|
|
|
The emission of Cerenkov radiation (CR) is a well-known relativistic effect that takes place when a charged particle travels in a dielectric medium with a velocity greater than the speed of light in the medium. The in vivo detection of CR is an emerging optical preclinical modality1,2 called Cerenkov luminescence imaging (CLI); more precisely, CLI is based on the detection of CR using radiopharmaceuticals labeled with beta plus1–4 and beta minus5,6 emitters. An overview of the different applications of CLI can be found in Ref. 7. Until now CLI has been applied only to small animal imaging; in this short communication we present the first in vivo application to a human patient. Human Cerenkov imaging is a challenging problem compared to small animals because of the patient size, organ depth, and low radiopharmaceutical activity concentration in the tissue. All these factors make the detection of the faint Cerenkov light a difficult task to achieve. As a proof of principle, we focused our attention in detecting CR from the thyroid gland of a patient treated orally for hyperthyroidism with 550 MBq of I–131. The main beta decay mode (89%) for I–131 is the emission of a beta minus particle with an end-point energy equal to 606 keV. The imaging of an animal thyroid using CLI was introduced in Ref. 8, injecting a mouse with I–124 and I–131; in this case the authors showed a good correlation with PET and SPECT imaging. In order to detect Cerenkov photons, we used a cooled () electron multiplied charge coupled device (EMCCD) (Photometrics, Evolve 512) coupled with a conventional , 8 mm C-mount lens (Edmund Optics, LV 0814). The EMCCD has a back illuminated sensor with pixel size equal to 16 μm and a peak quantum efficiency of 90%. The images were acquired using binning=1, corresponding to a matrix size of and the gain of the EMCCD was set to 400. All the Cerenkov images were acquired using 2 min of exposure time. Because of the direct interaction of gamma rays with the EMCCD sensor, a sparse spike noise pattern is generated during the Cerenkov image acquisition. The noise signal has been partially removed by applying a box median filter () on the CLI data. The EMCCD was placed inside a light tight dark room in order to avoid any visible light signal cross-contamination. More precisely, all the Cerenkov image acquisitions were performed in a hospital basement room with no windows. The neon lamps were removed and the entire door covered by a thick black curtain. The patient too has been surrounded by thick black curtains in order to stop any possible residue of external light. In order to investigate the absence of any external light cross-contamination in the room, a set of 5 min images with objects (phantom or human person) without any Cerenkov sources were acquired. In this case, no light signal was detectable by the EMCCD; the mean value of the dark image was 1300 arbitrary units (AU) and the standard deviation was 350 AU. The dark image has been subtracted from the Cerenkov image. The object to be imaged was placed at a distance equal to 50 cm from the EMCCD. Before acquiring Cerenkov images of patients, the system set-up has been tested using a syringe filled with physiologic solution containing 2-[18F]fluoro-2-deoxy-D-glucose (FDG). The syringe was filled with 80 MBq of FDG in order to take into account the 30% to 40% thyroid uptake at 24 h and also that the emitted Cerenkov light of F-18 is 2.5 higher than I–131 as shown in Ref. 4. The dimensions of the syringe active FDG region were equal to: . The EMCCD gain has been chosen by acquiring different images of the chicken phantom with different gain values. We found that a gain of 400 (range 0 to 1000) is a good compromise between signal and number of striking artifacts due to direct gamma interactions with the CCD. The syringe has been covered with a 1 cm slice of chicken breast in order to simulate optical photons attenuation due to the tissues. In order to provide a simple anatomical reference, a photographic image was always acquired before any Cerenkov image. In this case, the gain was set to 0, the exposure time was 0.5 s, the matrix dimensions were , and the object was illumined by a LED lamp. The matrices of both photographic and Cerenkov images were the same, and since nothing was moved between the two acquisitions, the two images are intrinsically co-registered. Figure 1(a) shows a Cerenkov image of the FDG syringe without chicken phantom, and Fig. 1(b) shows the image obtained by placing a 1 cm slice of chicken breast on the syringe. As one can see, there is good localization between the CR emission escaping the chicken breast and the syringe containing FDG. This is an encouraging result considering the non-negligible thickness of the chicken breast traveled by the Cerenkov photons. The signal-to-noise ratio was, respectively equal to 13.1 and 3.3 with and without median filtering. Fig. 1(a) A Cerenkov image of the FDG syringe without the chicken slice; the dimensions of the syringe active FDG region were equal to: . (b) The image obtained by placing a 1 cm slice of chicken breast on the syringe. The color scale is in arbitrary units. 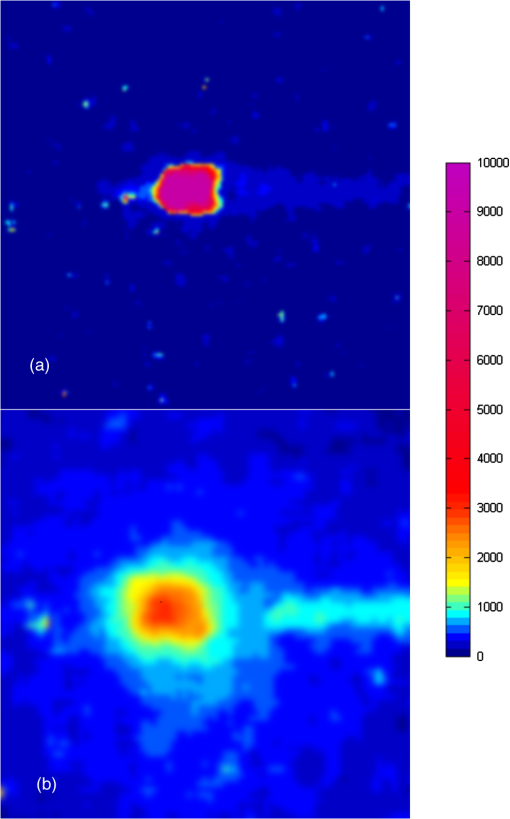 After having acquired the results presented in Fig. 1, we then imaged the thyroid region of a patient treated for hyperthyroidism with 550 MBq of I–131 after an uptake of 24 h. The patient was not fixed and, thus, any movement can introduce a kinematic blurring on the Cerenkov image. However, given that the acquisition time is comparable (or smaller) with respect to conventional gamma camera imaging performed without fixing the patient, we estimate a similar kinematic blur for the two methodologies. Figure 2(a) shows the Cerenkov image of the patient and Fig. 2(b) shows the overlay between the photographic image and the Cerenkov image. As one can see there is a good localization of the visible Cerenkov light signal within the thyroid region. The localization has been performed by a nuclear medicine physician (MF) with a long experience in thyroid imaging. Fig. 2(a) The Cerenkov image of the thyroid gland image. (b) The overlay between the photographic image (black and white) and the Cerenkov image of the patient. It is possible to notice a good correspondence between the region where CR is emitted and the thyroid. The color scale is in arbitrary units. 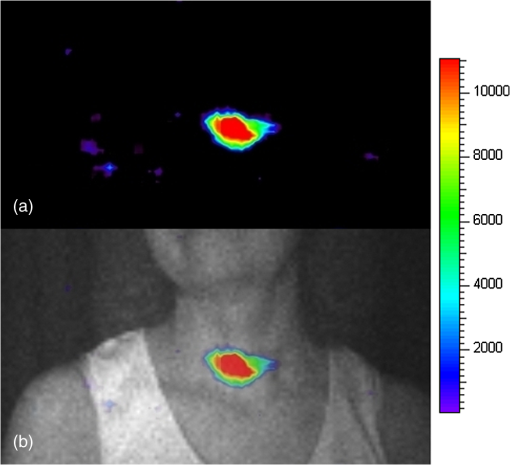 To our knowledge, this is the first experimental evidence showing that it is possible to obtain a planar image of Cerenkov photons escaping from a human tissue. We called this new imaging method Cerenkography to underline the peculiar source of visible light. The novel and interesting aspect of Cerenkography is that it provides a fast approach to image superficial organs (1 to 2 cm depth) of patients treated with beta minus radiopharmaceuticals. An interesting clinical application of Cerenkography is monitoring the iodine uptake in the thyroid in order to estimate the radiation dose given to the organ. Generally speaking, Cerenkography can also be used to monitor the radiation dose of any other therapy for superficial organs using beta emitters like Y-90 and Lu-177. Cerenkography can also be extended to the imaging of beta plus emitters. As shown also by recent simulations,9 a CCD detector optimized for the near infrared wavelength range could significantly improve the detection of CR escaping biological tissues. Considering the moderate costs of EMCCD’s with respect to the tomographs currently available in any nuclear medicine department, we believe that Cerenkography could became an interesting cost-effective medical tool in future years. AcknowledgmentsThe authors would like to acknowledge Prof. Carlo Tacchetti and Dr. Davide Mazza of the San Raffaele Centre for Experimental Imaging for providing the EMCCD used in our experiments. We also would like to acknowledge Prof. Alberto Del Guerra and Dr. Claudia Ceccarelli of Pisa University for the useful discussions. ReferencesR. Robertsonet al.,
“Optical imaging of Cerenkov light generation from positron-emitting radiotracers,”
Phys. Med. Biol., 54
(16), N355
–N365
(2009). http://dx.doi.org/10.1088/0031-9155/54/16/N01 PHMBA7 0031-9155 Google Scholar
A. E. Spinelliet al.,
“Cerenkov radiation allows in vivo optical imaging of positron emitting radiotracers,”
Phys. Med. Biol., 55
(2), 483
–495
(2010). http://dx.doi.org/10.1088/0031-9155/55/2/010 PHMBA7 0031-9155 Google Scholar
F. Boschiet al.,
“In vivo (18)F-FDG tumour uptake measurements in small animals using Cerenkov radiation,”
Eur. J. Nucl. Med., 38
(1), 120
–127
(2011). http://dx.doi.org/10.1007/s00259-010-1630-y EJNMD9 0340-6997 Google Scholar
H. Liuet al.,
“Molecular optical imaging with radioactive probes,”
PLoS One, 5
(3), e9470
(2010). http://dx.doi.org/10.1371/journal.pone.0009470 1932-6203 Google Scholar
A. E. Spinelliet al.,
“Cerenkov radiation imaging of beta emitters: in vitro and in vivo results,”
Nucl. Instrum. Meth. A, 648
(Suppl. 1), S310
–S312
(2011). http://dx.doi.org/10.1016/j.nima.2010.11.038 NIMAER 0168-9002 Google Scholar
A. E. Spinelliet al.,
“Multispectral Cerenkov luminescence tomography for small animal optical imaging,”
Opt. Express, 19
(13), 12605
–12618
(2011). http://dx.doi.org/10.1364/OE.19.012605 OPEXFF 1094-4087 Google Scholar
A. E. Spinelliet al.,
“Optical imaging using radioisotopes a novel multimodal approach to molecular imaging,”
Q. J. Nucl. Med. Mol. Imaging, 56
(3), 280
–290
(2012). Google Scholar
S. Y. Jeonget al.,
“Combined Cerenkov luminescence and nuclear imaging of radioiodine in the thyroid gland and thyroid cancer cells expressing sodium iodide symporter: initial feasibility study,”
Endocr. J., 58
(7), 575
–583
(2011). http://dx.doi.org/10.1507/endocrj.K11E-051 EDJUE6 0969-711X Google Scholar
A. E. SpinelliF. Boschi,
“Optimizing in vivo small animal Cerenkov luminescence imaging,”
J. Biomed. Opt., 17
(4), 040506
(2012). http://dx.doi.org/10.1117/1.JBO.17.4.040506 JBOPFO 1083-3668 Google Scholar
|

