|
|
|
Progressive alternation of biomechanical/elastic property and tissue composition of the cardiac wall is closely associated with the cardiac development in the developing embryonic heart.1 There is substantial evidence2 suggesting that intrinsic genetic expression is regulated by the local biomechanical environment that is determined by the dynamic interaction between the cardiac wall and flowing blood. To understand the cardiac development, particularly the interaction between wall dynamics and hemodynamics in developing outflow tract (OFT, a distal heart portion that connects the ventricle to the arterial system), a knowledge of the biomechanical/elastic properties of the cardiac wall is of fundamental importance. Pulse wave velocity (PWV) of the cardiac wall is highly correlated with the elastic properties.3 The wall PWV may be used to determine the Young’s modulus of the cardiac wall. There is no technique currently available that is capable of measuring the mechanical properties of the cardiac wall in vivo, without harm to the developing heart. Optical coherence tomography (OCT) is capable of noninvasive, noncontact, volumetric imaging of turbid tissue with high temporal ( range) and spatial () resolution.4,5 In this letter, we propose to use the time-lapse sequence of OCT images that is captured at the OFT longitudinal section by an ultrafast OCT system to calculate the wall PWV, from which the elasticity of the OFT wall is deduced. To demonstrate the proof of the concept, we used the stage HH18 chick embryos as the animal model in this study. The chick embryo preparation and the OCT system setup has been elucidated in Ref. 6. To increase the imaging speed and the image definition, and to enhance the ability of capturing fast events of the embryonic beating heart, a dual-camera spectral domain optical coherence tomography (SD-OCT) configuration was used that operated at 1310 nm wavelength region, providing a A-line scan rate and spatial resolution.6 In order to quantify the longitudinal propagation of the wall pulse wave along the OFT wall, 1000 repeated B-frames (a time sequence of OFT longitudinal sections, ) were acquired at an imaging rate of frames per second (fps), covering cardiac cycles of the beating heart. The schematic of measuring the wall PWV is illustrated in Fig. 1. Along the OFT longitudinal section, we selected a number of radial lines that are almost perpendicular to the OFT wall (marked by the red lines a to g) with a known distance between each line as measured from the OCT images. After selecting these radial lines, the M-mode structure images (time lapse profiles along the marked lines) were then digitally extracted from the available three-dimensional (3-D) time-lapse longitudinal volumetric OCT images (refer to Figs. 2 and 3), from which the pulse wave of the myocardial wall motion was computed. From the pulse waves, the regional foot-to-foot wave velocity () was readily available by calculating the time delay () between the initial opening and the ultimate closing of the myocardial wall between a known distance () apart [refer to Fig. 3(c)]7: And the regional apparent phase velocity () was calculated using the apparent time delay (), which can be measured by differentiating the phases of corresponding fundamental harmonics () at the two locations7,8: where is the heart rate.Fig. 1OCT image of the longitudinal section of the OFT averaged over cardiac cycles, sketching the measurement of the PWV. Red lines (a–g) indicate the sites for extracting pulsatile motion of myocardial wall. Six segments in total are used for PWV measurement of myocardial wall in this case. V: ventricle; M: myocardium. 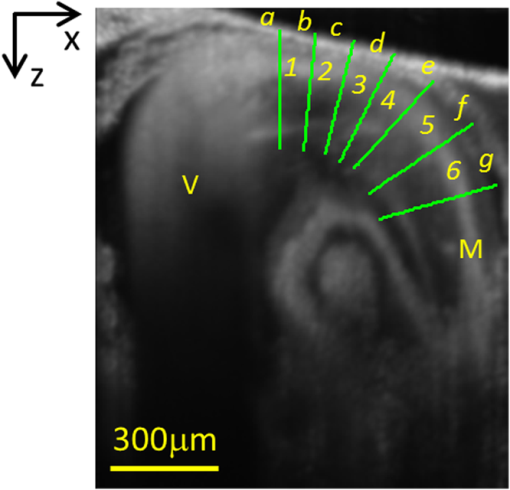 Fig. 2Simultaneous M-mode images of the instantaneous myocardial wall motion along the lines (a-g), respectively, distributed at seven descending OFT positions marked in Fig. 1. Solid lines depict the inner boundary of the myocardial wall. Dashed lines indicate one cardiac cycle in (a). M: myocardium; CJ: cardiac jelly; B: blood. 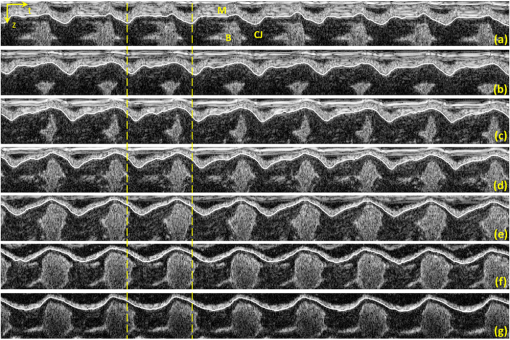 Fig. 3Pulse wave of the myocardial wall simultaneously obtained throughout OFT as extracted from the available 3-D volumetric time-lapse longitudinal dataset. (A, B), Pulse waves and fundamental harmonics of myocardial wall, respectively, corresponding to the positions of a–g in Figs. 1 and 2. (C, D) enlarged view of the wall pulse waveforms as marked in (A) and (B), respectively. (E-F) Typical OCT images represent the longitudinal sections of OFT corresponding to the time instants at V: ventricle; M: myocardium; CJ: cardiac jelly; B: blood. 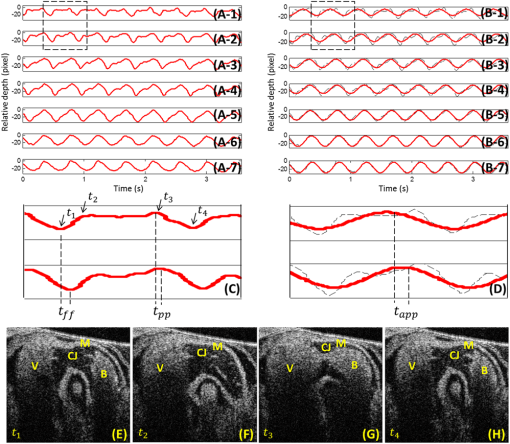 For a transverse wave on a viscoelastic conduit with a relatively small wall thickness (compared to its diameter) filled with an incompressible fluid, a simple model of the well-known Moens-Korteweg equation can be used to describe the relationship between the wave propagation and the elasticity property.9,10 Thus, the Young’s modulus () of the myocardial wall could be derived from the measured wall PWV (): where is the wall thickness, is the density of the wall (similar to the blood) and is the radius of the OFT.As shown in Fig. 2, the simultaneous M-mode images of the instantaneous myocardial wall motion were extracted from the time sequence of OFT longitudinal sections along the lines [Fig. 2(a) to 2(g)], respectively. These figures represent seven descending OFT positions marked as “a” to “g” in Fig. 1. Based on the image segmentation,11 the instantaneous positions of the myocardial wall boundary were obtained from the M-mode images as depicted by the curves in Fig. 2. Accordingly, the pulse waves of the wall motion were obtained at the seven descending OFT positions as shown in Fig. 3(a). The corresponding fundamental harmonic waves of the wall motion are plotted in Fig. 3(b). Figure 3(c) shows an enlarged view of a typical waveform of the myocardial wall motion. The arrows and in Fig. 3(c), respectively correspond to the longitudinal sections in Fig. 3(e) and 3(h), indicating the instantaneous times of the initial opening and the ultimate closing of the myocardial wall when the OFT is most contracted. The principal peak as marked by the arrow in Fig. 3(c) corresponds to the longitudinal section in Fig. 3(g), being the instant of blood ejection from the ventricle when the OFT is most expanded and filled with blood. It is interesting to note that there exists a secondary peak as marked by the arrow in Fig. 3(c). Such a secondary peak is apparent for the positions of “a” to “d” in Figs. 1 and 2 where the outflow cushion (OC) was present. This secondary peak is most likely due to the OC expansion, which has been reported to happen prior to the ventricle ejection for the stage HH18 chick embryos.6,12 Due to the high spatial resolution of OCT, the distance between each position was precisely calculated [Fig. 4(a)]. The corresponding time delays (both foot-to-foot and apparent ) are plotted in Fig. 4(b) as a function of the locations. Consequently, both the foot-to-foot PWV and the apparent PWV can be made readily available [Fig. 4(c)]. Fig. 4Plots of the distance (A), time delay (B) and PWV (C) as a function of the descending locations in OFT. 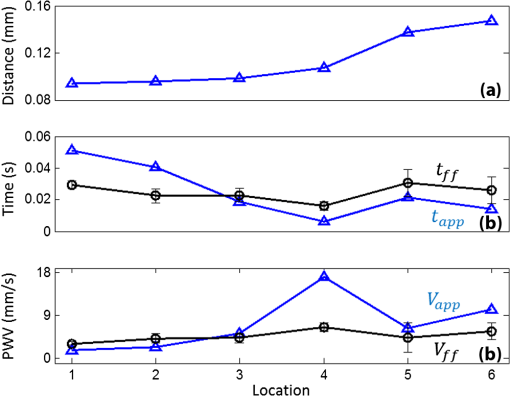 From Fig. 4, the mean foot-to-foot PWV was evaluated to be for the chick embryo we used in this study. The ratio of the OFT radius to the wall thickness was estimated to be 7.66 from the OCT M-mode images and the blood density was assumed to be . Based on these values, the Young’s modulus of the myocardial wall was then deduced from Eq. (3). In this letter, a method based on dynamic structure tracking has been developed to quantify the elastic properties of the myocardial wall, including PWV and Young’s modulus. In the study, it was assumed that the wall pulse wave from the seven positions in Fig. 1 was measured simultaneously. However, the OCT longitudinal section was acquired line by line (A scan-by-A scan). The maximal lateral spatial interval in the six wall regions in Fig. 1 is , thus there exists a maximal inherent time delay between each pulse wave, as shown in Fig. 3(a). Fortunately, the minimal foot-to-foot time delay is and the minimal apparent time delay is [refer to Fig. 4(b)]. Accordingly, the inherent time delay due to the line-by-line imaging nature of the OCT system is relatively too small to account for noticeable errors in the evaluations. Comparing the foot-to-foot velocity and the apparent velocity for each wall segments, a significant difference exists at the location 4 [refer to Fig. 4(c)]. Such difference between the foot-to-foot velocity and the apparent velocity is very similar to the situation of the blood pulse wave propagating in the human aortic region at the level of the renal arteries, where the strong local reflection due to the renal branch was well documented to influence the apparent velocity.7 Thus, the significant difference in OFT at location 4 could be ascribed to the influence of the bend of the wall at this location. According to Eq. (3), for viscoelastic conduits with the same elastic property , the smaller radius would correspond to the higher pulse velocity . Referring to Fig. 1, from locations 1 to 6, the OFT radius gradually decreases, while the wall foot-to-foot velocity gradually increases [Fig. 4(c)]. This observation is in good agreement with a prior study where the foot-to-foot velocity along the descending human aorta reported in Fig. 5 of Ref. 7 was increased with the decrease of the aorta radius. The measured foot-to-foot PWV ranged from 3.2 to with a mean of , and the averaged Young’s modulus was calculated to be 0.36 Pa. Due to the lack of corresponding OFT value in the literature, the tissue wave propagation in atrioventricular (AV) canals of HH17 and HH24 embryonic chick was taken for comparison. According to Ref. 1, in the AV canals of HH17 and HH24 embryonic chick, the tissue wave speed was approximately and , and the effective modulus was 0.15 and 0.85 Pa, respectively. Generally, our measurement was on the same scale as that reported in Ref. 1. The discrepancy was most likely due to the different embryonic stages, different tissue, and different measuring methods used. In this study, the wall PWV measurement is based on the dynamic structure tracking. Although this method served well for the purpose of proof-of-concept demonstration, the accuracy of pulse wave evaluation, particularly the high frequency components, is highly dependent upon the accuracy of image segmentation. Further improvement can be realized by measuring the pulsatile strain rate of the myocardial wall (based on the Doppler method) for timing the propagation of the wall pulse wave.6 Beside the cardiac development, possible applications of the wall PWV may also be found in the intravascular OCT for characterizing the elastic property of arterial wall. ReferencesJ. T. Butcheret al.,
“Transitions in early embryonic atrioventricular valvular function correspond with changes in cushion biomechanics that are predictable by tissue composition,”
Circ. Res., 100
(5), 1503
–1511
(2007). http://dx.doi.org/10.1161/CIRCRESAHA.107.148684 CIRUAL 0009-7330 Google Scholar
J. R. Hoveet al.,
“Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis,”
Nature, 421
(6919), 172
–177
(2003). http://dx.doi.org/10.1038/nature01282 NATUAS 0028-0836 Google Scholar
W. W. NicholsM. F. O’RourkeC. Vlachopoulos, McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles, 6th ed.Hodder Arnold, London
(2011). Google Scholar
A. F. Fercheret al.,
“Optical coherence tomography—principles and applications,”
Rep. Prog. Phys., 66
(1), 239
–303
(2003). http://dx.doi.org/10.1088/0034-4885/66/2/204 RPPHAG 0034-4885 Google Scholar
P. H. TomolinsR. K. Wang,
“Theory, developments and applications of optical coherence tomography,”
J. Phys. D: Appl. Phys., 38
(7), 2519
–2535
(2005). http://dx.doi.org/10.1088/0022-3727/38/15/002 JPAPBE 0022-3727 Google Scholar
P. Liet al.,
“In vivo functional imaging of blood flow and wall strain rate in outflow tract of embryonic chick heart using ultrafast spectral domain optical coherence tomography,”
J. Biomed. Opt., 17
(9), 096006
(2012). http://dx.doi.org/10.1117/1.JBO.17.9.096006 JBOPFO 1083-3668 Google Scholar
R. D. Lathamet al.,
“Regional wave travel and reflections along the human aorta: a study with six simultaneous micromanometric pressures,”
Circulation, 72
(6), 1257
–1269
(1985). http://dx.doi.org/10.1161/01.CIR.72.6.1257 CIRCAZ 0009-7322 Google Scholar
S. Solomonet al.,
“Determination of vascular impedance in the peripheral circulation by transcutaneous pulsed Doppler ultrasound,”
Chest, 108
(2), 515
–521
(1995). http://dx.doi.org/10.1378/chest.108.2.515 CHETBF 0012-3692 Google Scholar
M. Pernotet al.,
“ECG-gated, mechanical and electromechanical wave imaging of cardiovascular tissues in vivo,”
Ultrasound Med. Biol., 33
(7), 1075
–1085
(2007). http://dx.doi.org/10.1016/j.ultrasmedbio.2007.02.003 USMBA3 0301-5629 Google Scholar
D. J. Korteweg,
“Ueber die Fortpflanzungsgeschwindigkeit des Schalles in elastischen Röhren,”
Annalen der Physik, 241
(12), 525
–542
(1878). http://dx.doi.org/10.1002/(ISSN)1521-3889 ANPYA2 0003-3804 Google Scholar
X. Yinet al.,
“Extracting cardiac shapes and motion of the chick embryo heart outflow tract from four-dimensional optical coherence tomography images,”
J. Biomed. Opt., 17
(9), 096005
(2012). http://dx.doi.org/10.1117/1.JBO.17.9.096005 JBOPFO 1083-3668 Google Scholar
A. M. Oosterbaanet al.,
“Doppler flow velocity waveforms in the embryonic chicken heart at developmental stages corresponding to 5–8 weeks of human gestation,”
Ultrasound in Obstet. Gynecol., 33
(6), 638
–644
(2009). http://dx.doi.org/10.1002/uog.v33:6 0960-7692 Google Scholar
|


