|
|
1.IntroductionHarmonic generation microscopy (HGM) has emerged as an important imaging modality in biological study,1–9 especially in in vivo applications10–17 because of its nonlinear and noninvasive nature. Our preliminary in vivo clinical studies indicated that HGM can provide a sub-micron spatial resolution, low photodamage and phototoxicity, reduced dye toxicity as a result of the minimized use of external fluorophores, and low photobleaching. In human skin, third-harmonic generation (THG) microscopy can provide intrinsic contrasts in elastic fibers,17 cytoplasmic membrane,13 nucleus,13 actin filaments,18 lipid bodies,19 hemoglobin,20 and melanin.21 With the combination of THG and second-harmonic generation (SHG) processes, multi-harmonic generation microscopy (HGM) is an ideal imaging tool for morphological visualizations of human skin.21–26 Despite the visible advantages of using HGM for label-free imaging, exogenous contrast agents27–31 are still developed for a higher structural or molecular specificity in applications such as biomedical molecular imaging and cancer diagnostics. Many previous studies have reported that metal nanoparticles can be used as contrast agents to enhance THG signals through surface plasmon resonance (SPR).27–29 Using metal nanoparticles as THG contrast agents requires matching the THG or the excitation wavelength to the SPR wavelength. The effect fixes the size of the metal nanoparticles and therefore limits their THG efficiency.27 Therefore, a THG contrast agent that can be functional for a broad excitation wavelength range is highly desirable. The nonlinear THG susceptibility can be greatly enhanced when one of the transition frequencies closely matches one of the virtual transition frequencies of the THG process.32 Thus, quantum dots and hematoxylin have been used as THG contrast agents for biomolecular imaging through multiphoton resonance.30,31 CdSe quantum dots can efficiently generate THG signals 20 times stronger than fluorescence even with an epi-collection scheme,30 thus reducing the photodamage to biological specimens. However, the bio-safety of using quantum dots is noted for in vivo human imaging. In this paper, we demonstrate the potential of the commonly adopted tattoo dye as a THG contrast agent for in vivo optical virtual biopsy of human skin through multiphoton resonance enhancement. This demonstration was performed on cultured cells transfected with tattoo dyes, in tattooed mouse skin in vivo, and in tattooed human skin in vivo. Strong resonance-enhanced THG was generated from the tattoo dyes. Compared with hematoxylin,31 nanoparticles27–29 or lipid-enclosed quantum dots30 and iron oxide nanoparticles,33 tattoo dyes are widely adopted in human skin so that future clinical biocompatibility evaluation is relatively achievable. Combined with the demonstrated THG enhancement effect, tattoo dyes show their promise for future clinical imaging applications. 2.Methods and ResultsThe practice of skin tattoos has been adopted for several centuries worldwide. In the United States, tattoo dyes are subject to regulation by the U.S. Food and Drug Administration (FDA) as cosmetics and color additives.34 Various tattoo dye colors exist in the commercial market. Black dye is an essential color for tattooing because of its versatility. Many tattooists use black dye to apply the initial outline and various shades of a tattoo design to the client’s skin. In this study, we used a commercial black dye. Based on the commercial factors, the ingredients and concentrations of the tattoo dyes are not indicated. Therefore, we dissolved 5 μL of the black tattoo dye (Scream ink SI01, Tat2king Tattoo Supply Co., Ltd., China) in 5 mL of de-ionized water to measure the absorption spectrum [Fig. 1(a)]. The absorption coefficient at the wavelength of 1230 nm was approximately for the diluted tattoo dye solution. The spectrum indicated that the black tattoo dye has a broad range of absorption at the visible and near-infrared light bands. This absorption characteristic provides the single-, two-, and three-photon resonance enhancement criteria32 for the THG process as illustrated in Fig. 1(b). Therefore, we suggest that the black tattoo dye is suitable as a THG contrast agent through the multiphoton resonance enhancement. Fig. 1(a) Absorption spectrum of a black tattoo dye dissolved in deionized water. (b) Schematic of transition levels in single-, two-, and three-photon resonant enhancement of the THG process. 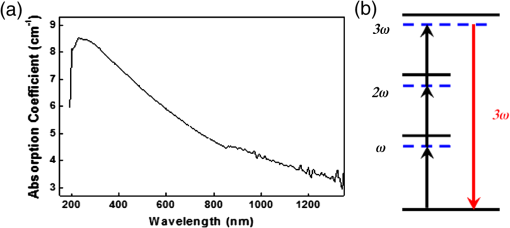 Our study used a Cr:forsterite laser with a central wavelength of 1230 nm, a 100 fs pulse width, and a repetition rate of 110 MHz as the excitation source. The average excitation power after the objective was 100 mW. The experimental setup for the in vitro experiment can be obtained in our previous study.35 Band-pass filters with different center wavelengths and bandwidths (HQ615/30 for SHG and D410/30 for THG, Chroma) were inserted before the PMTs to filter out the background noise to increase the signal-to-noise ratio. A 625 nm long pass filter (E625lp, Chroma) was used for the collection of the two-photon fluorescence (TPF). A549 cells are a well-characterized standard among the human cell lines used in molecular biology. Thus, we used lipofectamine (Invitrogen) to transfect the black tattoo dye into the A549 cells to evaluate the THG enhancement effect of the tattoo dyes in living cells. Based on the recommended protocol of the product,36 we added lipofectamine into the black tattoo dye for 20 min at room temperature. The A549 cells were cultured in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum (FBS) and 1% Penicillin and Streptomycin37 and exposed to the lipofectamine/dye mixture. Control samples consisted of A549 cells exposed to the same medium without the lipofectamine/dye mixture. The THG (purple) and fluorescence (green) images of the A549 cells with and without tattoo dyes are shown in Fig. 2. In some areas, the intensity of the THG signals in the A549 cells with the tattoo dye [Fig. 2(c) (arrows)] was at least 2.5 times stronger than that in the A549 cells without the tattoo dye [Fig. 2(a)] under the same photomultiplier tube (PMT) voltage. The strong THG intensity may have originated from the presence of the tattoo pigments in the cytoplasm of the A549 cells. From the TPF channel, we also found a weak fluorescence from the tattoo pigments in the cytoplasm of the A549 cells [Fig. 2(d) (arrows)], but no fluorescence was observed from the A549 cells without the tattoo dye [Fig. 2(b)]. These results indicate that the enhanced THG and weak TPF signals were contributed by the tattoo dye. Fig. 2Nonlinear images of the A549 cells with and without tattoo dye under the same PMT voltage. (a) THG and (b) two-photon fluorescence images of the A549 cells without tattoo dye. (c) THG and (d) two-photon fluorescence images of the A549 cell transfected with tattoo dye revealed the tattoo pigments located in cytoplasm (arrows). Scale bar: 5 μm. 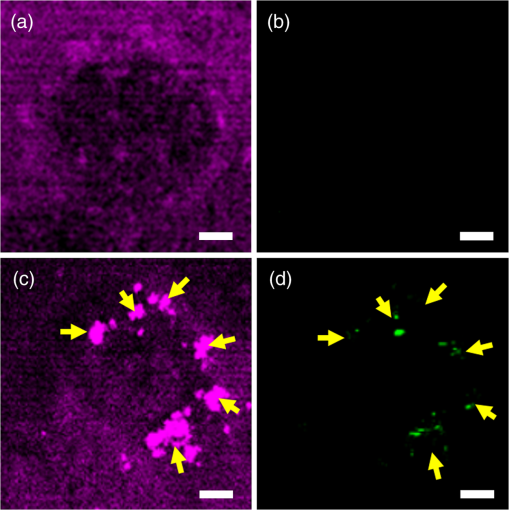 The capability of the THG enhancement in vivo was evaluated by conducting studies in animal models and human. Previous reports have indicated that tattoo pigments in human skin stain the cytoplasm of fibroblasts deep in the dermis layer.38,39 Tattoo dyes thus serve as in vivo THG contrast agents of fibroblasts in mouse and human skin in our studies. For a fair comparison, the intensities of the in vivo THG images were obtained under the same PMT voltage. The experimental setup of the mouse skin in vivo and human skin in vivo can be found in our previous reports.13,21 For animal studies, the experimental protocols were approved by the National Taiwan University Institutional Animal Care and Use Committee (NTU-IACUC). We used seven-week-old female imprinting control region mice. We tattooed the undiluted black dye to the mouse skin and performed in vivo HGM on the tattooed skin after six months. Figure 3(a) and 3(b) shows examples of the horizontally sectioned SHG (green) and THG (purple) images at a depth of 30 μm below the skin surface taken from the dermal layer of the normal and tattooed mouse skin, respectively. Compared with the normal mouse skin [Fig. 3(a)], the enhanced THG signals from the tattoo pigments [Fig. 3(b) (arrows)], which provides the contrast of fibroblasts,38,39 was revealed within the collagen network. The contrast of the collagen network was provided by the SHG signals. In addition, in vivo HGM was also performed on human tattooed skin. The image acquisition process was performed under the informed consent approved by the Research Ethics Committee of the National Taiwan University Hospital. A volunteer with black tattoo was included in this study. The safety of the in vivo HGM imaging for human skin has been demonstrated previously.21,25,26 The tattooed volunteer felt no pain nor had other unpleasant feeling during and after the experiments. The observed site was also immediately examined by a physician after in vivo observation to check for clinical adverse symptoms. No skin changes can be found on the observed sites. We do not know the content of the black tattoo dye from the tattooed volunteer. However, results similar to those from the in vivo animal study were also obtained from the horizontally sectioned HGM images of the dermal layer in the normal and tattooed skin. The HGM images of the normal skin [Fig. 3(c)] were taken from the untattooed skin adjacent to the tattooed skin at a depth of 60 μm below the skin surface. The strongly enhanced THG signals were again observed in the tattooed region in the dermis layer, providing the contrast of fibroblasts,18,19 within the collagenous network as shown in Fig. 3(d) (arrows). Fibroblasts are associated with cancer cells at all stages of cancer progression,40 and their structural and functional contributions need to be studied in detail. Our reported in vivo study thus indicates the potential of the tattoo dye as an in vivo THG contrast agent to specify the fibroblast in human and animal skin for clinical and preclinical imaging. Fig. 3The HGM images at a depth of 30 μm below the mouse skin surface (a) without and (b) with tattoo as well as at a depth of 60 μm below the human skin surface (c) without and (d) with tattoo. The THG intensity from the tattoo pigments was strongly enhanced within the collagenous network (arrows). All images were taken under the same PMT voltage. THG: purple; SHG: green. Scale bar: 20 μm. 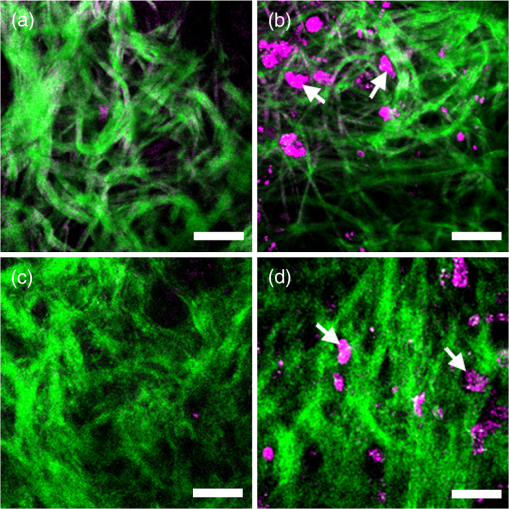 3.Discussion and ConclusionIn contrast to previously reported nanoparticles,27–29 lipid-enclosed quantum dots and iron oxides,30,33 or the hematoxylin dye31 as THG exogenous contrast agents, tattoo dye is the only exogenous contrast agent currently allowed to be applied on human skin so that the establishment of the clinical database for future biocompatibility evaluation is relatively achievable. Comparing the THG approach for visualizing exogenous dyes with other forms of nonlinear microscopies, including coherent anti-Stokes Raman scattering microscopy,41 four-wave mixing microscopy,42 and pump-probe microscopy,43 THG microscopy is a single-beam technique so that it is with a simpler system. Single-beam based technologies also allow us to position the single excitation beam at the lowest photodamage wavelength. For multiphoton fluorescence techniques, we found that tattoo dyes are with a weak fluorescence [as shown in Fig. 2(d)] and are less desirable compared with other available fluorescent dyes.44 In contrast to these nonlinear microscopies, THG is also the first imaging modality for visualizing tattoo dyes in human skin in vivo. In summary, we demonstrated the potential of tattoo dyes as a contrast agent in THG microscopy of culture cells, mouse skin in vivo and human skin in vivo. The intensity of THG can be greatly enhanced 2.5 times using black tattoo dyes in living cells. In vivo THG imaging capability was simply identified through the comparison between the images with and without tattoo in animal and human skin. After the study of its biocompatibility and modification in ligand conjugation, tattoo dyes have the potential to serve as a general THG contrast for various molecules and cells for future biological and clinical imaging applications. AcknowledgmentsThis work is sponsored by the National Health Research Institute of Taiwan (NHRI-EX101-9936EI), National Science Council (NSC-101-2120-M-002-005), and National Taiwan University Molecular Imaging Center. ReferencesD. YelinY. Silberberg,
“Laser scanning third-harmonic-generation microscopy in biology,”
Opt. Exp., 5
(8), 169
–175
(1999). http://dx.doi.org/10.1364/OE.5.000169 OPEXFF 1094-4087 Google Scholar
P. J. CampagnolaL. M. Loew,
“Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms,”
Nat. Biotechnol., 21
(11), 1356
–1360
(2003). http://dx.doi.org/10.1038/nbt894 NABIF9 1087-0156 Google Scholar
C.-K. Sunet al.,
“Higher harmonic generation microscopy for developmental biology,”
J. Struct. Biol., 147
(1), 19
–30
(2004). http://dx.doi.org/10.1016/j.jsb.2003.10.017 JSBIEM 1047-8477 Google Scholar
C.-S. Hsiehet al.,
“Higher harmonic generation microscopy of in vitro cultured mammal oocytes and embryos,”
Opt. Exp., 16
(15), 11574
–11588
(2008). OPEXFF 1094-4087 Google Scholar
J. Linet al.,
“Assessment of liver steatosis and fibrosis in rats using integrated coherent anti-Stokes Raman scattering and multiphoton imaging technique,”
J. Biomed. Opt., 16
(11), 116024
(2011). http://dx.doi.org/10.1117/1.3655353 JBOPFO 1083-3668 Google Scholar
C.-K. Sun,
“Higher harmonic generation microscopy,”
Adv. Biochem. Eng./Biotechnol., 95 17
–56
(2005). http://dx.doi.org/10.1007/b14097 ABEBDZ 0724-6145 Google Scholar
S.-Y. ChenC.-Y. S. HsuC.-K. Sun,
“Epi-third and second harmonic generation microscopic imaging of abnormal enamel,”
Opt. Exp., 16
(15), 11670
–11679
(2008). OPEXFF 1094-4087 Google Scholar
W.-J. Leeet al.,
“Virtual biopsy of rat tympanic membrane using higher harmonic generation microscopy,”
J. Biomed. Opt., 15
(4), 046012
(2010). http://dx.doi.org/10.1117/1.3469848 JBOPFO 1083-3668 Google Scholar
S.-W. Chuet al.,
“Studies of tensors in submicron-scaled bio-tissues by polarization harmonics optical microscopy,”
Biophys. J., 86
(6), 3914
–3922
(2004). http://dx.doi.org/10.1529/biophysj.103.034595 BIOJAU 0006-3495 Google Scholar
M. J. Farraret al.,
“In vivo imaging of myelin in the vertebrate central nervous system using third harmonic generation microscopy,”
Biophys. J., 100
(5), 1362
–1371
(2011). http://dx.doi.org/10.1016/j.bpj.2011.01.031 BIOJAU 0006-3495 Google Scholar
S.-Y. Chenet al.,
“Noninvasive harmonics optical microscopy for long-term observation of embryonic nervous system development in vivo,”
J. Biomed. Opt., 11
(5), 054022
(2006). http://dx.doi.org/10.1117/1.2363369 JBOPFO 1083-3668 Google Scholar
E. Brownet al.,
“Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation,”
Nat. Med., 9
(6), 796
–800
(2003). http://dx.doi.org/10.1038/nm879 1078-8956 Google Scholar
S.-P. Taiet al.,
“In vivo optical biopsy of hamster oral cavity with epi-third-harmonic-generation microscopy,”
Opt. Exp., 14
(13), 6178
–6187
(2006). http://dx.doi.org/10.1364/OE.14.006178 OPEXFF 1094-4087 Google Scholar
S.-W. Chuet al.,
“In vivo developmental biology study using noninvasive multi-harmonic generation microscopy,”
Opt. Exp., 11
(23), 3093
–3099
(2003). http://dx.doi.org/10.1364/OE.11.003093 OPEXFF 1094-4087 Google Scholar
C.-S. Hsiehet al.,
“In vivo long term continuous observation of gene expression in zebrafish embryos nerve systems by using harmonic generation microscopy and morphant technology,”
J. Biomed. Opt., 13
(6), 064041
(2008). http://dx.doi.org/10.1117/1.3050423 JBOPFO 1083-3668 Google Scholar
M.-R. Tsaiet al.,
“In vivo optical virtual biopsy of human oral mucosa with harmonic generation microscopy,”
Biomed. Opt. Express, 2
(8), 2317
–2328
(2011). http://dx.doi.org/10.1364/BOE.2.002317 BOEICL 2156-7085 Google Scholar
C.-H. Yuet al.,
“In vivo and ex vivo imaging of intra-tissue elastic fibers using third-harmonic-generation microscopy,”
Opt. Exp., 15
(18), 11167
–11177
(2007). http://dx.doi.org/10.1364/OE.15.011167 OPEXFF 1094-4087 Google Scholar
M.-R. Tsaiet al.,
“Characterization of oral squamous cell carcinoma based on higher-harmonic generation microscopy,”
J. Biophotonics, 5
(5–6), 415
–424
(2012). http://dx.doi.org/10.1002/jbio.v5.5/6 JBOIBX 1864-063X Google Scholar
D. Debarreet al.,
“Imaging lipid bodies in cells and tissues using third-harmonic generation microscopy,”
Nat. Methods, 3
(1), 47
–53
(2006). http://dx.doi.org/10.1038/nmeth813 1548-7091 Google Scholar
C.-F. ChangC.-H. YuC.-K. Sun,
“Multi-photon resonance enhancement of third harmonic generation in human oxyhemoglobin and deoxyhemoglobin,”
J. Biophotonics, 3
(10–11), 678
–658
(2010). http://dx.doi.org/10.1002/jbio.201000045 JBOIBX 1864-063X Google Scholar
S.-Y. Chenet al.,
“In vivo virtual biopsy of human skin by using noninvasive higher harmonic generation microscopy,”
IEEE J. Sel. Top. Quant. Electron., 16
(20), 478
–492
(2010). http://dx.doi.org/10.1109/JSTQE.2009.2031987 IJSQEN 1077-260X Google Scholar
C.-K. Sunet al.,
“Multiharmonic generation biopsy of skin,”
Opt. Lett., 28
(24), 2488
–2490
(2003). http://dx.doi.org/10.1364/OL.28.002488 OPLEDP 0146-9592 Google Scholar
S.-P. Taiet al.,
“Optical biopsy of fixed human skin with backward-collected optical harmonics signals,”
Opt. Exp., 13
(20), 8231
–8242
(2005). http://dx.doi.org/10.1364/OPEX.13.008231 OPEXFF 1094-4087 Google Scholar
T.-H. Tsaiet al.,
“Optical signal degradation study in fixed human skin using confocal microscopy and higher-harmonic optical microscopy,”
Opt. Exp., 14
(2), 749
–758
(2006). http://dx.doi.org/10.1364/OPEX.14.000749 OPEXFF 1094-4087 Google Scholar
Y.-H. Liaoet al.,
“In vivo harmonic generation biopsy for quantitative evaluation in chronological aged skin keratinocytes,”
Biomed. Opt. Express, 4
(2), 77
–88
(2013). http://dx.doi.org/10.1364/BOE.4.000077 BOEICL 2156-7085 Google Scholar
S.-Y. ChenH.-Y. WuC.-K. Sun,
“In vivo harmonic generation biopsy of human skin,”
J. Biomed. Opt., 14
(6), 060505
(2009). http://dx.doi.org/10.1117/1.3269676 JBOPFO 1083-3668 Google Scholar
M. LippitzM. A. van DijkM. Orrit,
“Third-harmonic generation from single gold nanoparticles,”
Nano Lett., 5
(4), 799
–802
(2005). http://dx.doi.org/10.1021/nl0502571 NALEFD 1530-6984 Google Scholar
S.-P. Taiet al.,
“Molecular imaging of cancer cells using plasmon-resonant-enhanced third-harmonic-generation in silver nanoparticles,”
Adv. Mater., 19
(24), 4520
–4523
(2007). http://dx.doi.org/10.1002/(ISSN)1521-4095 ADVMEW 0935-9648 Google Scholar
T.-M. Liuet al.,
“Measuring plasmon-resonance enhanced third-harmonic of a Ag nanoparticles,”
Appl. Phys. Lett., 89
(4), 043122
(2006). http://dx.doi.org/10.1063/1.2240738 APPLAB 0003-6951 Google Scholar
C.-F. Changet al.,
“Cell tracking and detection of molecular expression in live cells using lipid-enclosed CdSe quantum dots as contrast agents for epi-third harmonic generation microscopy,”
Opt. Exp., 16
(13), 9534
–9548
(2008). http://dx.doi.org/10.1364/OE.16.009534 OPEXFF 1094-4087 Google Scholar
C.-H. Yuet al.,
“Molecular third-harmonic-generation microscopy through resonance enhancement with absorbing dye,”
Opt. Lett., 33
(4), 387
–389
(2008). http://dx.doi.org/10.1364/OL.33.000387 OPLEDP 0146-9592 Google Scholar
R. B. Boyd, Nonlinear Optics, 2nd ed.Academic Press, Orlando, FL
(2003). Google Scholar
C.-F. Changet al.,
“Direct Backward Third-Harmonic Generation in Nanostructures,”
Opt. Exp., 18
(7), 7397
–7406
(2010). http://dx.doi.org/10.1364/OE.18.007397 OPEXFF 1094-4087 Google Scholar
U. S. Food and Drug Administration, “Think before you ink: Are tattoos safe?,”
http://www.fda.gov/forconsumers/consumerupdates/ucm048919.htm Google Scholar
S.-W. Chuet al.,
“High-resolution simultaneous three-photon fluorescence and third-harmonic-generation microscopy,”
Microsc. Res. Technol., 66
(4), 193
–197
(2005). http://dx.doi.org/10.1002/(ISSN)1097-0029 MRTEEO 1059-910X Google Scholar
Lipofectamine™ 2000, “User manuals,”
http://tools.invitrogen.com/content/sfs/manuals/lipofectamine2000_man.pdf Google Scholar
R. A. Fraseret al.,
“Efficient incorporation of transfected blastodermal cells into chimeric chicken embryos,”
Int. J. Dev. Biol., 37
(3), 381
–385
(1993). IJDBE5 0214-6282 Google Scholar
T. Høgsberget al.,
“Tattoo inks in general usage contain nanoparticles,”
Brit. J. Dermatol., 165
(6), 1210
–1218
(2011). http://dx.doi.org/10.1111/bjd.2011.165.issue-6 BJDEAZ 1365-2133 Google Scholar
H. Fujitaet al.,
“The uptake and long-term storage of India ink particles and latex beads by fibroblasts in the dermis and subcutis of mice, with special regard to the non-inflammatory defense reaction by fibroblasts,”
Arch. Histol. Cytol., 51
(3), 285
–294
(1988). http://dx.doi.org/10.1679/aohc.51.285 AHCYEZ 0914-9465 Google Scholar
R. KalluriM. Zeisberg,
“Fibroblasts in cancer,”
Nat. Rev. Cancer, 6
(5), 392
–401
(2006). http://dx.doi.org/10.1038/nrc1877 NRCAC4 1474-175X Google Scholar
S. Schlückeret al.,
“Immuno-surface-enhanced coherent anti-stokes Raman scattering microscopy: immunohistochemistry with target-specific metallic nanoprobes and nonlinear Raman microscopy,”
Anal. Chem., 83
(18), 7081
–7085
(2011). http://dx.doi.org/10.1021/ac201284d ANCHAM 0003-2700 Google Scholar
A. V. Kachynskiet al.,
“Zinc oxide nanocrystals for non-resonant nonlinear optical microscopy in biology and medicine,”
J. Phys. Chem. C Nanomater. Interfac., 112
(29), 10721
–10724
(2008). http://dx.doi.org/10.1021/jp801684j Google Scholar
P. Samineniet al.,
“Pump-probe imaging of historical pigments used in paintings,”
Opt. Lett., 37
(8), 1310
–1312
(2012). http://dx.doi.org/10.1364/OL.37.001310 OPLEDP 0146-9592 Google Scholar
A. V. Zvyaginet al.,
“Imaging of zinc oxide nanoparticle penetration in human skin in vitro and in vivo,”
J. Biomed. Opt., 13
(6), 064031
(2008). http://dx.doi.org/10.1117/1.3041492 JBOPFO 1083-3668 Google Scholar
|

