 JLCRD4 1099-1344 Google Scholar
M. J. Uddinet al.,
“[I]-Celecoxib analogues as SPECT tracers of cyclooxygenase-2 in inflammation,”
ACS Med. Chem. Lett., 2
(2), 160
–164
(2011). http://dx.doi.org/10.1021/ml100232q AMCLCT 1948-5875 Google Scholar
M. J. Uddinet al.,
“Selective visualization of cyclooxygenase-2 in inflammation and cancer by targeted fluorescent imaging agents,”
Cancer Res., 70
(9), 3618
–3627
(2010). http://dx.doi.org/10.1158/0008-5472.CAN-09-2664 CNREA8 0008-5472 Google Scholar
M. J. UddinL. J. Marnett,
“Synthesis of 5- and 6-carboxy-X-rhodamines,”
Org. Lett., 10
(21), 4799
–4801
(2008). http://dx.doi.org/10.1021/ol801904k ORLEF7 1523-7060 Google Scholar
A. Chopraet al.,
“Molecular Imaging and Contrast Agent Database (MICAD): evolution and progress,”
Mol. Imag. Biol., 14
(1), 4
–13
(2012). http://dx.doi.org/10.1007/s11307-011-0521-3 1536-1632 Google Scholar
K. Leunget al.,
“Essential parameters to consider for the characterization of optical imaging probes,”
Nanomedicine (Lond), 7
(7), 1101
–1107
(2012). http://dx.doi.org/10.2217/nnm.12.79 1743-5889 Google Scholar
J. S. Damianoet al.,
“Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines,”
Blood, 93
(5), 1658
–1667
(1999). BLOOAW 0006-4971 Google Scholar
T. Naritaet al.,
“Nonsteroidal anti-inflammatory drugs induce hypermotilinemia and disturbance of interdigestive migrating contractions in instrumented dogs,”
J. Vet. Pharmacol. Ther., 29
(6), 569
–577
(2006). http://dx.doi.org/10.1111/jvp.2006.29.issue-6 JVPTD9 1365-2885 Google Scholar
A. S. Kalgutkaret al.,
“Ester and amide derivatives of the nonsteroidal antiinflammatory drug, indomethacin, as selective cyclooxygenase-2 inhibitors,”
J. Med. Chem., 43
(15), 2860
–2870
(2000). http://dx.doi.org/10.1021/jm000004e JMCMAR 0022-2623 Google Scholar
R. J. Flower,
“The development of COX2 inhibitors,”
Nat. Rev. Drug Discov., 2
(3), 179
–191
(2003). http://dx.doi.org/10.1038/nrd1034 NRDDAG 1474-1776 Google Scholar
Y. Kugeet al.,
“Synthesis and evaluation of a radioiodinated lumiracoxib derivative for the imaging of cyclooxygenase-2 expression,”
Nucl. Med. Biol., 36
(8), 869
–876
(2009). http://dx.doi.org/10.1016/j.nucmedbio.2009.07.006 NMBIEO 0969-8051 Google Scholar
L. E. Krachet al.,
“Clinical tolerance and toxicity of intravenous baclofen: a pilot study in a canine model,”
J. Pediatr. Rehabil. Med., 4
(2), 89
–98
(2011). 1874-5393 Google Scholar
T. Knudsenet al.,
“Pharmacokinetics, pharmacodynamics and safety of recombinant canine FVIIa in a study dosing one haemophilia A and one haemostatically normal dog,”
Haemophilia, 17
(6), 962
–970
(2011). http://dx.doi.org/10.1111/hae.2011.17.issue-6 1351-8216 Google Scholar
J. W. Bartgeset al.,
“Bioavailability and pharmacokinetics of intravenously and orally administered allopurinol in healthy beagles,”
Am. J. Vet. Res., 58
(5), 504
–510
(1997). AJVRAH 0002-9645 Google Scholar
M. C. Paoloniet al.,
“Rapamycin pharmacokinetic and pharmacodynamic relationships in osteosarcoma: a comparative oncology study in dogs,”
PLoS One, 5
(6), e11013
(2010). http://dx.doi.org/10.1371/journal.pone.0011013 1932-6203 Google Scholar
J. R. Vane,
“Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs,”
Nat. New Biol., 231
(25), 232
–235
(1971). http://dx.doi.org/10.1038/newbio231232a0 Google Scholar
P. GruetW. SeewaldJ. N. King,
“Evaluation of subcutaneous and oral administration of robenacoxib and meloxicam for the treatment of acute pain and inflammation associated with orthopedic surgery in dogs,”
Am. J. Vet. Res., 72
(2), 184
–193
(2011). http://dx.doi.org/10.2460/ajvr.72.2.184 AJVRAH 0002-9645 Google Scholar
F. D. HartP. L. Boardman,
“Indomethacin: a new non-steroid anti-inflammatory agent,”
Br. Med. J., 2
(5363), 965
–970
(1963). http://dx.doi.org/10.1136/bmj.2.5363.965 BMJOAE 0007-1447 Google Scholar
D. R. BrocksF. Jamali,
“Etodolac clinical pharmacokinetics,”
Clin. Pharmacokinet, 26
(4), 259
–274
(1994). http://dx.doi.org/10.2165/00003088-199426040-00003 CPKNDH 0312-5963 Google Scholar
N. M. Davieset al.,
“Clinical pharmacokinetics and pharmacodynamics of celecoxib: a selective cyclo-oxygenase-2 inhibitor,”
Clin. Pharmacokinet, 38
(3), 225
–242
(2000). http://dx.doi.org/10.2165/00003088-200038030-00003 CPKNDH 0312-5963 Google Scholar
N. M. DaviesN. M. Skjodt,
“Choosing the right nonsteroidal anti-inflammatory drug for the right patient: a pharmacokinetic approach,”
Clin. Pharmacokinet, 38
(5), 377
–392
(2000). http://dx.doi.org/10.2165/00003088-200038050-00001 CPKNDH 0312-5963 Google Scholar
J. K. Takemotoet al.,
“Clinical pharmacokinetic and pharmacodynamic profile of etoricoxib,”
Clin. Pharmacokinet, 47
(11), 703
–720
(2008). http://dx.doi.org/10.2165/00003088-200847110-00002 CPKNDH 0312-5963 Google Scholar
K. Pasloskeet al.,
“Pharmacokinetics of ketorolac after intravenous and oral single dose administration in dogs,”
J. Vet. Pharmacol. Ther., 22
(5), 314
–319
(1999). http://dx.doi.org/10.1046/j.1365-2885.1999.00225.x JVPTD9 1365-2885 Google Scholar
J. N. Kinget al.,
“Robenacoxib in the dog: target species safety in relation to extent and duration of inhibition of COX-1 and COX-2,”
J. Vet. Pharmacol. Ther., 34
(3), 298
–311
(2011). http://dx.doi.org/10.1111/jvp.2011.34.issue-3 JVPTD9 1365-2885 Google Scholar
D. W. KnappD. J. Waters,
“Naturally occurring cancer in pet dogs: important models for developing improved cancer therapy for humans,”
Mol. Med. Today, 3
(1), 8
–11
(1997). http://dx.doi.org/10.1016/S1357-4310(96)20031-0 MMTOFK 1357-4310 Google Scholar
E. P. Spugniniet al.,
“COX-2 overexpression in canine tumors: potential therapeutic targets in oncology,”
Histol. Histopathol, 20
(4), 1309
–1312
(2005). HIHIES 0213-3911 Google Scholar
E. G. Macewen,
“Spontaneous tumors in dogs and cats: models for the study of cancer biology and treatment,”
Cancer Metastasis Rev., 9
(2), 125
–136
(1990). http://dx.doi.org/10.1007/BF00046339 CMRED4 0167-7659 Google Scholar
S. I. Mohammedet al.,
“Expression of cyclooxygenase-2 (COX-2) in human invasive transitional cell carcinoma (TCC) of the urinary bladder,”
Cancer Res., 59
(22), 5647
–5650
(1999). CNREA8 0008-5472 Google Scholar
E. M. Pestili De Almeidaet al.,
“Expression of cyclo-oxygenase-2 in naturally occurring squamous cell carcinomas in dogs,”
J. Histochem. Cytochem., 49
(7), 867
–875
(2001). http://dx.doi.org/10.1177/002215540104900707 JHCYAS 0022-1554 Google Scholar
S. I. Mohammedet al.,
“Effects of the cyclooxygenase inhibitor, piroxicam, in combination with chemotherapy on tumor response, apoptosis, and angiogenesis in a canine model of human invasive urinary bladder cancer,”
Mol. Cancer Ther., 2
(2), 183
–188
(2003). Google Scholar
S. I. Mohammedet al.,
“Expression of cyclooxygenase-1 and 2 in naturally-occurring canine cancer,”
Prostaglandins Leukot. Essent. Fatty Acids, 70
(5), 479
–483
(2004). http://dx.doi.org/10.1016/j.plefa.2003.10.002 PLEAEU 0952-3278 Google Scholar
J. Y. Leeet al.,
“Expression of cyclooxygenase-2, P-glycoprotein and multi-drug resistance-associated protein in canine transitional cell carcinoma,”
Res. Vet. Sci., 83
(2), 210
–216
(2007). http://dx.doi.org/10.1016/j.rvsc.2006.12.012 RVTSA9 0034-5288 Google Scholar
A. J. Dannenberget al.,
“Cyclooxygenase-2 and epidermal growth factor receptor: pharmacologic targets for chemoprevention,”
J. Clin. Oncol., 23
(2), 254
–266
(2005). http://dx.doi.org/10.1200/JCO.2005.09.112 JCONDN 0732-183X Google Scholar
D. W. Knappet al.,
“Phase I trial of piroxicam in 62 dogs bearing naturally occurring tumors,”
Cancer Chemother. Pharmacol., 29
(3), 214
–218
(1992). http://dx.doi.org/10.1007/BF00686255 CCPHDZ 0344-5704 Google Scholar
D. W. Knappet al.,
“Piroxicam therapy in 34 dogs with transitional cell carcinoma of the urinary bladder,”
J. Vet. Intern. Med., 8
(4), 273
–278
(1994). JVIMEM Google Scholar
B. R. Schmidtet al.,
“Evaluation of piroxicam for the treatment of oral squamous cell carcinoma in dogs,”
J. Am. Vet. Med. Assoc., 218
(11), 1783
–1786
(2001). http://dx.doi.org/10.2460/javma.2001.218.issue-11 JAVMA4 0003-1488 Google Scholar
M. J. Edelmanet al.,
“Eicosanoid modulation in advanced lung cancer: cyclooxygenase-2 expression is a positive predictive factor for celecoxib + chemotherapy–Cancer and Leukemia Group B Trial 30203,”
J. Clin. Oncol., 26
(6), 848
–855
(2008). http://dx.doi.org/10.1200/JCO.2007.13.8081 JCONDN 0732-183X Google Scholar
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||