|
|
|
In fluorescence tomography (FT), two parameters can be spatially resolved: fluorescence concentration and lifetime.1,2 The fluorescence concentration depends on the accumulation of fluorescent contrast agents that provide the location and molecular status of the targeted tissue. Fluorescence lifetime, on the other hand, depends on environmentally induced physiological changes such as local pH, blood supply, and temperature. These microenvironmental factors have been shown to vary with tumor growth and metastasis.3 In recent years, there has been an increasing interest in developing fluorescent probes whose lifetime differs in diseased tissue.4,5 For example, Ardeshirpour et al. recently showed that the fluorescence lifetime could be used to detect the binding of targeted optical probes to the extracellular receptors on tumor cells in vivo.6 Since both fluorescence parameters are essential, an ideal FT system should be able to resolve both parameters. Currently, most FT systems utilize steady-state measurements, which can only provide fluorescence concentration alone.7 However, time-resolved measurements, such as frequency domain (FD) or time domain (TD), are required to resolve the fluorescence lifetime parameter. Consequently, various frequency- and time-domain systems have recently been developed.8–17 The recovered fluorescence parameters by stand-alone FT system, however, are highly dependent on the size and depth of the object. This is mainly due to the ill-posed inverse problem as a result of strong photon scattering in turbid media.18,19 To overcome this limitation, structural a priori information from another high-resolution anatomical imaging modality has been used to guide and constrain the reconstruction algorithm. In fact, a similar approach has also been applied to improve the performance of nuclear imaging, which is also a low-resolution functional/molecular imaging modality. Examples of this method can be found in combined positron emission tomography (PET) and x-ray CT systems, hybrid PET-MRI, as well as MRI compatible single-photon computed tomography (SPECT) systems.20–23 Extensive effort has been spent to integrate diffuse optical tomography (DOT) with other imaging modalities to obtain accurate optical property maps of the tissue under investigation.24,25 Meanwhile, FT systems have also been integrated with x-ray CT as well as MRI systems to recover accurate fluorescence concentration images.18,26–31 However, most of these hybrid systems use steady-state measurements except for the recent ones developed by Tichauer et al. and Darne et al., which integrated FT with x-ray CT.30,31 Our group recently developed a first-of-its-kind hybrid FT-DOT-MRI system that could provide quantitative fluorescence concentration and lifetime maps and confirmed its performance with phantom studies.32 In this letter, we present first in vivo results using this hybrid system. In addition to the use of structural a priori information, background optical heterogeneity correction is also essential for quantitative FT. Two methods are commonly used for this purpose: Born normalization and utilizing DOT functional a priori information. Born normalization is an approximate method for background optical correction.33 In this method, the fluorescence measurements are normalized to the corresponding excitation measurements. The data is then processed as if the optical background is homogeneous. An alternative method is implementing DOT functional a priori information.34,35 In this case, optical properties, absorption, and scattering coefficients are reconstructed from DOT measurements at both excitation and emission wavelengths. The reconstructed background optical property maps are then used as the functional a priori information in order to rigorously correct the fluorescence light propagation at both the excitation and emission wavelengths. In this paper, these two background heterogeneity correction methods are also compared. 1.MethodA picture of the combined FT-DOT-MRI interface is shown in Fig. 1(a). Optical fibers transmitted light to and from the optic interface located in the MRI bore. The optical interface had eight source and eight detector positions with radial adjustable holders. A 16-leg birdcage coil was built into the interface for transmitting and receiving RF signals. MRI and optical measurements were obtained simultaneously with this hybrid system. Fig. 1(a) Optic fiber interface integrated with a birdcage MRI coil; (b) the schematic diagram of the frequency-domain FT system. 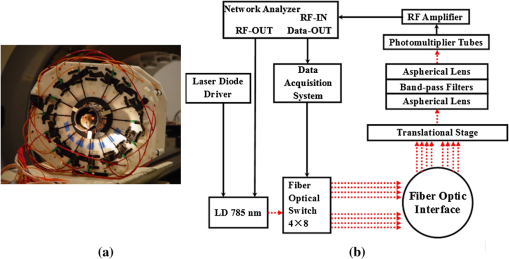 An FD photomultiplier tube-based FT system was used for the experiments [Fig. 1(b)]. The details of the instrumentation were previously given by Lin et al.32 A network analyzer was used to provide the RF signal for the laser diode amplitude modulation as well as measure the amplitude and phase of the detected fluorescence-emission signals. The fluorophore ICG (IC-Green, Akorn, Inc.) was excited by a 785-nm laser diode. Two cascaded bandpass filters (830 nm, MK Photonics, Albuquerque, New Mexico) were used to eliminate the excitation light. The optical properties of the animal were obtained through DOT measurements at the excitation wavelength. Since the emission and excitation wavelength were close to each other and on the opposite sides of the isobetic point (800 nm) for oxy- and deoxy-hemoglobin, the properties were assumed to be the same at the emission wavelength. The DOT measurements were obtained using our fully automatic FD system reported earlier by Gulsen et al.36 In this study, however, the detector fibers were sequentially connected to the FT and DOT systems that operated at a modulation frequency of 100 MHz, using a computer controlled translation stage. For optical data analysis, the FD diffusion equation was used to model light propagation in tissue. A Levenberg-Marquardt nonlinear optimization algorithm was used for reconstructing both fluorescence concentration and lifetime. When structural a priori information from the MRI was available, Laplacian-type soft a priori was utilized to guide and constrain the FT reconstruction algorithm. More details on the mathematical framework are described in our previous studies.37 For the MRI data acquisition, a 4T system was used to acquire high-resolution spin-echo T1-weighted MR images. The MR acquisition parameters were 300-ms repetition time, 14-ms echo time, 90-deg flip-angle, 120-mm field of view, 4-mm slice-thickness, and matrix size. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of California, Irvine. Transparent thin-wall NMR tubes were implanted in a six-week-old Fischer rat. The tube had an inner diameter of 4.2 mm and a wall thickness of 0.3 mm. 1% Intralipid and 669 nM ICG were added as the scatterer and fluorophore, respectively. The rat was anesthetized using combination of ketamine and xylazine, and positioned on a custom-made holder after the surgical placement of the tube. The tube was placed deep inside the abdominal cavity. The MRI image showed that the tube was 15 mm under the skin (Fig. 2). This was a very difficult case due to the position of the inclusion, which was located deep in heterogeneous tissue. Fig. 2The results for different combinations of functional a priori information. The MRI image of the animal is shown at the top of the figure. The ICG inclusion is indicated by the arrow and can be identified as the dark circle in the MR image. The reconstructed images for each case, with and without MR a priori information is provided. 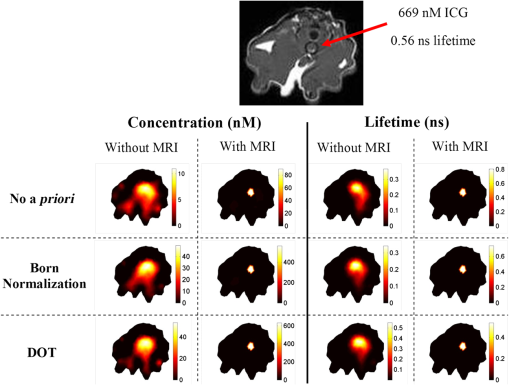 The image co-registration procedure is critical to utilize MRI for anatomical guidance during the FT reconstruction. Fiducial markers made from agar powder were used for accurate co-registration of the MRI and optical images. The exterior boundary of the animal was obtained from MRI images and used to generate the finite element mesh for optical analysis as described in our earlier studies.32 Following the generation of the FEM mesh, FT reconstructions were performed with three different combinations of functional a priori. Two different methods of optical background heterogeneity correction were compared: Born normalization method and reconstructed DOT optical maps. The details of the three cases are summarized in Table 1. Table 1Three different combinations of functional a priori information.
For each case, the fluorescence parameters were reconstructed with and without MRI structural a priori information. When MRI a priori information was used, the fluorophore location obtained from the MR image was utilized to guide and constrain the FT reconstruction. The results obtained from these three cases were compared to demonstrate the benefit of using DOT functional and/or MRI anatomical a priori information for obtaining quantitative FT reconstruction results. 2.Results and DiscussionThe MRI image of the animal is shown in Fig. 2 and the location of the tube filled with the fluorophore is indicated by the red arrow. The results of the reconstructed concentration and lifetime maps for all cases are shown in Fig. 2, and the recovered ICG values are listed in Table 2. As shown in the first row of Fig. 2, the ICG inclusion can be clearly located in the reconstructed concentration and lifetime maps using FT data. However, the recovered ICG concentration and lifetime has 98% and 52% error, respectively. Even when structural a priori information from MRI is utilized, the recovered ICG concentration and lifetime still has 87% and 45% error, respectively. When the Born normalization is used to correct for background optical heterogeneity, the recovered ICG concentration error is reduced to 94%, while the recovered ICG lifetime maintains 55% error. On the other hand, when structural a priori information from the MRI is used, the error is dramatically reduced to 16% and 30% for recovered concentration and lifetime, respectively. When background optical heterogeneity is corrected using the absorption and reduced scattering coefficient maps obtained from the DOT measurements, error in the recovered parameters further reduced to 94% and 27%. Finally, when both MRI structural and DOT functional a priori information are used together, the error in the recovered ICG concentration and lifetime are both reduced to 5%. Table 2The recovered ICG concentration and lifetime for the animal study.
In conclusion, a hybrid frequency domain FT-DOT-MRI small animal system was evaluated in vivo. A 4.2-mm fluorescence inclusion embedded deep inside a rat was accurately recovered using this system. Without MRI structural a priori information, the recovered ICG concentration and lifetime maps are only qualitative. Both ICG concentration and lifetime can be accurately recovered in a heterogeneous medium using both DOT functional a priori information and MRI structural a priori information. It has been demonstrated that a stand-alone FT system cannot recover fluorescence parameters accurately. Several studies have shown the recovered fluorophore concentration is highly dependent on the sizes and depth of the inclusion.18,19 To overcome this limitation, much effort has been spent to integrate FT with XCT or MRI to incorporate structural a priori information for FT reconstruction guidance. To the best of our knowledge, all current FT-MRI systems operate in the continuous wave mode. These systems are not capable of resolving fluorophore lifetime parameters. In order to obtain both fluorescence parameters accurately, we built a first-of-its-kind FD hybrid FT-MRI system that can resolve both fluorophore concentration and lifetime accurately. Furthermore, two background-optical-heterogeneity correction methods are compared in this study. The Born normalization method is more straightforward and simpler to implement, and already used in many FT studies. However, background optical heterogeneity correction using recovered optical property map from DOT is demonstrated to provide much better accuracy than the Born normalization method.38 Our study also confirms that better quantitative accuracy is achieved when the absorption and scattering maps of the medium are reconstructed from DOT and used in FT reconstruction. Perhaps in most small animal imaging applications, the Born normalization method is sufficient to correct background optical heterogeneity in FT reconstruction. However, this is not the case for breast imaging due to large tissue volume. In breast imaging, multi-wavelength DOT measurements are taken for tissue chromophore reconstruction to extract physiologically relevant parameters such as the total hemoglobin concentration, oxygen saturation, water concentration, and fat content. The FT measurements can be taken in addition to DOT measurements to provide complementary information about the fluorescence contrast agents or targeted molecular probes. In such a case, functional a priori information from DOT reconstruction should be used for quantitative imaging. In our current setting, the DOT measurements need to be acquired using a separate system. This is not only inconvenient but also greatly increases the data acquisition time. The next step is to integrate the fluorescence detection unit to the DOT system so that the high-speed DOT and FT measurements can be taken with the same instrument. AcknowledgmentsThis research is supported in part by National Institutes of Health (NIH) grants R01EB008716, R21/33 CA120175, R21 CA121568, P30CA062203 and Susan Komen for the Cure Foundation grant KG101442. We also want to express our thanks to Lena Zhang for her outstanding help in animal studies. ReferencesM. S. PattersonB. W. Pogue,
“Mathematical model for time-resolved and frequency-domain fluorescence spectroscopy in biological tissues,”
Appl. Opt., 33
(10), 1963
–1974
(1994). http://dx.doi.org/10.1364/AO.33.001963 APOPAI 0003-6935 Google Scholar
D. Y. Paithankaret al.,
“Imaging of fluorescent yield and lifetime from multiply scattered light reemitted from random media,”
Appl. Opt., 36
(10), 2260
–2272
(1997). http://dx.doi.org/10.1364/AO.36.002260 APOPAI 0003-6935 Google Scholar
M. Y. Berezinet al.,
“Near infrared dyes as lifetime solvatochromic probes for micropolarity measurements of biological systems,”
Biophys. J., 93
(8), 2892
–2899
(2007). http://dx.doi.org/10.1529/biophysj.107.111609 BIOJAU 0006-3495 Google Scholar
R. Alfordet al.,
“Fluorescence lifetime imaging of activatable target specific molecular probes,”
Contrast Media Mol. Imag., 5
(1), 1
–8
(2010). http://dx.doi.org/10.1002/cmmi.360 1555-4317 Google Scholar
T. Nakajimaet al.,
“Real-time monitoring of in vivo acute necrotic cancer cell death induced by near infrared photoimmunotherapy using fluorescence lifetime imaging,”
Cancer Res., 72
(18), 4622
–4628
(2012). http://dx.doi.org/10.1158/0008-5472.CAN-12-1298 CNREA8 0008-5472 Google Scholar
Y. Ardeshirpouret al.,
“In vivo fluorescence lifetime imaging monitors binding of specific probes to cancer biomarkers,”
PloS One, 7
(2), e31881
(2012). http://dx.doi.org/10.1371/journal.pone.0031881 1932-6203 Google Scholar
F. Leblondet al.,
“Pre-clinical whole-body fluorescence imaging: review of instruments, methods and applications,”
J. Photochem. Photobiol. Biol., 98
(1), 77
–94
(2010). http://dx.doi.org/10.1016/j.jphotobiol.2009.11.007 JPPBEG 1011-1344 Google Scholar
V. Y. Solovievet al.,
“Fluorescence lifetime imaging by using time-gated data acquisition,”
Appl Opt., 46
(30), 7384
–7391
(2007). http://dx.doi.org/10.1364/AO.46.007384 APOPAI 0003-6935 Google Scholar
A. Mayet al.,
“Whole-body, real-time preclinical imaging of quantum dot fluorescence with time-gated detection,”
J. Biomed. Opt., 14
(6), 060504
(2009). http://dx.doi.org/10.1117/1.3269675 JBOPFO 1083-3668 Google Scholar
A. T. N. Kumaret al.,
“A time domain fluorescence tomography system for small animal imaging,”
IEEE Trans. Med. Imag., 27
(8), 1152
–1163
(2008). http://dx.doi.org/10.1109/TMI.2008.918341 ITMID4 0278-0062 Google Scholar
A. GodavartyE. M. Sevick-MuracaM. J. Eppstein,
“Three-dimensional fluorescence lifetime tomography,”
Med. Phys., 32
(4), 992
–1000
(2005). http://dx.doi.org/10.1118/1.1861160 MPHYA6 0094-2405 Google Scholar
J. McGintyet al.,
“In vivo fluorescence lifetime tomography of a FRET probe expressed in mouse,”
Biomed. Opt. Express, 2
(7), 1907
–1917
(2011). http://dx.doi.org/10.1364/BOE.2.001907 BOEICL 2156-7085 Google Scholar
J. ChenV. VenugopalX. Intes,
“Monte Carlo based method for fluorescence tomographic imaging with lifetime multiplexing using time gates,”
Biomed. Opt. Express, 2
(4), 871
–886
(2011). http://dx.doi.org/10.1364/BOE.2.000871 BOEICL 2156-7085 Google Scholar
R. E. Nothdurftet al.,
“In vivo fluorescence lifetime tomography,”
J. Biomed. Opt., 14
(2), 024004
(2009). http://dx.doi.org/10.1117/1.3086607 JBOPFO 1083-3668 Google Scholar
A. Laidevantet al.,
“Fluorescence time-resolved imaging system embedded in an ultrasound prostate probe,”
Biomed. Opt. Express, 2
(1), 194
–206
(2011). http://dx.doi.org/10.1364/BOE.2.000194 BOEICL 2156-7085 Google Scholar
B. MontcelP. Poulet,
“An instrument for small-animal imaging using time-resolved diffuse and fluorescence optical methods,”
Nucl. Instrum. Meth. Phys. Res. Sec., 569
(2), 551
–556
(2006). http://dx.doi.org/10.1016/j.nima.2006.08.133 0168-9002 Google Scholar
V. Venugopalet al.,
“Full-field time-resolved fluorescence tomography of small animals,”
Opt. Lett., 35
(19), 3189
–3191
(2010). http://dx.doi.org/10.1364/OL.35.003189 OPLEDP 0146-9592 Google Scholar
Y. Linet al.,
“Quantitative fluorescence tomography using a combined tri-modality FT/DOT/XCT system,”
Opt. Express, 18
(8), 7835
–7850
(2010). http://dx.doi.org/10.1364/OE.18.007835 OPEXFF 1094-4087 Google Scholar
D. S. Kepshireet al.,
“Subsurface diffuse optical tomography can localize absorber and fluorescent objects but recovered image sensitivity is nonlinear with depth,”
Appl. Opt., 46
(10), 1669
–1678
(2007). http://dx.doi.org/10.1364/AO.46.001669 APOPAI 0003-6935 Google Scholar
S. BasuA. Alavi,
“Revolutionary impact of PET and PET-CT on the day-to-day practice of medicine and its great potential for improving future health care,”
Nucl. Med. Rev. Cent. East. Eur., 12
(1), 1
–13
(2009). Google Scholar
M. S. Judenhoferet al.,
“Simultaneous PET-MRI: a new approach for functional and morphological imaging,”
Nat. Med., 14
(4), 459
–465
(2008). http://dx.doi.org/10.1038/nm1700 1078-8956 Google Scholar
J. H. Market al.,
“Development of an MR-compatible SPECT system (MRSPECT) for simultaneous data acquisition,”
Phys. Med. Biol., 55
(6), 1563
–1575
(2010). http://dx.doi.org/10.1088/0031-9155/55/6/002 PHMBA7 0031-9155 Google Scholar
H. Seunghoonet al.,
“Development of a new RF coil and -ray radiation shielding assembly for improved MR image quality in SPECT/MRI,”
Phys. Med. Biol., 55
(9), 2495
–2504
(2010). http://dx.doi.org/10.1088/0031-9155/55/9/005 PHMBA7 0031-9155 Google Scholar
B. Brooksbyet al.,
“Magnetic resonance-guided near-infrared tomography of the breast,”
Rev. Sci. Instrum., 75
(12), 5262
–5270
(2004). http://dx.doi.org/10.1063/1.1819634 RSINAK 0034-6748 Google Scholar
A. P GibsonJ. C. HebdenS. R. Arridge,
“Recent advances in diffuse optical imaging,”
Phys. Med. Biol., 50
(4), R1
–R43
(2005). http://dx.doi.org/10.1088/0031-9155/50/4/R01 PHMBA7 0031-9155 Google Scholar
D. Kepshireet al.,
“A microcomputed tomography guided fluorescence tomography system for small animal molecular imaging,”
Rev. Sci. Instrum., 80
(4), 043701
(2009). http://dx.doi.org/10.1063/1.3109903 RSINAK 0034-6748 Google Scholar
S. C. Daviset al.,
“Magnetic resonance-coupled fluorescence tomography scanner for molecular imaging of tissue,”
Rev. Sci. Instrum., 79
(6), 064302
(2008). http://dx.doi.org/10.1063/1.2919131 RSINAK 0034-6748 Google Scholar
A. Aleet al.,
“Imaging performance of a hybrid x-ray computed tomography-fluorescence molecular tomography system using priors,”
Med. Phys., 37
(5), 1976
–1986
(2010). http://dx.doi.org/10.1118/1.3368603 MPHYA6 0094-2405 Google Scholar
A. Aleet al.,
“FMT-XCT: in vivo animal studies with hybrid fluorescence molecular tomography-x-ray computed tomography,”
Nat. Methods, 9
(6), 615
–620
(2012). http://dx.doi.org/10.1038/nmeth.2014 1548-7091 Google Scholar
K. M. Tichaueret al.,
“Imaging workflow and calibration for CT-guided time-domain fluorescence tomography,”
Biomed. Opt. Express, 2
(11), 3021
–3036
(2011). http://dx.doi.org/10.1364/BOE.2.003021 BOEICL 2156-7085 Google Scholar
C. D. Darneet al.,
“Hybrid PET/CT and frequency-domain based NIRF optical tomography modality for preclinical studies,”
in Biomedical Optics Conf., Biomed Poster Session II (BTu3A),
BTu3A.70
(2012). Google Scholar
Y. Linet al.,
“A photo-multiplier tube-based hybrid MRI and frequency domain fluorescence tomography system for small animal imaging,”
Phys. Med. Biol., 56
(15), 4731
–4747
(2011). http://dx.doi.org/10.1088/0031-9155/56/15/007 PHMBA7 0031-9155 Google Scholar
V. NtziachristosR. Weissleder,
“Experimental three-dimensional fluorescence reconstruction of diffuse media by use of a normalized Born approximation,”
Opt. Lett., 26
(12), 893
–895
(2001). http://dx.doi.org/10.1364/OL.26.000893 OPLEDP 0146-9592 Google Scholar
Y. Linet al.,
“Fluorescence diffuse optical tomography with functional and anatomical a priori information: feasibility study,”
Phys. Med. Biol., 52
(18), 5569
–5585
(2007). http://dx.doi.org/10.1088/0031-9155/52/18/007 PHMBA7 0031-9155 Google Scholar
Y. Linet al.,
“Quantitative fluorescence tomography with functional and structural a priori information,”
Appl. Opt., 48
(7), 1328
–1336
(2009). http://dx.doi.org/10.1364/AO.48.001328 APOPAI 0003-6935 Google Scholar
G. Gulsenet al.,
“Combined diffuse optical tomography (DOT) and MRI system for cancer imaging in small animals,”
Technol. Cancer. Res. Treat., 5
(4), 351
–363
(2006). 1533-0346 Google Scholar
Y. Linet al.,
“Quantitative fluorescence tomography with functional and structural a priori information,”
Appl. Opt., 48
(7), 1328
–1336
(2009). http://dx.doi.org/10.1364/AO.48.001328 APOPAI 0003-6935 Google Scholar
L. Hervéet al.,
“Noncontact fluorescence diffuse optical tomography of heterogeneous media,”
Appl. Opt., 46
(22), 4896
–4906
(2007). http://dx.doi.org/10.1364/AO.46.004896 APOPAI 0003-6935 Google Scholar
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

