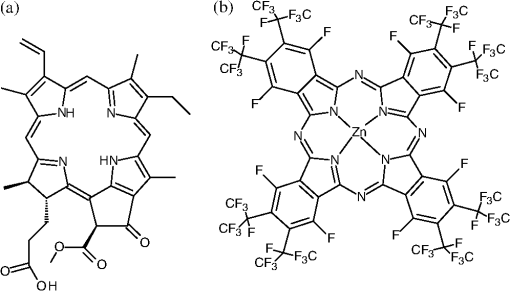
|
|
1.IntroductionThe majority of living organisms on Earth utilize oxygen for respiration and energy conversion. Molecular oxygen in its lowest excited electronic state, singlet oxygen [], is a highly reactive species generated spontaneously in vivo upon exposure of endogenous photosensitizers (PS), such as flavins, to ultraviolet to visible radiation.1–3 is involved in many chemical and biological processes such as signal transduction for the expression of several proteins, gene regulation via the transcription factor activator protein-2 as well as activation of protein kinases.4–6 Moreover, is identified as one of the main factors contributing to skin aging and skin cancer.7,8 Photodynamic therapy (PDT) exploits the photosensitized generation of . The administration of a PS to a patient followed by localized light (red or near infrared) exposure, for example of malignant tissue, results in damage to the cancer cells. This therapy is quite selective since the PS and the low energy light are not toxic by themselves. They generate the toxic effect only if present simultaneously and advantageously at the selected location of the targeted tissue. Neighboring, healthy tissue that is not illuminated is not damaged.9–11 The distribution of PSs in biological environments and their ability to generate are features critically important for PDT applications. The electronic and structural parameters that determine distribution properties can be determined via classical chemical and physical methods, while the methodologies of detection in vitro and in vivo are subjects to continuous improvements.12 The evaluation of the efficiency of novel PSs could benefit greatly from in vivo time-resolved detection and, when applicable, in situ will greatly improve PDT treatments. Singlet oxygen detection, even in vitro, often relies on indirect methods. For example, the time-dependent concentration variation of quenchers such as uric acid or dichlorofluorescein diacetate.7,13 The reliability of indirect methods, however, is poor in inhomogeneous systems such as biological samples. Specifically, the diffusion in cells is limited by its lifetime to less than 0.3 μm, a distance which is at most as large as the dimension of some cell organelles.14–17 Consequently, the accumulation loci of the quencher, presumably present throughout organelles, may exceed the diffusion area which leaves a fraction of the quencher out of reach. Since the quencher and PS may have different distribution patterns, no reliable information can be gained from indirect measurements. Thus, using such reporter substances in skin in vivo is difficult or impossible.1 Singlet oxygen measurements in vivo are even more difficult. Indirect methods, such as ESR detection of nitroxyl spin probesare common.18 Fluorescence microscopy using a fluorescent probe, for example singlet oxygen sensor green, has been applied.19 Even though this would be a convenient method, results obtained have limited value due to a variety of side effects that may occur, especially in mammalian cells.20 Furthermore, a major limitation of indirect methods is the inability to resolve kinetics. Thus, little information about the environment where the is generated can be gained due to the fact that only the total amount of that has reacted with the probe is measured. A similar drawback affects direct measurement techniques that track only the total amount of without time resolution.11,21 Imaging techniques, with a resolution on the order of millimeters, have been applied in vivo using scanning techniques or a camera.22,23 Using these methods, approaches have also tracked only the total amount of luminescence intensity is observed without time resolution. The direct approach of time-resolved detection is usually based on detection of the characteristic luminescence at around 1270 nm.24 Despite the low quantum yield and consequent low-intensity, luminescence-based measurements have been pursued intensely. Recently, the detection of luminescence kinetics, from cells in suspension as well as from single cells under a microscope, has become possible.11,16,17,21,25 Using an innovative technological approach, Lee et al. measured the decay time-resolved after excitation of a PS with a microsecond light pulse. This way, short rise times of the luminescence signal cannot be usually resolved, as illustrated in Sec. 2.1.26 In some heterogeneous systems, such as biological cells, this short luminescence rise time is determined by the decay time.27 Using laser excitation in the nanosecond regime, direct, time-resolved measurements of in vivo on rats, photosensitized by systemic drug administration, have been reported, yet the low signal-to-noise ratio limited the ability to determine the kinetics accurately.11 In the best case thus far, the direct, time-resolved measurements of in skin generated by UVA laser excitation at 355 nm irradiation of endogenous PSs, yielded a weak and complex signal where semi-quantified decay time components were attributed. These time components include a time below 2 μs, a decay of 8 μs fitted to the luminescence signal in the time region from 2 to 16 μs, and a long lived decay time greater than 16 μs.25 Time-resolved luminescence measurements in vivo are still very difficult and require refinement to provide a diagnostic tool for use in clinical protocols or research.1 Recently, we reported an experimental set-up with very high sensitivity that allows the use of low illumination doses. This facilitated the discovery that, in the case of cells in suspension, the and PS triplet decay times are a function of light exposure.28,29 Additionally, the SNR of the signals of , generated in membranous systems, is sufficiently high to allow distinguishing homogenous environments from heterogeneous, membranous systems by evaluating the kinetics of luminescence.27 Here, we report the comparative investigation of two PSs applied to pig ear skin. Pig ear skin ex vivo is considered a good model for human skin in various aspects.30 The time-resolved luminescence measurements provide insight into the interactions of the PS with its environment. In conjunction with additional spectroscopic measurements, we show that significant differences in the accumulation of the two PSs can consistently be observed in the luminescence kinetics. This methodology also allows tracking the oxidation of chemical quenchers and saturation. 2.Materials and Methods2.1.PhotosensitizersPheophorbide-a (Pheo), Fig. 1(a), a dye initially extracted from leaves of Urtica urens, was obtained from spirulina Arthrospira platensis.31 1,4,8,11,15,18,22,25-octakis-fluoro-2,3,9,10,16,17,23,24-octakis-perfluoro(isopropyl)phthalocyanine zinc (), Fig. 1(b), was prepared using a microwave assisted procedure.32 For the PS in an oxygen containing environment, the fluorescence intensity is correlated to the quantum yield.33 Therefore, in the investigated samples, the localization pattern determined with fluorescence microscopy equals that of generation. 2.2.Sample Preparation and HandlingPhysiogel® hypoallergen vanishing crème was obtained from Stiefel Laboratorium GmbH, Germany. Pheo was suspended in Physiogel® (0.1% by weight) to yield a molar concentration of . For the : crème composition, a ratio of was used to yield the same molar concentration. Thin layers of the crème mixtures were placed on glass slides for experiments involving only the PS in the crème. The crème itself exhibited no luminescence in the spectral region of phosphorescence. Pig ear skin samples, from animals sacrificed the previous day, were obtained from the Dermatological Clinic, Charité Hospital, Berlin, Germany. The ears were washed and the hair was removed using a razorblade or scalpel. Samples from the ear were cut out. On these skin samples, the crème was applied, topically, without exertion of any force at an amount of approximately 20 to . Residual (not absorbed) crème was removed from the skin surface after 60 min by washing the sample with water. Lipids were extracted by separating a peripheral epidermal layer from the skin, drying them, and then extracting them with petroleum ether.34 No further action was taken to ensure isolation of only the stratum corneum. Fatty tissue from lower layers was also washed with petroleum ether to extract the lipids. The solvent was removed and the lipid residues were re-suspended in solutions of the PS in dichloromethane (DCM). Following homogenization, the mixture was evaporated on glass slides to yield lipid films which are used for spectroscopic measurements. The area of the lipid film was chosen to be equal to the skin area used for lipid extraction. The amount of PS in the lipid film was chosen a factor of 20 less that the amount applied in crème to the same area. Due to inhomogeneity of the lipid film and uncertainties of the crème application, these values have estimated errors in the order of a factor of 2 to 4. The residue left after washing stratum corneum layers was ground into a mortar along with a solution of PS in DCM. The keratinous residue, obtained after the evaporation of DCM, was placed on adhesive tape in order to fix its position. The tape was shown not to interfere with measurements. The epidermis of the samples, used for the biopsies in Fig. 2, was impaired by stripping once with adhesive tape with cyanoacrylate before application of the PS. The procedure was similar to common tape stripping used in dermatology, described in Ref. 35 All samples were handled in almost total darkness by using only low intensity light of wavelengths not absorbed by the PS. Fig. 2Fluorescence microscopy image of skin samples prepared with the photosensitizer-crème according to Sec. 2. The Photosensitizer is (a) Pheo and (b) . Pheo shows fluorescence only in the stratum corneum while is also fluorescing in deeper layers of the epidermis. 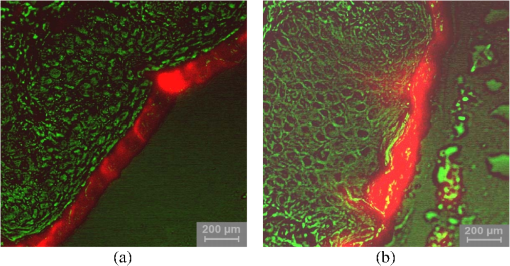 2.3.Singlet Oxygen Kinetics MethodologyThe time-dependency, , of the amount of PS-generated in a homogeneous environment is simplified described by Eq. (1): Considering a microsecond time scale, the PS molecules can be assumed to reach the triplet state instantaneously following a nanosecond laser pulse excitation. The fitting constant C accounts for the number of initially excited PS molecules, while and represent the and PS triplet state decay times, respectively. The sign of the preexponential factor can change depending on the relative values of the two decay times. A signal rise is determined mainly by the faster process the decay by the slower one.25 Hence, the firm assignment of PS triplet and decay times to the fitting parameters of the luminescence signal requires additional experiments.12 The PS triplet lifetime can be determined directly by flash photolysis or indirectly by the addition of quenchers in variable concentrations followed by the monitoring of the changes in decay time.36 Considering the high quantum yields, the PS triplet decay time is mainly determined by the efficiency of the energy transfer to . Thus, reducing the concentration leads to an increased PS triplet decay time while the decay time remains constant allowing a quantitative differentiation between the two processes and assigning of and to the fitted parameters.37It must be noted, that Eq. (1) is applicable only for homogenous environments. In a first approximation, this model can be extended to heterogeneous systems comprising a number of homogenous environments, therefore, neglecting the cross-diffusion between them. A sum of two (or more) distinct homogenous environment, each described by Eq. (1), is then fitted to the data.24 The consideration of such multiple environments results in the determination of more accurate fit parameters if the geometry of the system is known.27,38 2.4.Singlet Oxygen Luminescence Detection: Time Correlated Multiphoton CountingThe excitation source comprised a frequency-doubled diode pumped laser and a customized dye laser. The light intensity, adjustable up to 20 mW average power, was maintained at 5 mW, measured with a LabMax/J 10MT- 10 kHz, (Coherent). The repetition rate of 12.2 kHz resulted in approx. 0.4 μJ pulse energy. Due to the shape of the laser beam, the intensity at the sample is approx. . The acquisition time for a single measurement was 20 s, thus, one measurement delivers approximately to the sample. The excitation wavelength was chosen to match the absorption maximum of the lowest energy absorption band of the PS. For Pheo, excitation was done at 666 nm, for at 683 nm. Luminescence was detected with a photomultiplier H-10330-45 (HAMAMATSU Germany) that sensitive from 950 to 1400 nm with quantum efficiency of 2%. An lens transfers collimated light onto the effective area of the anode (1.6 mm diameter). The rise-time of 900 ps and the transit time spread of 300 ps may be neglected in the luminescence decay time domain. The high sensitivity of the setup not only allows the detection of , but also the determination of rise and decay times with an accuracy and reproducibility of 0.1 μs in many biological samples. If larger error margins are given for absolute values, these are due to sample variance. The evolution of the luminescence signal function of on-going illumination was performed via repeated measurement cycles at constant time intervals, as reported previously.27–29 Since the luminescence emission is centred at 1270 nm, an interference filter, with a central wavelength at 1270 nm, was used to discriminate other emission. The fact that is the source of the luminescence signals reported here was verified by spectral analysis of the signal. For this investigation, the luminescence was recorded using interference filters with central wavelengths at 1200, 1250, and 1300 nm. The intensities of these signals were compared to the signal obtained with the interference filter centered at 1270. A clear intensity maximum at 1270 nm is observed with no signal contribution at 1200 nm, verifying as source of the luminescence (data not shown). 2.5.Flash Photolysis MeasurementsAn pumped OPO (Ekspla, 420 to 2500 nm) laser was used for excitation. Excitation energy was 2 mJ in a 10 ns pulse at a repetition rate of 10 Hz. A stabilized light emitting diode (LED) was used as probe light source. Time dependent, transient triplet–triplet absorption spectra were observed perpendicular to the excitation with a band pass interference filter selected the transient absorption wavelength. The transmitted light was detected using a Si-photodiode with an integrated fast preamplifier (Elektronik Manufaktur Mahlsdorf, EMM, Berlin) using a recording oscilloscope (HP5415). The setup allows the determination of PS triplet decay times as short as 0.1 μs. 3.Results and DiscussionPhotosensitized generation of in skin poses a highly complex system. To explain the luminescence kinetics of this system in the following, initially, the interaction of the PSs and the photosensitized generated with the carrier crème is elucidated. Secondly, measurement results of skin samples and, finally, measurement results of the components extracted from the skin are discussed. 3.1.Distribution of Photosensitizer Fluorescence in CrèmeThe suspension of the two PS’s in crème was monitored via fluorescence microscopy. Figure 3 reveals that there are significant differences in solubility. Pheo exhibits homogenous fluorescence at room temperature, suggesting a homogeneous distribution, even though the light scattering image clearly shows a structure. The is strongly partitioned in a pattern, which will be referred to as droplets. Incubation of 1 h at 37°C results in the Pheo migration within small droplets. No effect on the distribution of was observed, the size of the droplets was larger at the higher temperature. The high hydrophobicity of explains the exclusive accumulation in the droplets, pointing to the statement: The crème consists of lipid droplets suspended in a water phase. Fig. 3Fluorescence microscopy images of emulsified photosensitizers. The images are an overlay of the scattered light (black and white) and the PS fluorescence (red). The displayed areas have the approximate dimensions . (a) Pheophorbide-a at room temperature; (b) at room temperature; (c) pheophorbide-a after incubation at 37°C; and (d) after incubation at 37°C.  3.2.Singlet Oxygen Generation in CrèmeBoth PS-crème compositions exhibit strong photosensitizing activity as judged by the observation of intense luminescence signals, as illustrated in Fig. 4. In contrast to the fluorescence microscopy indicating a homogenous distribution of Pheo and an inhomogeneous distribution of in the crème at room temperature, the luminescence signal for the Pheo-crème sample could not be fit satisfactory with the model for homogenous environments [Fig. 4(a)], however, in the case of -crème, the simplified Eq. (1) for homogenous environments is suitable [Fig. 4(b)]. Fig. 4Luminescence decay of singlet oxygen photosensitized generated by (a) Pheo and (b) in vanishing crème. Black lines are the fitting curves. (a) Dark Blue dots: measurement at 25°C; Light Red squares: measurement at 37°C. Fitted parameters: (a) 25°C: , : , : ; 37°C: , : . (b) , : . Insets: Flash photolysis measurements of triplet decay times (a) (25°C) (b) . See the text for details. 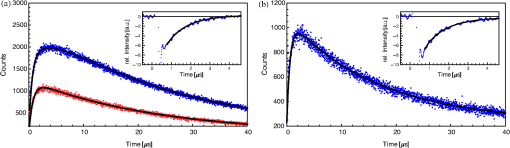 To fit the luminescence signal of the Pheo-crème at room temperature, we would rather use a model that assumes the distribution of in two different environments within the crème. Fitting the data with this model yields two sets of decay times, namely and in one environment and in the other. A decay time of 3.5 μs is consistent with literature values for the decay time in water.24 This observation is consistent with the statement that the crème is an emulsion in which lipid droplets are surrounded by a water phase. However, this explanation needs to be treated with caution since Pheo aggregates in pure water and neither fluoresces nor generates .39 Thus, one cannot assume that Pheo is generating in a pure water phase. Rather, we propose Pheo might be present in a lipoid system, which is suspended in the water phase and, thus, Pheo is still able to generate single oxygen. The term lipoid, in contrast to lipid, will be used here to distinguish lipids organized in membranous systems such as liposomes or micelles from lipids that do not form these structures and, thus, cannot be suspended in water. The flash photolysis measurements support this hypothesis. A single PS triplet decay time can be observed, thus, no generation in radically different environments is expected. In consideration of the amphiphilic properties of Pheo, the spontaneous formation of lipoids, which are suspended in the water phase, is likely. It is known, for example, that Pheo can easily be incorporated into lipoid structures such as liposomes or micelles where the preparation method is based on self-organized formation of lipoids.40 The decay parameter values in Ref. 28 must be placed in the context of the temperature dependence of the Pheo-in-crème system. The luminescence signals of Pheo-crème at 37°C can be fit with the standard double-exponential model, yielding a single decay time of and a PS triplet time of which indicates a homogenous environment of generation and decay. Considering the size of the droplets visible with fluorescence microscopy [Fig. 4(c) and 4(d)] and the diffusion length of , this interpretation is consistent with the experimental data. If is generated only in the lipid droplet and then diffuses out into a water phase, this would affect the kinetics in the investigated crème only in a negligible way. In a completely lipid environment, the diffusion length of would be in the order of 100 nm.41 The average droplet size of 5 μm is large compared to 100 nm diffusion length of . Taking 100 nm as upper limit of the diffusion length for the water phase and a sphere with a diameter of 5 μm as the droplet size, a maximum of the generated within a droplet can leave the droplet and be quenched outside. Thus, the observed formation of droplets should have a negligible influence on the kinetics. The kinetics shown in Fig. 4(a) (red) and 4(b) deviate negligibility from the kinetics of a homogenous environment. This does not justify the use of more complex fitting models. The influence of diffusion on luminescence kinetics have been examined in detail in Ref. 27 for cell suspensions. However, we refrain from further investigating the crème system here as it is only used as a carrier for the PS and not further related to the discussion of the luminescence kinetics of the skin samples. The decay parameters of luminescence were assigned to the and PS triplet decay times with the help of flash photolysis measurements. The values for the PS triplet decay time are for Pheo and for (Fig. 4, insets). The assignments of for Pheo and are in accordance with the notion that the shorter time reflects the PS triplet decay. The flash photolysis measurement yields longer PS triplet decay times here, compared to those obtained via luminescence measurement. This may reflect the fact that the pulse energy for a flash photolysis measurement is much higher than for the measurement. Singlet oxygen measurements are done using a laser with a repetition rate of 12.2 kHz and 0.4 μJ energy per pulse. The flash photolysis uses a laser with 10 Hz repetition rate and 10 ns pulse length with energy of 1 mJ. The comparably higher pulse energy may lead to local oxygen depletion which results in a longer PS triplet decay time. It is interesting to note that, unlike the case of Pheo, protons attached to the nitrogen atoms of the inner ring of the scaffold exhibit a high acidity. This effect also enhances the Lewis acidity of coordinated metal centers, including the of , which was shown to coordinate ligands exhibiting electron lone pairs such as oxygen of acetone, methanol and even water.42 Considering the strong hydrophobic nature of the fluoro groups of the , it is not surprising that it is easily soluble in organic solvents including polar alcohols and acetone. The high hydrophobicity causes a binding constant to liposomes (formed by amphiphilic lipoids) smaller than .43 The results shown in Fig. 3, however, indicate favorable solubility in lipids, probably neutral fats or fatty acids not forming a highly structured distribution such as membrane-like structures. This result can be understood considering the overall hydrophobicity of scaffold and the neutral nature of lipids. In contrast, membranes are more amphiphilic in nature due to their lipoid components. From a PS materials point of view, the above mentioned properties of suggest that other scaffolds bearing perfluoroalkyl groups might also exhibit favorable PDT properties in case no accumulation in cells is needed. 3.3.Distribution of Photosensitizer Fluorescence in SkinThe localization of PSs within skin samples was investigated using fluorescence microscopy, as shown in Fig. 2. Pheo is localized exclusively in the stratum corneum, as shown in Fig. 4. Pheo was applied after the stratum corneum barrier was disrupted by tape stripping, nevertheless, no fluorescence in deeper epidermal layers is observed. In contrast, fluoresces not only in the stratum corneum, but also in the deeper layers of the epidermis. The ultrastructure of the stratum corneum cannot be resolved using fluorescence microscopy. In order to investigate a possible influence of temperature upon partition effects, pig ears were stored after application of crème both at 37°C and at room temperature. Differences in the localization within the epidermis, depending on the temperature, have not been observed in the skin samples. 3.4.Singlet Oxygen Generation in Ex Vivo Pig SkinThe time-dependent luminescence signals of generated by the two PSs are shown in Fig. 5. Similar to the measurements for cells in vitro, reported previously,27 a change of the luminescence kinetics with illumination is observed here. The blue and red curves in Fig. 5 show the initial data and a postillumination measurement, respectively. Fig. 5Luminescence decay of singlet oxygen photosensitized generated by (a) Pheo and (b) in pig skin. Dark Blue dots: initial measurement (), Light Red squares: measurement after (a) resp. (b) . Black lines are the fitting curves. Fitted parameters (a) (): , , (): , , (b) (): , , (): , . 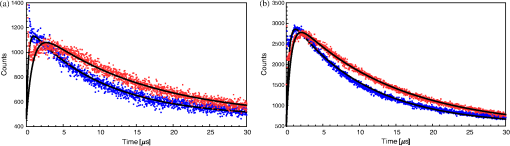 Specifically, in the Pheo PS case shown in Fig. 5(a), the PS triplet decay time of the first measurement is and increases after an illumination of the sample with to , while the decay time increases from to . Figure 5(b) shows that is characterized by a similar behavior, with slightly different parameters, suggesting a slower evolution of decay times with illumination. The decay times of the sample increase from and to and after delivery of . This discrepancy could be attributed to the distributions and accumulation of the two PSs in different microenvironments. The amplitude difference of a factor of about 2 to 3 is insufficient for deriving further conclusions about the efficiency of generation as this parameter is subject to a high, sample-dependent variance. The stated errors for the decay time parameters include sample variability and exhibit a lower relative variance. The initial decay times for fresh samples of Pheo, 12.5 μs and , 10.2 μs, are about half of those observed in the vanishing crème alone (26 μs for Pheo and 17 μs for ). The significant reduction indicates a strong interaction between the PS and the stratum corneum. The applicability of the fitting procedure, for homogenous environments, supports the notion that no residual crème remained on the skin during the measurements as the crème’s high luminescence signal would show up as a second component of the luminescence signal with different parameters. Small deviations between the fit and the data remain, especially for the sample. This will be discussed below. Yet, the deviations are too small to justify the introduction of a different theoretical model at this moment. In addition the scattering signal, specific for luminescence measurements, cannot be theoretically modeled and is overlapping the luminescence shortly after the laser pulse.12 The systematic change in the luminescence signal, as function of the illumination, has been tracked up to a total dose of , as illustrated in Fig. 6. For both PSs, the triplet decay times initially rise fast and then remain fairly constant after illumination with . The variation of the PS triplet decay time is attributed to initial oxygen retention within the sample that yields a very short PS triplet decay time at the beginning of the measurement followed by the consumption of oxygen within the skin by chemical reactions. A lower oxygen concentration is reducing the efficiency of the energy transfer from the PS triplet state to oxygen, a process that is proportional to the oxygen concentration. Consequently, the PS triplet decay time increases and equilibrium is reached when the rate of oxygen diffusion from the environment equals the rate of its local consumption. Fig. 6Variation of and Pheo triplet decay times in pig ear skin as a function of light doses (the scale is proportional to a time scale of 0 to 7 min). The Photosensitizer is (a) Pheo and (b) . Fitted parameters: (a) initial measurement (): , ; final measurement (): , . (b) initial measurement (): , ; final measurement (): , . 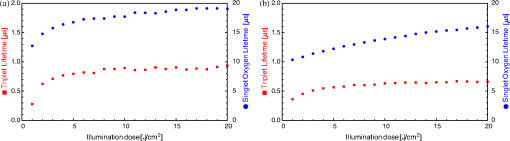 A significant difference between Pheo and is observed not only in the kinetics of a single measurement, but also in the change of the decay time with illumination. While, in the Pheo case, the decay time initially rises fast, the decay time of the sample shows a smoother increase with illumination. In biological system, the increasing decay time can be associated with a consumption of chemical quenchers.27 As is generated, it reacts with chemical quenchers such as endogenous antioxidants, proteins, unsaturated lipids, or other oxidative targets occurring in the skin. Since the chemical reaction rate should be proportional to the number of free reaction sites, one would expect their exponential decrease. Practically all major components of the skin such as keratins, proteins, lipids and several antioxidants are known to react with oxygen.44,45 A preliminary elucidation of the localization within the ultrastructure of the stratum corneum follows at the end of this section. Control experiments were performed by saturating samples with in order to verify the signal origin and parameter assignments of the observed luminescence, as shown in Fig. 7 as in Ref. 28. Purging oxygen by pure followed by the admission of normal air into the sample results in the recovery of the 1270 nm luminescence signal, which was not observed under . This is the expected behavior if external oxygen is the sole source of the luminescence emission. Fig. 7Change of singlet oxygen and photosensitizer triplet decay time of a pig-ear skin sample prepared with photosensitizer in crème. was purged by flushing the sample chamber with . At the beginning of the experiment air was allows to re-enter the sample. The singlet oxygen time remains fairly constant, except for a slight increase, the photosensitizer triplet decay time decreases, as expected. See the text for details. 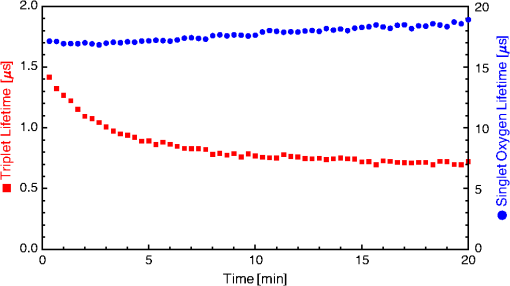 The assignments of rise and decay time of the luminescence signal for the skin samples are further verified with this method. Thus, upon allowing normal air to gradually contact the sample following its initial removal with , the signal recovery exhibits a significant decrease in the rise time as the oxygen level increases, while the longer time remains fairly constant. The increase in oxygen level increases the efficiency of the energy transfer from the PS triplet state to oxygen which results in a lower lifetime of this state. Consequently, the shorter time constant can be unambiguously assigned to the PS triplet decay time. The slight increase of the longer decay time follows the general tendency of the decay time to increase with the illumination dose. It can be noted, that a self-quenching effect of by molecular (triplet) oxygen has been reported, which might quantitatively affect the decay times.46 However, this is negligible here: Considering the variation of both times under atmosphere exchange, the assignment made is perspicuous. Since the longer time still increases while the shorter time decreases, the shorter time must be the triplet decay time of the PS. This assignment is alike for Pheo and . 3.5.Singlet Oxygen Generation in Extracted Lipid Components of Pig Ear SkinSinglet oxygen emission from the lipid films was observed for both PSs in both lipid films, as illustrated in Figs. 8 and 9. Singlet oxygen luminescence measurements of the lipid films from subcutaneous tissue show that these lipids contain oxidative targets as well, as shown in Figs. 8 and 10. The dynamics of the change of and PS triplet decay times in skin (Fig. 6) and in subcutaneous tissue (Fig. 10) are alike the dynamics found in lipid films of stratum corneum lipids (Fig. 9). As in the case of stratum corneum lipids, Pheo exhibits an initially fast rise reaching an inflection point at which the speed of increase slows significantly while, for the sample, the decay time increases more smooth and continues to rise at the end of the measurement. The fact that Pheo is generating from these extracted lipids does not contradict the fact that it accumulates only within the stratum corneum since the amphiphilic Pheo cannot diffuse through it in the intact skin to reach these areas. Fig. 8Luminescence decay of singlet oxygen photosensitized generated by (a) Pheo and (b) in a film of lower tissue lipids. Shown data applies to measurement no. 10 after already illumination with . Fits were obtained using the biexponential model according to Eq. (1). Fitted parameters: (a) , . (b) , . Insets show flash photolysis measurements conducted after the end of the singlet oxygen measurements. Data was fitted with a single decay time (a) and (b) . 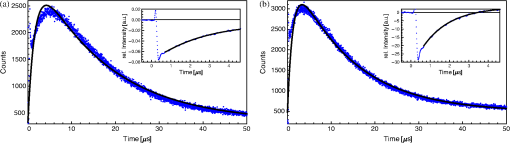 Fig. 9Variation of singlet oxygen and photosensitizer triplet decay times for (a) Pheo and (b) in a film of stratum corneum lipids as a function of light dose. Fitted parameters: a) initial measurement: , , final measurement (): , . b) initial measurement , , final measurement: , . 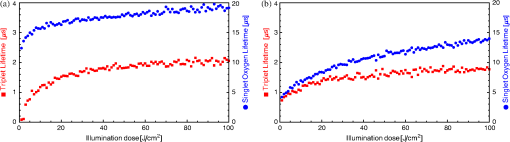 Fig. 10Variation of singlet oxygen and photosensitizer triplet decay times in a film of subcutaneous tissue lipid as a function of light dose (the scale is proportional to a time scale of 0 to 7 min). The photosensitizer is (a) Pheo and (b) Fitted parameters: (a) initial measurement: , , final measurement (): , . (b) initial measurement , , final measurement: , . 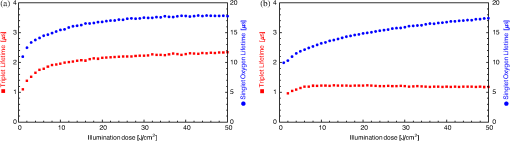 A dependence of the decay times on the light dose can be observed in the lipid films of stratum corneum lipids as well. Significant structural differences between the extracted lipid and the lipid system present in skin, however, preclude a detailed analysis of the shape of the luminescence signal. As stated above, the simple bi-exponential model, suitable for homogenous environments, can be improved for heterogeneous systems if the structure of the system is known. In case of the lipid films investigated here (Fig. 8), the inhomogeneity of the system is indicated by the deviation of the simple model from fit of the data and by the luminescence kinetics. The films, obtained with extracted lipids, clearly do not structurally resemble the native lipids present in the stratum corneum or in deeper tissues.47 This investigation, even if limited by employing the simple biexponential model, allows the identification of common chemical reactions of that take place in the stratum corneum and the extracted lipids. Similar differences in the change of the luminescence kinetics between the Pheo and samples can be noticed in the lipid samples as well as in skin. The rate of change of and PS triplet decay time with illumination is steadier for compared with Pheo. The initial change in decay times for Pheo is rapid, reaching a point of equilibrium or much slower growth. The change in kinetics shown is in the same order-of-magnitude as the change observed in the skin samples which indicates the PS and lipid concentrations used for preparing the lipid films closely resemble those in the pig ear. Since Pheo and are present in equimolar concentrations in the lipid film, the different change in kinetics should not be attributed solely to possible differences in absorption or a lower generation. As indicated already by the measurements in crème and as it can be assumed due to the properties of the PS, we rather propose that this difference in change of kinetics is related to the microenvironment of the PS. Thus, the PS triplet decay time equilibrium values of 2.1 μs and 1.7 μs for films of Pheo and , respectively, indicate that Pheo in the lipid film is relatively less accessible to oxygen which could indicate that Pheo is incorporated into a lipoid structure. It is likely that the preparation method of the films also influences the PS microenvironment which causes the appearance of lipoid, self-organized structures such as micelles, in case of Pheo, as suggested by deviations of the luminescence signals from the fitted model for homogenous environments. Without further proof of the effective micro-environment, however, a better theoretical model cannot be proposed. The generally shorter equilibrium PS triplet decay times in the pig ear, compared to the lipid films, indicates that the surrounding environment of the PS in skin is relatively more accessible by oxygen. This is due to several differences. For example, the lipid films are placed on a glass surface which hinders oxygen access from one side. In addition, the structure of the lipids, in the lipid-only film, is more compact compared to that of lipids in the skin where they occupy only the intercellular space. 3.6.Singlet Oxygen Generation in the Keratinous Component of Pig Ear SkinSamples of the keratinous residue, prepared with , exhibit luminescence. The time-resolved signals, however, could not be fit with the biexponential model due to the low signal intensity and, possibly, the presence of PS phosphorescence. Thus, a simplifying approach was employed to model the luminescence intensity. Specifically, the intensities were averaged over the first 20 μs after the laser pulse and the last 10 μs within the measurement time window of 80 μs after excitation. The difference obtained by subtracting the average intensity of the last 10 μs from the average intensity of the first 20 μs was attributed to a “short-lived” luminescence signal. The average value of the last 10 μs forms a long-lived intensity component. The change of the signal intensity of the short lived component, Fig. 11, reveals that this signal almost vanishes upon flushing of the sample chamber. Complete oxygen removal cannot be achieved, thus, a small signal may remain. Thus, a luminescence emission with a lifetime below 20 μs appears which suggests that a signal is emitted from the sample. The relatively short lifetime of 20 μs also indicates that the detected is not decaying in air where it could live up to hours. Fig. 11Signal intensity of a short lived luminescence from keratinous scraps obtained from pig-ear stratum corneum prepared with . The sample was flushed with from the beginning to point A. After A until point B air was allowed to re-enter the sample. At point B the sample was briefly flushed with compressed air. Between B and C the sample was exposed to normal atmosphere. This was followed by flushing with in the time between points C and D. After that the sample was exposed to normal atmosphere again. A signal intensity loss and recovery is observed under and exposure to air, respectively. 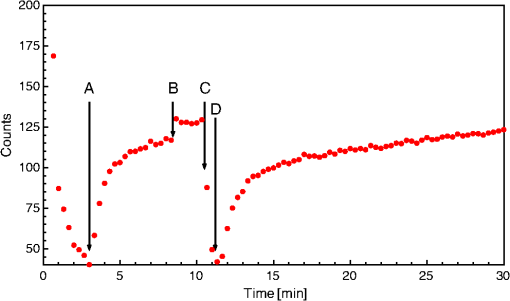 Quantification of the long lived component suggests that this long-lived luminescence may be due to the phosphorescence of the PS. The intensity of this component increases under flushing and is not spectrally centred at 1270 nm. It can also be detected at 1200 nm where the short lived component disappears. A possible emission in air is outshined by the phosphorescence. In the case of Pheo luminescence signals remain below the system’s sensitivity limit. The changes in the amplitude of the short lived component under change of atmosphere, in comparison to the long lived component, indicate that no emission can be tracked in this system. Also, no phosphorescence can be identified. Due to the preparation procedure, Pheo is present in the sample. However, when it dries out, it aggregates and the aggregates neither fluoresce nor produce .48 The keratin has no ability to cause the monomerization of Pheo. In conclusion, the sample is not producing . In contrast, cannot form aggregates and, thus, production remains possible in dry condition. The short lived component is, therefore, caused by which is quenched. Possible quenchers may either be keratin, which can be oxidized, or residual quenchers present within the keratinous residue.44 3.7.Correlation Between Photosensitizer Localization and Singlet Oxygen Luminescence KineticsThe complexity of skin composition prompted us to investigate the generation site of within the stratum corneum. The intercellular lipids are mainly organised as a lamellar system within a keratinous structure of high complexity.49 Cellular membranes, in general, constitute a major accumulation site for Pheo in living cells in vitro.36,40 Considering the lipoid structure of the intercellular lipids of the stratum corneum, as proposed previously, the incorporation of Pheo in this structure is likely even though the structure and composition of the lipid membranes in cells are different from the structure of the intercellular lipids.50 Since fluorescence microscopy cannot reveal the PS localization within this ultrastructure of the stratum corneum, the isolation of the skin’s major components was undertaken. The lipids from the stratum corneum were extracted as well the keratinous residue. Even though production in hair may occur, hair itself has not been further investigated here, as our studies are aimed at eventual PDT tumour treatment and, thus, focus on PS effects within the skin.51 Lipids from the tissue below the stratum corneum were also extracted and investigated as was found there as well. The residue of the deeper layers was not investigated as these layers comprise viable cells which are severely affected by the lipid extraction procedure. All extracted lipids show emission when prepared with any of the PS. From samples of the keratinous residue, luminescence is observed only for . This means, in case of pheo, only the intercellular lipids can serve as generation site for within the stratum corneum. Skin samples treated with Pheo show a faster increase of the decay time with illumination at the beginning of the measurement, however, only a very slow rise after illumination with light dose. In contrast, samples treated with show a slower increase in decay time which still rises even after a light dose of . The samples prepared with generally exhibit shorter decay times compared to those prepared with Pheo. In equal micro-environments (the volume that can be affected by via diffusion), this would result in a faster increase of decay time with illumination dose for the samples. As this is not observed, the micro-environments of the two PS must differ. For the skin samples, several explanations can be used to model this behavior. Initially, the diffusion length of is limited. In a purely lipid system, 100 nm have been reported.41 This value may be an upper limit for the investigated systems. After consumption of chemical quencher within the micro-environment, physical quenching determines the equilibrium value of the decay time. The amount of chemical quenchers available for oxidation is limited to those within the micro-environment. Due to its chemical properties, is distributed more evenly. Thus, the overall amount of quenchers available for oxidation is larger compared to the membrane-incorporable Pheo. For Pheo, the micro-environment spans only the lipoid structures. Therefore, the decay time of the Pheo samples more quickly reach an equilibrium state. Secondly, is generating below the stratum corneum as well. Thus, the affected volume of the skin is also larger on a macro scale. Consequently. the affected skin volume of the samples contains a higher total amount of quenchers which offers more reaction sites for and decreasing the rate of change of rise and decay times with illumination. Thirdly, the luminescence kinetics and generation loci are in good agreement considering the typical water concentration profile in the epidermis.52 At the junction between stratum corneum and the deeper layers of viable cells, the water content shows a sharp rise. The higher water content, in this environment, could explain the shorter decay time obtained in samples since water is an efficient physical quencher. The luminescence signals were fit biexponentially despite the fact that this is valid only in a homogenous environment. The additional generation of , with a short decay time in the layer of viable cells, would result in a lower fitting parameter for the decay time of the simple model. This explains the lower fitting parameter for the decay time of the sample compared to the Pheo sample where the is generated only in the stratum corneum. Due to the physical quenching by water, the rate of chemical quenching is reduced. A quantification of the contribution of the respective factors is difficult. Nevertheless, the characteristic difference in change of the luminescence rise and decay times is also observed in the lipid extracts even though these are structurally very different systems. For the lipids especially, the possibility of the formation of different microenvironments within the lipids can cause differences in the behavior of the two PSs, thus, making the first explanation the most likely one. 4.Conclusions and ProspectsSecond to none, the luminescence in tissue was detected time-resolved using low excitation energies of per measurement. Using a compositional formulation similar to that used for topical PS application in clinics, two PSs, an amphiphilic one, Pheo and a highly hydrophobic one, have been applied to pig ear skin. A consistent picture of the PS localisation and its microenvironment can be presented. The amphiphilic Pheo generates only in the stratum corneum. The hydrophobic was found to generate in the stratum corneum and as well in deeper layers of the epidermis, below the stratum corneum. Furthermore, lipids extracted from the epidermis show emission when prepared with any of the PS. From samples of the keratinous residue, luminescence is observed only for . In consideration of the fluorescence measurements for Pheo, only the intercellular lipids can serve as generation site for within the stratum corneum. In contrast, generation within the corneocytes cannot be excluded for . Considerations of the luminescence decay times and their dependence on illumination dose give further conclusions about the microenvironment of the PS. Samples prepared with Pheo show a faster increase of luminescence decay time with increasing illumination dose. In comparison, samples prepared with exhibit a slower increase. For the membrane-incorporable Pheo, the oxidation of local quenchers occurs at a higher rate which is likely due to the closer vicinity to the quencher molecules. This is not given for samples prepared with . The distribution of is more diffuse. This leads to a model for the microenvironment of the PSs within the ultrastructure of the stratum corneum:. Pheo is incorporated into lipoid structures formed by the intercellular lipids. In contrast, is distributed more evenly and does not provoke the formation of a structured microenvironment. The low excitation energies are crucial to reveal the kinetics of PS triplet state decay as well as that of under PDT relevant conditions. These times depend on the used PS and reflect the different accumulation loci and the PS microenvironment. We show that the light-dose induced variations of the decay times, after high illumination doses, also differ for both PSs which indicates different equilibrium values. The described experimental and technical conditions demonstrate the possibility to use the time-resolved luminescence detection as a diagnostic tool. Light-dose induced variations of the luminescence kinetics can be tracked at light levels commonly applied for PDT treatment. Monitoring the interaction with tissue and the saturation level facilitates the improvement of clinical protocols for treatment and reduces stress for patients. Differences in PS efficacy, due to accumulation properties, can be assed using luminescence detection. This provides an expeditious way to examine newly designed PS for their applicability in PDT. AcknowledgmentsThe authors would like to thank Prof. J. Lademann from the Charité Berlin for supplying pig-ear samples and Heike Richter for carrying out the biopsies. We also thank Hans Scholz, head of the electronics workshop of the Institute of Physics and Steffen Fahnauer and Michael Löbe from the mechanical workshop. We also thank Norbert Klose from Hamamatsu Germany. Hemantbhai Patel and Sergiu M. Gorun thank the US Army for financial support. ReferencesW. Baumleret al.,
“UVA and endogenous photosensitizers—the detection of singlet oxygen by its luminescence,”
Photochem. Photobiol. Sci., 11
(1), 107
–117
(2012). http://dx.doi.org/10.1039/c1pp05142c PPSHCB 1474-905X Google Scholar
J. Baieret al.,
“Singlet oxygen by uva exposure of endogenous photosensitizers,”
Biophys. J., 91
(4), 1452
–1459
(2006). http://dx.doi.org/10.1529/biophysj.106.082388 BIOJAU 0006-3495 Google Scholar
H. Masaki,
“Role of antioxidants in the skin: anti-aging effects,”
J. Dermatol. Sci., 58
(2), 85
–90
(2010). http://dx.doi.org/10.1016/j.jdermsci.2010.03.003 JDSCEI 0923-1811 Google Scholar
A. Mahnset al.,
“Contribution of UVB and UVA to UV-dependent stimulation of cyclooxygenase-2 expression in artificial epidermis,”
Photochem. Photobiol. Sci., 3
(3), 257
–262
(2004). http://dx.doi.org/10.1039/b309067a PPSHCB 1474-905X Google Scholar
S. Grether-Becket al.,
“Activation of transcription factor ap-2 mediates uva radiation and singlet oxygen-induced expression of the human intercellular adhesion molecule 1 gene,”
Proc. Natl. Acad. Sci. U.S.A., 93
(25), 14586
–14591
(1996). http://dx.doi.org/10.1073/pnas.93.25.14586 PNASA6 0027-8424 Google Scholar
G. Kicket al.,
“Strong and prolonged induction of c-jun and c-fos proto-oncogenes by photodynamic therapy,”
Br. J. Cancer, 74
(1), 30
–36
(1996). http://dx.doi.org/10.1038/bjc.1996.311 BJCAAI 0007-0920 Google Scholar
R. S. Cavalcanteet al.,
“A combination of techniques to evaluate photodynamic efficiency of photosensitizers,”
Laser Phys. Lett., 6
(1), 64
–70
(2009). http://dx.doi.org/10.1002/lapl.v6:1 1612-2011 Google Scholar
G. F. VileR. M. Tyrrell,
“UVA radiation-induced oxidative damage to lipids and proteins in vitro and in human skin fibroblasts is dependent on iron and singlet oxygen,”
Free Radic. Biol. Med., 18
(4), 721
–730
(1995). http://dx.doi.org/10.1016/0891-5849(94)00192-M FRBMEH 0891-5849 Google Scholar
M. C. DeRosaR. J. Crutchley,
“Photosensitized oxygen and its applications,”
Coord. Chem. Rev., 233–234 351
–371
(2002). http://dx.doi.org/10.1016/S0010-8545(02)00034-6 CCHRAM 0010-8545 Google Scholar
J. C. KennedyR. H. PottierD. C. Pross,
“Photodynamic therapy with endogenous protoporphyrin ix: basic principles and present clinical experience,”
J. Photochem. Photobiol. B, 6
(1–2), 143
–148
(1990). http://dx.doi.org/10.1016/1011-1344(90)85083-9 JPPBEG 1011-1344 Google Scholar
M. NiedreM. S. PattersonB. C. Wilson,
“Direct near-infrared luminescence detection of singlet oxygen generated by photodynamic therapy in cells in vitro and tissues in vivo,”
Photochem. Photobiol., 75
(4), 382
–391
(2002). http://dx.doi.org/10.1562/0031-8655(2002)0750382DNILDO2.0.CO2 PHCBAP 0031-8655 Google Scholar
J. R. Kanofsky,
“Measurement of singlet-oxygen in vivo: progress and pitfalls,”
Photochem. Photobiol., 87
(1), 14
–17
(2011). http://dx.doi.org/10.1111/php.2010.87.issue-1 PHCBAP 0031-8655 Google Scholar
Y. Y. Tianet al.,
“Mitochondria-involved apoptosis induced by mppa mediated photodynamic therapy,”
Laser Phys. Lett., 5
(10), 746
–751
(2008). http://dx.doi.org/10.1002/lapl.v5:10 1612-2011 Google Scholar
R. W. RedmondI. E. Kochevar,
“Spatially resolved cellular responses to singlet oxygen,”
Photochem. Photobiol., 82
(5), 1178
–1186
(2006). http://dx.doi.org/10.1562/2006-04-14-IR-874 PHCBAP 0031-8655 Google Scholar
J. Baieret al.,
“Time-resolved investigations of singlet oxygen luminescence in water, in phosphatidylcholine, and in aqueous suspensions of phosphatidylcholine or HT29 cells,”
J. Phys. Chem. B, 109
(7), 3041
–3046
(2005). http://dx.doi.org/10.1021/jp0455531 JPCBFK 1520-6106 Google Scholar
J. W. Snyderet al.,
“Optical detection of singlet oxygen from single cells,”
Phys. Chem. Chem. Phys., 8
(37), 4280
–4293
(2006). http://dx.doi.org/10.1039/b609070m PPCPFQ 1463-9076 Google Scholar
A. Jimenez-Banzoet al.,
“Kinetics of singlet oxygen photosensitization in human skin fibroblasts,”
Free Radic. Biol. Med., 44
(11), 1926
–1934
(2008). http://dx.doi.org/10.1016/j.freeradbiomed.2008.02.011 FRBMEH 0891-5849 Google Scholar
T. HerrlingK. JungJ. Fuchs,
“Measurements of UV-generated free radicals/reactive oxygen species (ROS) in skin,”
Spectrochim. Acta A Mol. Biomol. Spectrosc., 63
(4), 840
–845
(2006). http://dx.doi.org/10.1016/j.saa.2005.10.013 SAMCAS 1386-1425 Google Scholar
C. Florset al.,
“Imaging the production of singlet oxygen in vivo using a new fluorescent sensor, Singlet Oxygen Sensor Green,”
J. Exp. Bot., 57
(8), 1725
–1734
(2006). http://dx.doi.org/10.1093/jxb/erj181 JEBOA6 1460-2431 Google Scholar
A. Gollmeret al.,
“Singlet oxygen sensor green(R): photochemical behavior in solution and in a mammalian cell,”
Photochem. Photobiol., 87
(3), 671
–679
(2011). http://dx.doi.org/10.1111/php.2011.87.issue-3 PHCBAP 0031-8655 Google Scholar
M. J. Niedreet al.,
“Singlet oxygen luminescence as an in vivo photodynamic therapy dose metric: validation in normal mouse skin with topical amino-levulinic acid,”
Br. J. Cancer, 92
(2), 298
–304
(2005). http://dx.doi.org/10.1038/sj.bjc.6602331 BJCAAI 0007-0920 Google Scholar
M. J. Niedreet al.,
“Imaging of photodynamically generated singlet oxygen luminescence in vivo,”
Photochem. Photobiol., 81
(4), 941
–943
(2005). http://dx.doi.org/10.1562/2005-03-15-TSN-462R.1 PHCBAP 0031-8655 Google Scholar
S. Leeet al.,
“A singlet molecular oxygen imaging sensor for photodynamic therapy,”
(2008). Google Scholar
C. SchweitzerR. Schmidt,
“Physical mechanisms of generation and deactivation of singlet oxygen,”
Chem Rev, 103
(5), 1685
–1757
(2003). http://dx.doi.org/10.1021/cr010371d CHREAY 0009-2665 Google Scholar
J. Baieret al.,
“Direct detection of singlet oxygen generated by UVA irradiation in human cells and skin,”
J. Invest. Dermatol., 127
(6), 1498
–1506
(2007). http://dx.doi.org/10.1038/sj.jid.5700741 JIDEAE 0022-202X Google Scholar
S. Leeet al.,
“A singlet oxygen monitor as an in vivo photodynamic therapy dosimeter,”
SPIE, 738046
–738049 SPIE, San Jose
(2009). Google Scholar
S. Hackbarthet al.,
“New insights to primary photodynamic effects—Singlet oxygen kinetics in living cells,”
J. Photochem. Photobiol. B, 98
(3), 173
–179
(2010). http://dx.doi.org/10.1016/j.jphotobiol.2009.11.013 JPPBEG 1011-1344 Google Scholar
J. Schlothaueret al., In vivo detection of time-resolved singlet oxygen luminescence under PDT relevant conditions, 755106 SPIE, San Francisco
(2010). Google Scholar
J. SchlothauerS. HackbarthB. Röder,
“A new benchmark for time-resolved detection of singlet oxygen luminescence—revealing the evolution of lifetime in living cells with low dose illumination,”
Laser Phys. Lett., 6
(3), 216
–221
(2009). http://dx.doi.org/10.1002/lapl.v6:3 1612-2011 Google Scholar
U. Jacobiet al.,
“Porcine ear skin: an in vitro model for human skin,”
Skin Res. Technol., 13
(1), 19
–24
(2007). http://dx.doi.org/10.1111/srt.2007.13.issue-1 SRTEFN 0909-752X Google Scholar
A. WillstätterR. Stoll, Untersuchungen über Chlorophyll, Springer, Berlin
(1913). Google Scholar
R. Gerdeset al.,
“Rational design of a reactive yet stable organic-based photocatalyst,”
Dalton Trans., 7
(7), 1098
–1100
(2009). http://dx.doi.org/10.1039/b822111c DTARAF 1477-9226 Google Scholar
J. Zimmermannet al.,
“Determination of the electron transfer parameters of a covalently linked porphyrin-quinone with mesogenic substituents—optical spectroscopic studies in solution,”
J. Photochem. Photobiol. B, 40
(3), 209
–217
(1997). http://dx.doi.org/10.1016/S1011-1344(97)00058-4 JPPBEG 1011-1344 Google Scholar
V. KassisJ. Sondergaard,
“Heat-separation of normal human skin for epidermal and dermal prostaglandin analysis,”
Arch. Dermatol. Res., 273
(3–4), 301
–306
(1982). http://dx.doi.org/10.1007/BF00409259 ADREDL 0340-3696 Google Scholar
M. Breternitzet al.,
“Acute barrier disruption by adhesive tapes is influenced by pressure, time and anatomical location: integrity and cohesion assessed by sequential tape stripping. A randomized, controlled study,”
Br. J. Dermatol., 156
(2), 231
–240
(2007). http://dx.doi.org/10.1111/bjd.2007.156.issue-2 BJDEAZ 1365-2133 Google Scholar
A. Paulet al.,
“Comparative study on the photosensitization of jurkat cells in vitro by Pheophorbide-a and a pheophorbide-a diaminobutane poly-propylene-imine dendrimer complex,”
Laser Phys., 13
(1), 22
–29
(2003). LAPHEJ 1054-660X Google Scholar
J. Baieret al.,
“Theoretical and experimental analysis of the luminescence signal of singlet oxygen for different photosensitizers,”
J. Photochem. Photobiol. B, 87
(3), 163
–173
(2007). http://dx.doi.org/10.1016/j.jphotobiol.2007.02.006 JPPBEG 1011-1344 Google Scholar
J. Schlothauer,
“Zeitaufgelöster Nachweis von Singulett-Sauerstoff,”
49
–55 Technische Universität Berlin, Berlin
(2009). Google Scholar
A. A. Krasnovskyet al.,
“Photophysical Studies of pheophorbide-a and pheophytin-a phosphorescence and photo-sensitized singlet oxygen luminescence,”
J. Photochem. Photobiol. B, 5
(2), 245
–254
(1990). http://dx.doi.org/10.1016/1011-1344(90)80009-M JPPBEG 1011-1344 Google Scholar
B. Rödeet al.,
“Photophysical properties of pheophorbidea in solution and in model membrane systems,”
J. Porphyrins Phthalocyanines, 4
(1), 37
–44
(2000). http://dx.doi.org/10.1002/(SICI)1099-1409(200001/02)4:1<37::AID-JPP183>3.0.CO;2-O JPPHFZ 1088-4246 Google Scholar
B. EhrenbergJ. L. AndersonC. S. Foote,
“Kinetics and yield of singlet oxygen photosensitized by hypericin in organic and biological media,”
Photochem. Photobiol., 68
(2), 135
–140
(1998). http://dx.doi.org/10.1111/php.1998.68.issue-2 PHCBAP 0031-8655 Google Scholar
B. A. Benchet al.,
“Introduction of bulky perfluoroalkyl groups at the periphery of zinc perfluorophthalocyanine: chemical, structural, electronic, and preliminary photophysical and biological effects,”
Angew. Chem. Int. Ed. Engl., 41
(5), 747
–750
(2002). http://dx.doi.org/10.1002/1521-3773(20020301)41:5<747::AID-ANIE747>3.0.CO;2-J ACIEAY 0570-0833 Google Scholar
R. Minneset al.,
“Enhanced acidity, photophysical properties and liposome binding of perfluoroalkylated phthalocyanines lacking C-H bonds,”
Photochem. Photobiol., 82
(2), 593
–599
(2006). http://dx.doi.org/10.1562/2005-11-08-RA-732 PHCBAP 0031-8655 Google Scholar
J. J. Thieleet al.,
“Protein oxidation in human stratum corneum: susceptibility of keratins to oxidation in vitro and presence of a keratin oxidation gradient in vivo,”
J. Invest. Dermatol., 113
(3), 335
–339
(1999). http://dx.doi.org/10.1046/j.1523-1747.1999.00693.x JIDEAE 0022-202X Google Scholar
J. J. Thiele,
“Oxidative targets in the stratum corneum. A new basis for antioxidative strategies,”
Skin Pharmacol. Appl. Skin Physiol., 14
(Suppl. 1), 87
–91
(2001). http://dx.doi.org/10.1159/000056395 SPAPFF 1422-2868 Google Scholar
R. Dedicet al.,
“Singlet oxygen quenching by oxygen in tetraphenyl-porphyrin solutions,”
J. Lumin., 119 209
–213
(2006). http://dx.doi.org/10.1016/j.jlumin.2005.12.032 JLUMA8 0022-2313 Google Scholar
S. H. WhiteD. MirejovskyG. I. King,
“Structure of lamellar lipid domains and corneocyte envelopes of murine stratum corneum. An X-ray diffraction study,”
Biochemistry, 27
(10), 3725
–3732
(1988). http://dx.doi.org/10.1021/bi00410a031 MIRBD9 0144-0578 Google Scholar
B. RöderH. Wabnitz,
“Investigation on hematoporphyrin, mesoporphyrin, pheophorbide a and chlorin e6 in ethanol and aqueous solution by time-resolved fluorescence spectroscopy,”
J. Photochem. Photobiol. B., 1
(1), 103
–113
(1987). http://dx.doi.org/10.1016/1011-1344(87)80010-6 JPPBEG 1011-1344 Google Scholar
L. Norlen,
“Stratum corneum keratin structure, function and formation—a comprehensive review,”
Int. J. Cosmet. Sci., 28
(6), 397
–425
(2006). http://dx.doi.org/10.1111/ics.2006.28.issue-6 IJCMDW 0142-5463 Google Scholar
J. R. HillP. W. Wertz,
“Molecular models of the intercellular lipid lamellae from epidermal stratum corneum,”
Biochim. Biophys. Acta, 1616
(2), 121
–126
(2003). http://dx.doi.org/10.1016/S0005-2736(03)00238-4 BBACAQ 0006-3002 Google Scholar
O. Chiarelli-Netoet al.,
“Generation and suppression of singlet oxygen in hair by photosensitization of melanin,”
Free Radic. Biol. Med., 51
(6), 1195
–1202
(2011). http://dx.doi.org/10.1016/j.freeradbiomed.2011.06.013 FRBMEH 0891-5849 Google Scholar
R. R. WarnerM. C. MyersD. A. Taylor,
“Electron probe analysis of human skin: determination of the water concentration profile,”
J. Invest. Dermatol., 90
(2), 218
–224
(1988). http://dx.doi.org/10.1111/jid.1988.90.issue-2 JIDEAE 0022-202X Google Scholar
|