|
|
1.Confocal Microscopy and its Application in SkinConfocal laser scanning microscopy (CLSM) is a classical technique to generate images from cell or tissue samples by means of laser scanning on an optical platform. The images are obtained at a higher resolution with depth selectivity compared to conventional optical microscopy or fluorescence microscopy. The key function of CLSM is its ability for optical sectioning in which the images are reconstructed based on the point-by-point scanning. When the laser is able to penetrate the skin to “resolve” the interior details of the nonopaque specimens, the three dimensional structure of the superficial interior can be constructed. For opaque specimens, the surface of the samples can be imaged. Overall, the quality of the images is greatly enhanced due to the unique sectioning method of the laser, and the information from the out-of-focus field is not superimposed on the image in focus. There are other advantages with CLSM. Most of the traditional techniques used to visualize cells or tissues are accompanied by some distortion of the tissue, which may be caused by tissue fixation and sectioning and by duration of exposure to the beams of light. With CLSM, the intact and/or viable sample is exposed to the laser, and the samples can be imaged many times when stored properly. Additional details regarding the mechanisms and methods of CLSM may be readily found on the website of the microscope manufacturer. CLSM, invented in 1978, is a technique to use a laser to scan the three-dimensional volume of an object point-by-point by means of a focused laser beam, with a design similar to the scanning electron microscope.1 For the first time, investigators combined this laser scanning method with the detection of three-dimensional biological cells or tissues labeled with a fluorophore. However, it was not until a decade later that CLSM was used routinely in skin research. The first application of CLSM focused on three-dimensional reconstruction of fluorescence-labeled Langerhans’ cells in human skin.2 Since then, the application of CLSM in dermal research has increased tremendously. Recently, in vivo reflectance CLSM has been used for noninvasive evaluation of normal and diseased human skin near the limits of cellular resolution, although there are some limitations on fixation of the optical system on moist surfaces.3 Reflectance CLSM has been used in the quantification of epidermal pigmentation and dermal papilla density.4 A technique that is derived from CLSM and applied to skin research is multi-photon laser scanning microscopy (MPLSM). As a noninvasive quantitative method for skin alterations, Puschmann et al.5 utilized the high-resolution three-dimensional MPLSM to successfully quantify the age-associated alterations in the extracellular matrix. 2.NP Distribution and Penetration in the Skin or Skin CellsDue to its optical sectioning ability, CLSM is a unique method to locate nanoparticles (NP) in the skin. In our laboratory, we used several different methods to image individual native NP before topical application to the skin surface. Dark field microscopy is utilized to illuminate the sample with light and excludes the unscattered beam from the image. As a result, the field around the object produces the classic dark background while the NP is visualized as a bright object. As shown in Fig. 1(a), the 50 nm silver NP were visualized as bright spherical objects in the dark field, while NP outside of the focal plane were relatively gray. The focused silver NP in the CLSM reflection mode were very bright and easy to detect, while the out of focus silver NP were not visible [Fig. 1(b)]. The differential interference contrast (DIC) mode of the CLSM can also detect individual silver NP, but with very low contrast compared to the background [Fig. 1(c)]. Therefore, the CLSM reflection mode provides different images of silver NP compared to dark field microscopy and CLSM DIC mode. The only limitation of the laser scanning is that the NP appear nonspherical, probably due to a reconstruction artifact derived from the software or laser scanning vibration. NP in the confocal scattering mode as shown by Failla and Meixner6 provided images of gold nanorods as a means to determine the orientation. However, the size of these NP () in the confocal image did not reflect their actual sizes of as determined by transmission electron microscopy (TEM). Although the NP appeared extremely bright, the images from the reflection mode and scattering mode of CLSM may not reflect the true size or shape of these NP. However, this limitation does not influence the overall study of NP interaction with skin or skin cells due to the greater size of cells () or tissue thickness (sections usually more than 4 μm). Fig. 1Single NP detection by CLSM. (a) Silver NP, 50 nm, by dark field microscopy. (b) Same NP in the reflection mode of CLSM. (c) Same NP in the differential interference contrast (DIC) mode of CLSM. 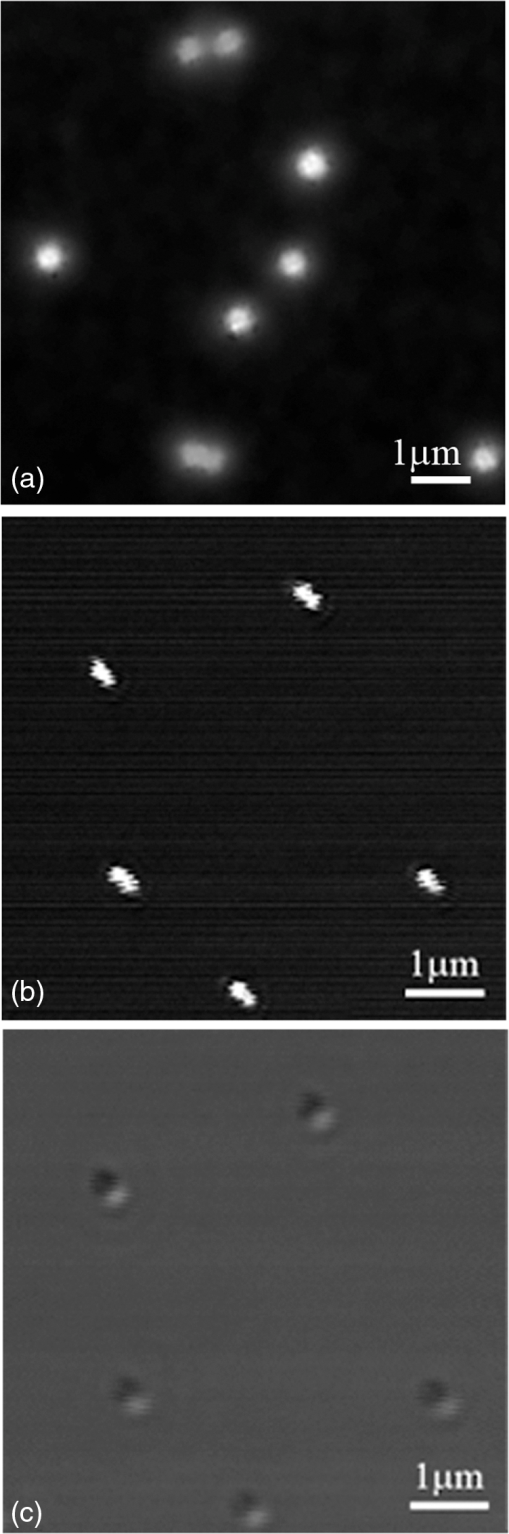 CLSM has been utilized to study the interaction between NP and skin for only a few years. As early as 2004, a polymerized bio-degradable block copolymer encapsulated with a fluorescent dye was applied to the skin of albino Hartley guinea pigs. After 12 h, these NP penetrated into the skin via shunt routes and were concentrated along hair follicles.7 Polystyrene NP were applied on the skin of the porcine ear and found to accumulate preferentially in the follicular openings.8 However, both studies included a phosphate buffered saline washing step following cryo-sectioning which may have altered the NP localization in the skin. In addition, methods such as electron microscopy and energy-dispersive x-ray (EDX) used to study NP within skin may create more artifacts due to tissue processing than unprocessed tissue analyzed by CLSM. Gold NP showed penetration through rat skin at low magnification by TEM, but they appeared as agglomerated gold and not individual NP.9 Studies using TEM illustrate how additional processing for electron microscopy may severely alter the localization of NP in skin. To study the actual location of NP in the skin, minimal washing and fixation with organic and inorganic reagents is required. But techniques that involve excessive washing or fixation may contribute to artifact. In addition, poor sectioning techniques may also contribute to NP contamination on the knife and thus the tissue during the sectioning process. However, our frozen skin samples were cut perpendicular to the knife with sectioning from the bottom of the dermis up through the epidermal layers. This prevents any contamination that may be due to dragging the NP from the surface to the dermal layers. This technique has been used in our laboratory for many years with radiolabeled compounds.10,11 Contamination can also be eliminated by optical sectioning within the tissue to distinguish whether the NP are on the surface of sections or within the section volume.12–16 Some of the factors that may affect the penetration of NP through skin have been described in two reviews.17,18 Several studies on the detection of NP in the skin by CLSM are described below. 2.1.NP Penetration in Skin Depends on NP Physiochemical PropertiesFluorescent dyes such as fluorescein 5-isothiocyanate (FITC) can be conjugated to NP, allowing researchers to investigate NP interactions with skin. However, traditional fluorescent dyes are not stable and may quench under the exposure of the lasers utilized with the confocal microscope. Another source of quenching may be due to the NP itself. Quantum dot (QD) NP have potential use in diagnostics, drug delivery and imaging in biomedicine or therapeutic applications due to their optical characteristics that result in strong fluorescence without photobleaching.19 QD are a very good model to determine the penetration of NP in skin. For such models, QD potential for toxicity and interactions within biological systems must be determined before nanomaterial risk assessments can be made. For imaging and optical sectioning, CLSM can precisely localize the QD (or other appropriate fluorophore-conjugated NP) within the optical sections to localize the NP in the different skin layers. The penetration of QD through skin has been addressed by our research group employing an ex vivo porcine skin model. QD565 and QD655 have a cadmium/selenide (CdSe) core surrounded by a zinc sulfide (ZnS) shell. By TEM, QD565 are spherical with a diameter of 4.6 nm, while QD655 are ellipsoid with a size of . The hydrodynamic diameters for QD565 are 35 nm for the QD coated with polyethylene glycol (PEG), uncharged, 14 nm for the QD coated with carboxylic acid (COOH), negatively charged, and 15 nm for the QD coated with PEG-amine (), positively charged. The hydrodynamic diameters of the coated QD655 were 45 nm (PEG), 18 nm (COOH), and 20 nm (). The penetration of QD 565 and 655 with the three different coatings were investigated in porcine flow-through diffusion cells, followed by skin sectioning and CLSM imaging.12 QD were excited by ultraviolet light or by 488 nm argon laser in CLSM; QD565 can be filtered and imaged at the emission wavelength of 565 nm, while QD655 image at the emission wavelength of 655 nm. It was noted that PEG and COOH coated QD 565 were localized within the epidermis at 8 h, while coated QD 565 were localized within the dermis. QD 655 with PEG and coatings were found within the epidermal layers after 8 h. In comparison, the penetration of QD655COOH coating was relatively slow and present within the epidermis at 24 h.12 Therefore, the surface coatings can greatly influence the depth of skin penetration of the NP. Also, we have performed in vitro flow-through cell diffusion studies with QD621, which has a different shape (nail shaped) and different shell coating (CdS shell).20 At the low concentration of 1 μM, the QD621 were located primarily in the normal intact stratum corneum layers of the skin, with no fluorescence detected in the stratum granulosum, stratum spinosum, or stratum basale layers of the epidermis. At the high concentration of 10 μM (Fig. 2), QD621 were primarily present within the stratum corneum layers or between the stratum granulosum-stratum corneum interface although a focal area of fluorescence was detected in the upper epidermal layers. Occasionally, QD621 were noted in the outer root sheath of the hair follicle.15 Fig. 2Topically applied QD621 to skin as imaged by CLSM. QD remained on the surface of the stratum corneum layer or within the outer root sheath of the hair follicle.15 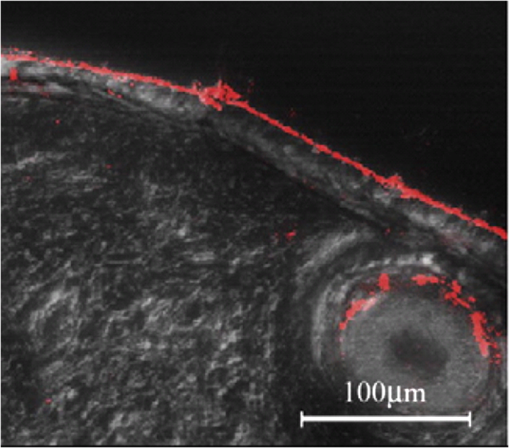 A recent study with poly (amidoamine) (PAMAM) dendrimers conjugated with rhodamine B isothiocyanate reported penetration in porcine skin.21 The authors used a Zeiss LSM 510 Meta confocal laser scanning microscope with a 1 mW helium—neon laser at 543 nm to image the localization of PAMAM in the skin. They hypothesized that these dendrimers at smaller sizes can penetrate the skin layers more efficiently than the larger dendrimers. In addition, dendrimers with surface-modification of either acetylation or carboxylation can increase skin permeation. In contrast, amine-terminated dendrimers show enhanced cell internalization and skin retention but reduced skin permeation. Compared to the QD studies performed in our lab, these results show differences between surface coatings and the ability to skin penetration. However, the authors claim that PAMAM dendrimers are skin-penetration enhancers, which could result in differences in skin penetration compared to QD. This illustrates that using CLSM, NP penetration in the skin is dependent on the composition, size, shape and surface coating of the NP. 2.2.NP Penetration in Skin Depends on Skin ConditionsIn the work place, a compromised skin barrier due to physical and/or chemical damage may increase dermal absorption of chemicals. The impaired skin barrier might lead not only to enhanced absorption of a specific chemical, but may increase the skin penetration and absorption of large molecules such as proteins and NP which are normally unable to penetrate intact skin.22 Additional studies investigated the penetration of QD655 and QD565 coated with COOH in rat skin for 8 and 24 h in flow-through diffusion skin cells.14 The skin was flexed, tape-stripped and abraded to determine if these mechanical actions could perturb the barrier and affect penetration. The QD655 or QD565 were localized on the surface of the stratum corneum in a homogeneous and continuous pattern, with minimal difference in QD penetration between flexed skin and intact skin. QD were also noted to be bound to the hairs. Rat skin was tape-stripped 10 times to remove the stratum corneum layers. In tape-stripped skin, QD were deposited evenly and homogeneously on the surface of the viable epidermal layers. Rat skin was abraded 60 times with sandpaper until the skin was slightly red but not bleeding. This mechanical action removed the stratum corneum layers and some of the viable epidermis so that penetration of QD was facilitated through skin. QD655 and QD565 showed slight penetration through the damaged skin into the dermis at both 8 and 24 h. Additional studies with QD in tape-stripped and intact human skin in flow-through cells found similar results, with QD penetration minimal.23 A previous study showed a fullerene amino acid-derivatized peptide NP of 3.5 nm was capable of penetrating the dermal layers of porcine skin flexed for 60 min and placed in flow-through diffusion cells for 8 h, while nonflexed control skin showed limited penetration to the upper epidermal layers.13 In another in vitro skin model, the isolated perfused porcine skin flap, QD were assessed to localize distribution in the skin by CLSM. Arterial extraction of QD was spectroscopically assayed for fluorescence after infusion into the perfused skin. QD-COOH had greater tissue deposition than did QD-PEG as assessed by CLSM.24 All of the above studies demonstrate that there are species differences, model differences and the condition of the skin barrier may play a role in the penetration of NP in skin. 2.3.NP Penetration into the Skin from Top to Bottom by CLSMAlvarez-Roman et al.8 investigated the skin distribution of fluorescent NP from the skin surface to deeper areas of the skin using CLMS at excitation wavelengths of 568 nm to scan the skin and at 488 nm to image the fluorescent NP, which reflected the NP interaction with the surface of the skin. This method allowed for the direct visualization of NP distribution along the skin surface and within follicular openings. Recently, investigators used a Zeiss CLSM to illuminate QD dosed on human skin in Franz static diffusion cells with a 488 nm argon excitation laser The QD were topically applied on the human skin followed by optical sectioning with the CLSM from the top (surface of the skin) to the bottom of the skin, a depth of more than 50 μm.16 This technique detected the presence of QD on the outer surface of the skin and determined whether the QD penetrated the skin. The authors concluded that some penetration occurred into the intact viable epidermis of skin by the QD-PEG with a pH 8.3, but not with QD-PEG with a pH 7.0. But with tape stripping, the stratum corneum layer was removed and QD penetrated through the viable epidermis and into the upper dermis within 24 h. This study showed that CLSM can determine the depth of NP interaction with the skin by sequentially analyzing optical sections in the stacked skin volume. 2.4.NP Interaction with Skin CellsIn vitro studies for NP interaction with cells have been well documented in recent years. Our lab has conducted numerous experiments with NP such as QD and fullerene () derivatives to dose human epidermal keratinocytes (HEK) and analyze the NP uptake by CLSM.25,26 Using CLSM, QD interactions with HEK is dependent upon the surface coating.27,28 Other than the traditional detection of fluorophore conjugated NP by CLSM, MWCNT can be visualized within HEK stained with cytoskeleton antibodies in the reflection mode to allow detection of individual MWCNT in the focal plane (Fig. 3).29 Additional in vitro models have utilized dendritic cells (DC) to study the cellular uptake of NP. Langerhans cells located within the epidermis of skin have similar functions to DC. They are a type of antigen-presenting immune cell for innate immunoresponse in the skin. Therefore, we established a technique using porcine monocyte-derived DC as an in vitro model. Pigs, unlike rodents, are genetically and physiologically similar to humans. Peripheral blood mononuclear cells were isolated from porcine blood by gradient centrifugation and purified to retain the CD14 positive monocytes. The monocytes were differentiated into DC with granulocyte macrophage colony-stimulating factor and interleukin-4 and then incubated with QD655-COOH. These DC were studied by CLSM and found to efficiently take up the QD655-COOH (Fig. 4).30 Fig. 3MWCNT interaction with human epidermal keratinocytes (HEK). MWCNT (yellow) at were dosed in HEK culture and visualized in the reflection mode of CLSM. F-actin (green) was stained to visualize the cell cytoskeleton.26 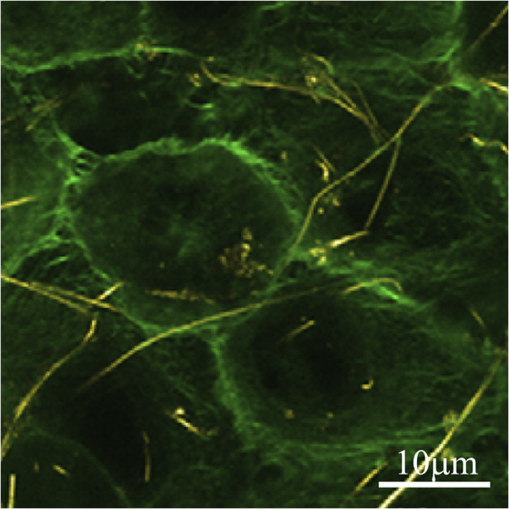 Fig. 4QD interaction with monocyte-derived dendritic cells (DC). QD655-COOH at 0.05 nM were incubated with DC for 30 min and viewed in CLSM. Note that QD can be internalized into the cell very quickly.30 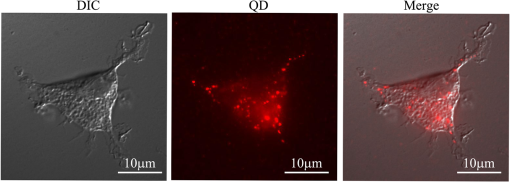 3.ConclusionIn summary, the penetration of NP into normal skin is minimal. The physicochemical parameters such as surface charge, size and functionalized surface coatings are important determinants that can modulate skin penetration. Species is also another determinant in NP penetration since the size of the keratinocytes and thickness of skin varies from species to species and with different body areas. While most studies conducted have been short-term, long-term studies are needed to evaluate skin penetration after chronic exposure. To date, there have been no reports on the adverse effects with NP on human skin in vivo. However, CLSM is an important tool to enhance the localization, visualization and penetration of NP in skin. In the next decade, CLSM in combination with other new noninvasive systems (metal detection, crystallization characterization system, etc.) might be a trend for nanomedicine to detect NP in the skin. ReferencesC. CremerT. Cremer,
“Considerations on a laser-scanning-microscope with high resolution and depth of field,”
Microsc. Acta., 81
(1), 31
–44
(1978). Google Scholar
A. ScheyniusP. Lundahl,
“Three-dimensional visualization of human Langerhans’ cells using confocal scanning laser microscopy,”
Arch. Dermatol. Res., 281
(8), 521
–525
(1990). http://dx.doi.org/10.1007/BF00412737 ADREDL 0340-3696 Google Scholar
S. Lange-Asschenfeldtet al.,
“Applicability of confocal laser scanning microscopy for evaluation and monitoring of cutaneous wound healing,”
J. Biomed. Opt., 17
(7), 076016
(2012). http://dx.doi.org/10.1117/1.JBO.17.7.076016 JBOPFO 1083-3668 Google Scholar
S. G. Lagarrigueet al.,
“In vivo quantification of epidermis pigmentation and dermis papilla density with reflectance confocal microscopy: variations with age and skin phototype,”
Exp. Dermatol., 21
(4), 281
–286
(2012). http://dx.doi.org/10.1111/exd.2012.21.issue-4 EXDEEY 0906-6705 Google Scholar
S. Puschmannet al.,
“Approach to quantify human dermal skin aging using multiphoton laser scanning microscopy,”
J. Biomed. Opt., 17
(3), 036005
(2012). http://dx.doi.org/10.1117/1.JBO.17.3.036005 JBOPFO 1083-3668 Google Scholar
A. V. FaillaA. J. Meixner,
“Confocal imaging of single metallic nanoparticles. Confocal microscopy in combination with higher-order laser modes can detect and distinguish individual metallic nanoparticles from a single scattering image,”
(2006) http://spie.org/x8588.xml Google Scholar
J. Shimet al.,
“Transdermal delivery of minoxidil with block copolymer nanoparticles,”
J. Control. Release, 97
(3), 477
–484
(2004). http://dx.doi.org/10.1016/j.jconrel.2004.03.028 JCREEC 0168-3659 Google Scholar
R. Alvarez-Románet al.,
“Skin penetration and distribution of polymeric nanoparticles,”
J. Control. Release, 99
(1), 53
–62
(2004). http://dx.doi.org/10.1016/j.jconrel.2004.06.015 JCREEC 0168-3659 Google Scholar
G. Sonavaneet al.,
“In vitro permeation of gold nanoparticles through rat skin and rat intestine: effect of particle size,”
Colloids Surf. B. Biointerfaces, 65
(1), 1
–10
(2008). http://dx.doi.org/10.1016/j.colsurfb.2008.02.013 CSBBEQ 0927-7765 Google Scholar
J. E. RiviereN. A. Monteiro-RiviereA. O. Inman,
“Determination of lidocaine concentrations in skin after transdermal iontophoresis: effects of vasoactive drugs,”
Pharm. Res., 9
(2), 211
–214
(1992). http://dx.doi.org/10.1023/A:1018985323001 PHREEB 0724-8741 Google Scholar
N. A. Monteiro-Riviereet al.,
“Topical penetration of piroxicam is dependent on the distribution of the local cutaneous vasculature,”
Pharm. Res., 10
(9), 1326
–1331
(1993). http://dx.doi.org/10.1023/A:1018973814456 PHREEB 0724-8741 Google Scholar
J. P. Ryman-RasmussenJ. E. RiviereN. A. Monteiro-Riviere,
“Penetration of intact skin by quantum dots with diverse physicochemical properties,”
Toxicol. Sci., 91
(1), 159
–165
(2006). http://dx.doi.org/10.1093/toxsci/kfj122 TOSCF2 1096-0929 Google Scholar
J. G. Rouseet al.,
“Effects of mechanical flexion on the penetration of fullerene amino acid-derivatized peptide nanoparticles through skin,”
Nano Lett., 7
(1), 155
–160
(2007). http://dx.doi.org/10.1021/nl062464m NALEFD 1530-6984 Google Scholar
L. W. ZhangN. A. Monteiro-Riviere,
“Assessment of quantum dot penetration into intact, tape-stripped, abraded and flexed rat skin,”
Skin Pharmacol. Physiol., 21
(3), 166
–180
(2008). http://dx.doi.org/10.1159/000131080 SPPKE6 1660-5527 Google Scholar
L. W. Zhanget al.,
“Biological interactions of quantum dot nanoparticles in skin and in human epidermal keratinocytes,”
Toxicol. Appl. Pharmacol., 228
(2), 200
–211
(2008). http://dx.doi.org/10.1016/j.taap.2007.12.022 TXAPA9 0041-008X Google Scholar
T. W. Prowet al.,
“Quantum dot penetration into viable human skin,”
Nanotoxicology, 6
(2), 173
–185
(2012). http://dx.doi.org/10.3109/17435390.2011.569092 1743-5390 Google Scholar
N. A. Monteiro-RiviereB. Baroli,
“Nanomaterial penetration,”
Toxicology of the Skin-Target Organ Series, 29 333
–346 Informa Healthcare, New York, NY
(2010). Google Scholar
N. A. Monteiro-RiviereF. Larese Filon,
“Effects of engineered nanomaterials on skin,”
Adverse Effects of Engineered Nanomaterials, 185
–207 Elsevier, NY
(2012). Google Scholar
X. Michaletet al.,
“Single-quantum dot imaging with a photon counting camera,”
Curr. Pharm. Biotechnol., 10
(5), 543
–58
(2009). http://dx.doi.org/10.2174/138920109788922100 CPBUBP 1389-2010 Google Scholar
W. W. Yuet al.,
“Forming biocompatible and nonaggregated nanocrystals in water using amphiphilic polymers,”
J. Am. Chem. Soc., 129
(10), 2871
–2879
(2007). http://dx.doi.org/10.1021/ja067184n JACSAT 0002-7863 Google Scholar
Y. Yanget al.,
“Effect of size, surface charge, and hydrophobicity of poly(amidoamine) dendrimers on their skin penetration,”
Biomacromolecules, 13
(7), 2154
–2162
(2012). http://dx.doi.org/10.1021/bm300545b BOMAF6 1525-7797 Google Scholar
S. KezicJ. B. Nielsen,
“Absorption of chemicals through compromised skin,”
Int. Arch. Occup. Environ. Health., 82
(6), 677
–688
(2009). http://dx.doi.org/10.1007/s00420-009-0405-x IAEHDW Google Scholar
N. A. Monteiro-RiviereA. O. Inman,
“Evaluation of quantum dot nanoparticle penetration in human skin,”
in The Toxicologist CD-An Official Journal of the Society of Toxicology,
211
(2008). Google Scholar
H. A. Leeet al.,
“Biodistribution of quantum dot nanoparticles in perfused skin: evidence of coating dependency and periodicity in arterial extraction,”
Nano Lett., 7
(9), 2865
–2870
(2007). http://dx.doi.org/10.1021/nl071563c NALEFD 1530-6984 Google Scholar
L. W. ZhangN. A. Monteiro-Riviere,
“Mechanisms of quantum dot nanoparticle cellular uptake,”
Toxicol. Sci., 110
(1), 138
–155
(2009). http://dx.doi.org/10.1093/toxsci/kfp087 TOSCF2 1096-0929 Google Scholar
L. W. ZhangN. A. Monteiro-Riviere,
“Lectins modulate multi-walled carbon nanotubes cellular uptake in human epidermal keratinocytes,”
Toxicol. In Vitro, 24
(2), 546
–551
(2010). http://dx.doi.org/10.1016/j.tiv.2009.11.007 TIVIEQ Google Scholar
J. P. Ryman-RasmussenJ. E. RiviereN. A. Monteiro-Riviere,
“Surface coatings determine cytotoxicity, and irritation potential of quantum dot nanoparticles in epidermal keratinocytes,”
J. Invest. Dermat., 127
(1), 143
–153
(2006). http://dx.doi.org/10.1038/sj.jid.5700508 JIDEAE 0022-202X Google Scholar
J. P. Ryman-RasmussenJ. E. RiviereN. A. Monteiro-Riviere,
“Variables influencing interactions of untargeted quantum dot nanoparticles with skin cells and identification of biochemical modulators,”
Nano Lett., 7
(5), 1344
–1348
(2007). http://dx.doi.org/10.1021/nl070375j NALEFD 1530-6984 Google Scholar
L. W. Zhanget al.,
“Endocytic mechanisms and toxicity of a functionalized fullerene in human cells,”
Toxicol. Lett., 191
(2–3), 149
–157
(2009). http://dx.doi.org/10.1016/j.toxlet.2009.08.017 TOLED5 0378-4274 Google Scholar
L. W. ZhangW. BaumerN. A. Monteiro-Riviere,
“Cellular uptake mechanisms and toxicity of quantum dots in dendritic cells,”
Nanomedicine., 6
(5), 777
–791
(2011). http://dx.doi.org/10.2217/nnm.11.73 1743-5889 Google Scholar
|

