|
|
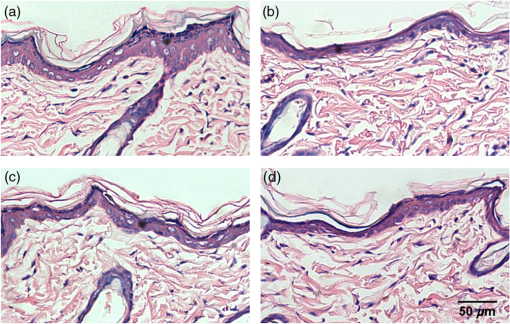
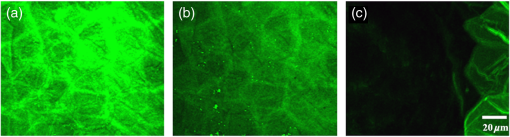
1.IntroductionSkin, the largest and outermost organ, provides the most accessible way to administer medicine. Transdermal drug delivery (TDD), as an easier and more targeted administration method, has attracted extensive investigations.1–3 However, the stratum corneum (SC) blocks the penetration of drugs into the dermis, which limits the treatment outcome. In order to breach the barrier function of SC, various physical or chemical methods have been proposed, such as ultrasound,4 iontophoresis,5 electroporation,6 microneedles,7 plasma,8 and chemical enhancers.9 These methods have been successfully used to enhance the transdermal delivery of small molecules, lipophilic-preferred and low-dose drugs, but the delivery of macromolecules, hydrophilic drugs, vaccines, and new genetic treatment employings such as DNA or small-interfering RNA is still challenging.1 With the development of medical laser technique, lasers have been shown to have great advantages in enhancing TDD of not only small molecules but also large and hydrophilic molecules. For instance, after having been irradiated by Er:YAG laser, the flux of vitamin C derivatives across laser-treated mouse skin was up to 189-fold than the flux across intact skin;10 the penetration of peptide and related vaccine were 3 to 140 fold higher than intact skin;11 the flux of 77 kDa dextran across mouse skin pretreated with laser was up to 100.82-fold higher than that of the passive control, and the transdermal delivery of hydrophilic permeants such as peptides were also enhanced;12 the absorption of topical lidocaine was significantly increased on in vivo skin.13 With Q-switched (QS) ruby laser irradiation, the insulin was allowed to pass through the SC into systemic circulation of diabetic rat to decrease blood glucose by ;14 the gene transfer of a plasmid DNA and the expression level for laser-treated rat skin were two orders higher than the controlled skin;15 the delivery of gene-coded plasmid into skin grafts was increased and the grafts adhesion was accelerated.16 Besides, after QS-Nd:YAG laser (532 and 1064 nm) irradiation, both the concentration and penetration depth of methylene blue into biofilms were enhanced;17 the penetration of 5-fluorouracil into irradiated rabbit ear skin was increased.18 In addition, 532-nm QS-Nd:YAG laser irradiation could facilitate the vaccine penetration and induce more immune responses.19 As for the mechanisms, it has been reported that laser-induced perturbation of the SC is responsible for laser-enhanced TDD. For instance, Er:YAG laser-induced ablation of the SC enhances macromolecular delivery;12 QS-ruby laser caused expansion of the lacunar spaces within the SC lipid bilayers provides the evidence for enhancing TDD.3,20 Both reports claimed that lasers enhancing TDD should be due to the changes in the SC induced by photomechanical action. From the above, it can be concluded that the changes in the SC depends on the laser wavelength, outmode, etc. After having compared the enhancing effects induced by various light sources, such as QS-Nd:YAG laser (532 nm), LP/QS-Nd:YAG laser (1064 nm), laser (10.6 μm), and intense pulsed light,21,22 it has been found that the 1064 nm-Nd:YAG lasers with LP or QS output modes could result in better enhancing effect than others on in vivo skin with minimal side effects. However, it is unclear whether lasers with the same wavelength (1064 nm-Nd:YAG) but with different output modes induce the same changes in the SC. What is more, the biological effect induced by laser–tissue interaction and the long-term enhancing effect on transdermal delivery by laser irradiation need to be further investigated. In this work, the physical and physiological mechanisms of LP/QS laser-enhancing TDD will be investigated. In order to prove whether there is laser–tissue photomechanical action, the hematoxylin-eosin (HE) histological analysis were applied to examine physiological microstructure of in vivo skin, and two-photon fluorescence microscopy (TPFM) was used to image the corneocytes and its molecular constitution information of the SC after irradiation by 1064 nm-Nd:YAG lasers with LP and QS output modes. Actually, laser–tissue interactions usually company the thermal effect of tissue, so an infrared thermography was used to monitor the dynamical temperature distribution on skin surface during different laser irradiation. Besides, the long-term enhancing efficacy for transdermal delivery caused by the lasers was evaluated. 2.Materials and Methods2.1.Animal Preparation and Laser IrradiationThe Experimental Animal Management Ordinance of Hubei Province, China, approved this study. Male Wistar rats (, ) were purchased from Hubei Health and Epidemic Prevention Station (Wuhan, China) and fed under specific pathogen-free (SPF) conditions at room temperature (22°C to 26°C) and humidity (30% to 50%). The average thickness of the SC for rat skin was about 20 to 25 μm.23 Animals were intraperitoneally anesthetized ( bodyweight) with a mixture of chloral hydrate () and ethylurethanm (). Then the dorsal hair was depilated. The dorsal skin of each rat was divided into two eudipleural areas and irradiated by LP-Nd:YAG laser (SHATL, China) or QS-Nd:YAG laser (MEDITECH, Korea), respectively. The fluence for LP laser was with pulse duration of 40 ms and pulse number of 10. The QS laser pulse with 100 mJ irradiated on a circular with diameter of 5 mm, and the calculated fluence for QS laser was with pulse duration of 8 ns and pulse number of 60. The parameters have been proved to be the optimal dose for enhancing penetration of glycerol and cause no side effects.21 2.2.Infrared Thermography for Monitoring the Temperature of SkinAn infrared thermographic system with infrared spectral range of 7.5 to 14 μm (VCr680HS, InfraTec GmbH, Germany) was used to dynamically monitor the temperature of skin surface of rats () during the laser irradiation. The spatial resolution of microbolometer detector array is pixels, and its measurement accuracy is between 0°C and 100°C. The sampling frequency used in this work was 50 Hz. And the measurements were also obtained before and 1 min after irradiation stopped. After laser irradiation, the HE histopathological examination method was used to test the two eudipleural areas irradiated by LP or QS laser on back of rats (), and a two-photon fluorescence microscopy was used to image the molecular constitution of the SC of rat skin (). In order to understand the effects of laser irradiation on skin, the intact skin on the same rats were selected as control. Other rats () were applied to evaluate the enhancing TDD, and then continue bred under SPF condition for further experiment. The examination and imaging methods are described in detail as follows. 2.3.Histopathological Examination of Microstructure of SkinSkin biopsies of rat skin were taken immediately, and on the 2nd, 5th, and 14th days to examine the short-term and long-term changes in skin structure, respectively. Five rats were sacrificed at each time point. The physiological microstructural alteration was evaluated by histological analyses using HE staining. The skin samples were fixed with 4% neutral formaldehyde and then were dehydrated using graded alcohol. After paraffin embedding, the samples were sliced with thickness of 4 to 5 μm and then stained with hematoxylin and eosin. Finally, each slice was imaged by a microscopy (IX71, Olympus, Japan) equipped with a color digital industrial camera (DFK 41BU02, The Imaging Source Europe GmbH, Germany). 2.4.Two-Photon Fluorescence Microscopy for Imaging Skin MoleculesThe intact and the irradiated skin of rats () were imaged by TPFM to obtain the change of molecular composition of the SC. The system was composed of a two-photon microscope (FV300, Olympus) equipped with a mode-locked ultrafast laser (Chameleon Ultra II, Coherent). The image was acquired by using a water immersion objective lens (, NA 0.8, Olympus). The Verdi-pumped Ti:Sapphire laser produced mode-locked, sub-200 femtosecond pulses at a 80 MHz repetition rate with an output power more than 2 W at the peak of the tuning curve. To detect the autofluorescence of skin, the excitation wavelength was set at 780 nm. 2.5.Fiber Spectroscopy for Evaluating Laser-Enhancing Transdermal DeliveryIn order to evaluate the enhancing effects of transdermal delivery by laser irradiation, the changes in skin reflectance induced by glycerol were measured by a visible and near infrared fiber spectrometer (USB-4000, Ocean Optics, USA). The reflectance spectroscopy has often been used to assess the transdermal extent of chemical agents such as glycerol. Glycerol is not only a typical adjuvant in medicine and cosmetic products but also a typical hydrophilic agent with hyperosmosis and high refractive index.21,22,24–26 The penetration of glycerol into dermis will lead to the refractive index matching and reduction of scattering coefficient of skin, which will increase light penetration depth, and then decrease the skin reflectance.21,22,26–29 Experimental procedure in details was the same as reported in previous studies.21,22 The initial reflectance spectrum of back skin was first measured. Then the tested area was irradiated by lasers and topically treated with glycerol. The control area was just treated with glycerol without irradiation. Eight to ten minutes later, glycerol was gently wiped off, and the reflectance spectrum was measured again. In order to observe the long-term enhancing effect, in the following two weeks, glycerol was repetitively applied to the tested area and control area, and then reflectance spectrum was obtained. 3.Results3.1.Thermal Responses of Laser-Irradiated SkinFigure 1(a) and 1(b) shows the typical temperature distribution at initial state, immediately and 1 min after laser irradiation. The darker shade represents lower temperature, and the brighter shade represents higher temperature. It can be seen that the LP laser irradiation induces significant temperature rise, as shown in Fig. 1 column (a); 1 min after laser irradiation, the temperature of the irradiated area is still higher than the surrounding areas. In contrast, the QS laser irradiation does not raise the skin temperature obviously, as shown in column (b) in Fig. 1. Fig. 1(a) and (b) Real-time measurements and (c) and (d) changes in the skin temperature induced by laser irradiation. Dosage for LP laser is , for QS laser, . C1 is enlarged view of the irradiated area; (c) and (d) show the temperature distribution of a lined-region, marked as L1. 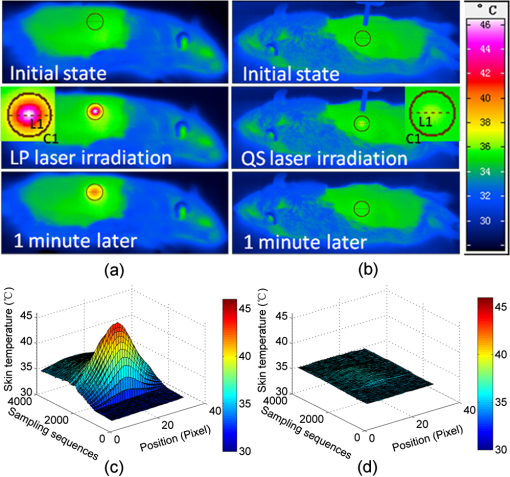 Figure 1(c) and 1(d) profiles the dynamical temperature distribution of the Line-1 area marked in Fig. 1(a) and 1(b). It can be seen that the temperature rises higher and higher as LP laser irradiation prolongs, and the temperature is the highest at the irradiated center. After LP laser irradiation stops, the temperature of skin surface decreases sharply, but still remains higher than the initial state [Fig. 1(c)]. In contrast, the changes in skin temperature during the QS laser irradiation are much lower [Fig. 1(d)]. According to quantitative calculation, the maximum temperature rise of irradiated skin caused by LP and QS laser is and , respectively. 3.2.Short-Term Effect of Laser Irradiation on Stratum CorneumIn order to examine the impact on skin microstructure, intact and laser-irradiated skin samples were subject to histopathological examination with HE staining. As shown in Fig. 2, the SC of intact skin sample remains integrated, sheet and multilayered, clinging tightly to the under homogeneous epidermis [Fig. 2(a) and 2(c)]. The SC of skin sample irradiated by LP laser is exfoliated and thinned, and the residual SC is incompact [Fig. 2(b)]. For the skin irradiated by QS laser, there is rupture in the SC [Fig. 2(d)]. 3.3.Changes in the Corneocytes and Molecular Composition of the SCChanges of corneocytes and molecular composition for the SC were imaged by TPFM. Figure 3 shows the typical images of skin autofluorescence of intact and irradiated skin areas with LP or QS laser. In Fig. 3(a), there are pentagonal or hexagonal corneocytes interlocked tightly with each other, which show exactly the canonical “brick” structure of the SC. And the border of corneocytes is distinctive and bright in the intact skin. After LP laser irradiation, the autofluorescence from corneocytes becomes weak, the morphology of corneocyte seems to be dim and obscured, and the border of some corneocytes is no longer clear. The bright spots on the SC may be keratins from corneocytes [Fig. 3(b)]. The QS laser irradiation makes the fluorescence of the corneocytes under laser pulses disappear, and the adjacent fluorescence was weakened [Fig. 3(c)]. 3.4.Long-Term Effect of Laser Irradiation on Enhancing Transdermal DeliveryFigure 4(a), 4(b), and 4(c) shows the representative reflectance spectrum of experimental and control skin on the 1st, 5th, and 14th days. Immediately after LP laser irradiation, topical application of glycerol could decrease the overall intensity of the skin reflectance obviously, but mono-treatment of glycerol on control skin could not decrease the reflectance spectrum. On the fifth day, glycerol application can still decrease the reflectance spectrum of experimental group in comparison with the control skin [Fig. 4(b)], but there is no difference between the two groups on the 14th day [Fig. 4(c)]. Furthermore, the relative change in reflectance at 615 nm was quantitatively calculated for both the experimental and control skin. Then the enhancing-fold for transdermal glycerol delivery was deduced through dividing the former by the latter, as Fig. 4(d) shows. It can be seen that LP and QS lasers possess similar enhancing capacity. The reflectance changes for experimental groups are more than 12 times of that for the control group on the first day, which means the penetration of glycerol into dermis is significantly increased after LP or QS laser irradiation. Then the enhancing effect is still retained but gradually faded in the next two weeks. Fig. 4(a) through (c) Typical reflectance spectrum (RS) of experimental and control skin on day 0, day 5, and day 14 after LP laser irradiation; (d) the relative enhancing-fold of transdermal glycerol delivery induced by laser irradiation compared with untreated control. 0 and 0 represent the initial RS of experimental and control skin, respectively; 1 and 1 represent the RS after glycerol treatment. 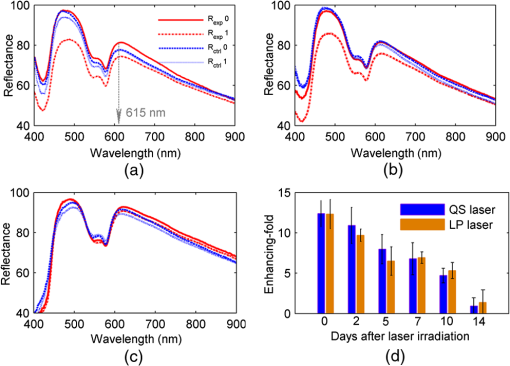 Figure 5 shows the typical results of histopathological examination of intact and irradiated skin on the fifth day [Fig. 5(a), 5(b), and 5(c)] and the 14th day [Fig. 5(d), 5(e), and 5(f)]. The SC of the intact skin has compact and multilayer structure [Fig. 5(a) and 5(d)]. On the fifth day, the SC of the skin irradiated by the LP laser seems to be thinned and loosened [Fig. 5(b)]; micropore is still visible on the QS laser-irradiated skin [Fig. 5(c)]. Meanwhile, there is no infection by bacteria and fungi. On the 14th day, the SC regrows from the viable epidermis for the skin irradiated by LP or QS laser. Fig. 5Long-term effect of laser irradiation on the SC examined by HE staining at day 5 (top row) and day 14 (bottom row): (a) and (c) intact skin without laser irradiation; (b) and (e) skin irradiated by LP laser; (c) and (f) skin irradiated by QS laser. The magnification times was . All the figures have the same bar, 50 μm. 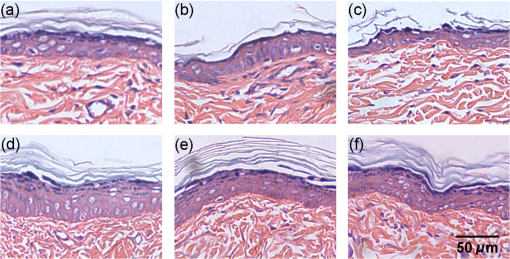 4.DiscussionLasers have been shown to have great advantages over other methods to enhance TDD, but previous investigations have shown that the enhancing mechanisms should be due to the photomechanical action which leads to the SC damage. Actually, laser–tissue interaction depends on the wavelength, the output, even the irradiation dose, etc. Therefore the mechanism of laser-enhancing TDD should be evaluated for a specific laser and specific irradiation parameters. Even though laser-enhanced TDD is realized by disrupting the SC, the issue of concern is how to choose a gentle and effective method to enhance TDD for different medicines. It is reported that Er:YAG laser irradiation ablates the SC completely;30 QS-ruby laser irradiation causes a transient damage to the SC of in vitro skin.3 All the changes in the SC are due to laser–tissue mechanical action. LP- and QS-Nd:YAG (1064 nm) lasers are commonly applied in clinical medicine and cosmetic dermatology31 and have also been proven to be effective in enhancing penetration of chemical agent into skin in vivo.21,22 In this work, not only the interactions between the lasers and in vivo skin but also the further biological effects were investigated to explain the physical and physiological mechanisms. The possible photomechanical effect was evaluated by examining the changes in micromorphology of skin and molecular constitution of the SC, and the dynamical heat response of skin surface was monitored during both LP and QS lasers irradiation by thermal imaging. Results from dynamic thermal imaging showed that the LP laser irradiation can lead to obvious temperature rise on skin surface, which is almost up to 13°C. Since the temperature of skin surface is usually 32°C, skin is tolerable for the temporal temperature rise caused by LP laser irradiation.32,33 In contrast, the QS laser irradiation only results in temperature rise of 1°C. Admittedly, the sampling frequency of the thermal imaging system is too low to record the transient temperature rise during the QS laser irradiation of 8 ns, but the continuous measuring can still evaluate whether there is obvious thermal effect in tissue to some extent. The differences in thermal responses of tissue to the two lasers should be due to pulse width. For instance, the thermal relaxation time is in microsecond-domain on the cell-specific scale,31 which is less than the impulse of LP laser (40 ms), but much larger than the ultrashort pulse of QS laser (8 ns). Histopathological examination can give microstructure information of full-thickness skin. Results have shown that LP laser irradiation can make the SC exfoliated and thinned, which may be the disruption of SC caused by transient photothermal effect. Previous investigation demonstrated that cold-plasma induced the temperature of skin surface to reach 45°C and detected the thermal damage in the upper cell layers of the SC;34 transient spray with hot water also disrupts the SC to enhance the TDD.35 The QS laser irradiation can break down local layer of the SC, which may be due to the giant pressure within ultrashort impulse irradiation on the skin with high-energy peak.36 In addition, the results showed that laser irradiation induced no obvious change in the gross structure of dermis, but it is still worth to further investigate whether there is influence on the junction between epidermis and dermis, and both in vitro histopathological examination with high-power microscope and in vivo monitoring with imaging system such as optical coherence tomography should be taken into account.37 The TPFM can provide molecular information since some endogenous chromophores such as keratin can produce autofluorescence under multiphoton excitation.38,39 As we know, the SC is composed of many keratin-abundant corneocytes and extracellular lipid matrix. Similar to collagenous fiber, the keratin filament is made up of multiple copies of keratin monomer with many intra- and intermolecular bonds. The pentagonal or hexagonal structure for intact skin is just the corneocytes filling with keratin. LP laser irradiation made the autofluorescence decrease. One reason is that some of the corneocytes have been exfoliated, resulting in less keratin to emit autofluorescence. Another may be that the abrupt rise in skin temperature breaks some kind of bonds within keratin fibrils, which may be for the similar mechanism as heating breaks more easily the bonds within collagen fibrils,40 leading to loosening of keratin molecules. By contrast, QS laser irradiation made the local autofluorescence extinguish to form micropores, which means the keratin molecules or even whole corneocytes were completely damaged. In addition, the results also showed that the enhancing effects by LP and QS laser irradiation can last for at least one week without infection, which is first reported. This is different from the transient-enhancing effect of photomechanical hypersonic wave.3 It may be because such low-level LP and QS lasers just selectively and mildly disrupt the SC, so neither cause irritation nor change the epidermal metabolism. Such long-lasting enhancement is much more convenient for long-term TDD instead of repetitiously breaching the SC, thus preventing patients from potential toxicity and tolerance. In addition, the LP and QS lasers have been widely used in cosmetic dermatology;31 the co-administration of drugs or other skin care products is strongly recommended to achieve good therapeutic effectiveness. The results also showed that the transcutaneous penetration of glycerol, a typical hydrophilic agent, was significantly enhanced by LP or QS laser irradiation, which further proved that the laser irradiation was capable of enhancing the transdermal delivery of hydrophilic molecules. On the whole, the Nd:YAG lasers irradiation with different output modes could produce different physical responses and then physiological changes on in vivo skin, but their enhancing effects on TDD are from disruption of the SC. As a result, the diffusion rate and efficiency of drug molecules can be improved due to the shortened diffusion length or the vertical diffusion micropores. From the standpoint of medicine administration, the LP laser irradiation should be more beneficial for hydrophilic and small molecules to diffuse into dermis; the QS laser should facilitate more molecules including macromolecules, vaccines, and new genetic treatment employings to penetrate directly into dermis, which is worthy of further confirmation. 5.ConclusionThis work investigated the physical and physiological mechanisms of 1064 nm-Nd:YAG lasers with different output modes enhancing transdermal delivery. The biological effects result from the laser–tissue physical interaction. It can be concluded that the mechanism for LP-Nd:YAG laser irradiation to enhance transdermal delivery is from interference of the SC by exfoliating the corneocytes and decompacting the keratin through obvious photonthermal effect. For the QS-Nd:YAG laser, the photon mechanical action induced perforation of the SC is the main mechanism to enhance transdermal delivery. In addition, both the penetration enhancing effect by LP- and QS-Nd:YAG lasers can persist for at least one week without infection. The long-term enhancing effect will be convenient for transdermal delivery administration. This work broadens the knowledge concerning the interaction of impulse laser with skin in the near-infrared domain and can be interesting to laser dermatology applications. The revealed physical and physiological mechanisms will provide theoretical basis for development and application of lasers in enhancing transdermal delivery. What is more, it will be significant for a larger field of cosmetic dermatology if lasers are applied to enhance skin care products to penetrate. AcknowledgmentsThis study was supported by grants of the National Major Scientific Research Program of China (Grant No. 2011CB910401), Science Fund for Creative Research Group of China (Grant No. 61121004), National Nature Science Foundation of China (Nos. 81171376, 91232710, 812111313), the Research Fund for the Doctoral Program of Higher Education of China (No. 20110142110073). ReferencesM. R. PrausnitzR. Langer,
“Transdermal drug delivery,”
Nat. Biotechnol., 26
(11), 1261
–1268
(2008). http://dx.doi.org/10.1038/nbt.1504 NABIF9 1087-0156 Google Scholar
R. K. Subediet al.,
“Recent advances in transdermal drug delivery,”
Arch. Pharm. Res., 33
(3), 339
–351
(2010). http://dx.doi.org/10.1007/s12272-010-0301-7 APHRDQ Google Scholar
A. G. DoukasaN. Kollias,
“Transdermal drug delivery with a pressure wave,”
Adv. Drug Deli. Rev., 56
(5), 559
–579
(2004). http://dx.doi.org/10.1016/j.addr.2003.10.031 ADDREP 0169-409X Google Scholar
S. PaliwalG. K. MenonS. Mitragotri,
“Low-frequency sonophoresis: ultrastructural basis for stratum corneum permeability assessed using quantum dots,”
J. Invest. Dermatol., 126
(5), 1095
–1101
(2006). http://dx.doi.org/10.1038/sj.jid.5700248 JIDEAE 0022-202X Google Scholar
Y. N. Kaliaet al.,
“Iontophoretic drug delivery,”
Adv. Drug Deliv. Rev., 56
(5), 619
–658
(2004). http://dx.doi.org/10.1016/j.addr.2003.10.026 ADDREP 0169-409X Google Scholar
A. R. DenetR. VanbeverV. Preat,
“Skin electroporation for transdermal and topical delivery,”
Adv. Drug Deliv. Rev., 56
(5), 659
–674
(2004). http://dx.doi.org/10.1016/j.addr.2003.10.027 ADDREP 0169-409X Google Scholar
S. Henryet al.,
“Microfabricated microneedles: a novel approach to transdermal drug delivery,”
J. Pharm. Sci., 87
(8), 922
–925
(1998). http://dx.doi.org/10.1002/(ISSN)1520-6017 JPMSAE 0022-3549 Google Scholar
O. Lademannet al.,
“Stimulation of the penetration of particles into the skin by plasma tissue interaction,”
Laser Phys. Lett., 8
(10), 758
–764
(2011). http://dx.doi.org/10.1002/lapl.v8.10 1612-2011 Google Scholar
A. C. WilliamsB. W. Barry,
“Penetration enhancers,”
Adv. Drug. Deliv. Rev., 56
(5), 603
–618
(2004). http://dx.doi.org/10.1016/j.addr.2003.10.025 ADDREP 0169-409X Google Scholar
C. Y. Hsiaoet al.,
“Skin pretreatment with lasers promotes the transdermal delivery of vitamin C derivatives,”
Laser. Med. Sci., 26
(3), 369
–376
(2011). http://dx.doi.org/10.1007/s10103-010-0863-0 LMSCEZ 1435-604X Google Scholar
W. R. Leeet al.,
“Erbium:YAG laser enhances transdermal peptide delivery and skin vaccination,”
J. Contr. Release, 128
(3), 200
–208
(2008). http://dx.doi.org/10.1016/j.jconrel.2008.03.003 JCREEC 0168-3659 Google Scholar
J. Y. Fanget al.,
“Transdermal delivery of macromolecules by erbium:YAG laser,”
J. Contr. Release, 100
(1), 75
–85
(2004). http://dx.doi.org/10.1016/j.jconrel.2004.08.009 JCREEC 0168-3659 Google Scholar
G. OniS. A. BrownJ. M. Kenkel,
“Can fractional lasers enhance transdermal absorption of topical lidocaine in an in vivo animal model,”
Laser. Surg. Med., 44
(2), 168
–174
(2012). http://dx.doi.org/10.1002/lsm.21130 LSMEDI 0196-8092 Google Scholar
S. Leeet al.,
“Photomechanical transdermal delivery of insulin in vivo,”
Laser. Surg. Med., 28
(3), 282
–285
(2001). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
M. Oguraet al.,
“In vivo targeted gene transfer in skin by the use of laser-induced stress waves,”
Laser. Surg. Med., 34
(3), 242
–248
(2004). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
K. Aizawaet al.,
“Accelerated adhesion of grafted skin by laser-induced stress wave–based gene transfer of hepatocyte growth factor,”
J. Biomed. Opt., 14
(16), 064043
(2009). http://dx.doi.org/10.1117/1.3253325 JBOPFO 1083-3668 Google Scholar
M. Oguraet al.,
“Photomechanical wave-assisted drug delivery in oral multispecies biofilms,”
World J. Microbiol. Biotechnol., 23
(11), 1637
–1646
(2007). http://dx.doi.org/10.1007/s11274-007-9411-x WJMBEY 0959-3993 Google Scholar
C. Gómezet al.,
“Laser treatments on skin enhancing and controlling transdermal delivery of 5- fluorouracil,”
Laser. Surg. Med., 40
(1), 6
–12
(2008). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
X. ChenM. X. Wu,
“Laser vaccine adjuvant for cutaneous immunization,”
Expet Rev. Vaccine., 10
(10), 1397
–1403
(2011). http://dx.doi.org/10.1586/erv.11.112 ERVXAX 1476-0584 Google Scholar
G. K. MenonN. KolliasA. G. Doukas,
“Ultrastructural evidence of stratum corneum permeabilization induced by photomechanical waves,”
J. Invest. Dermatol., 121
(1), 104
–109
(2003). http://dx.doi.org/10.1046/j.1523-1747.2003.12302.x JIDEAE 0022-202X Google Scholar
C. Liuet al.,
“Enhancement of skin optical clearing efficacy using photo-irradiation,”
Laser. Surg. Med., 42
(2), 132
–140
(2010). http://dx.doi.org/10.1002/lsm.v42:2 LSMEDI 0196-8092 Google Scholar
C. Liuet al.,
“Combined laser and glycerol enhancing skin optical clearing,”
Proc. SPIE, 7186 71860D
(2009). http://dx.doi.org/10.1117/12.811304 PSISDG 0277-786X Google Scholar
T. Ngawhirunpatet al.,
“Changes in electrophysiological properties of rat skin with age,”
Biol. Pharm. Bull., 25
(9), 1192
–1196
(2002). http://dx.doi.org/10.1248/bpb.25.1192 BPBLEO 0918-6158 Google Scholar
D. Zhuet al.,
“Imaging dermal blood flow through the intact rat skin with an optical clearing method,”
J. Biomed. Opt., 15
(2), 026008
(2010). http://dx.doi.org/10.1117/1.3369739 JBOPFO 1083-3668 Google Scholar
X. Wenet al.,
“In vivo skin optical clearing by glycerol solutions: mechanism,”
J. Biophoton., 3
(1), 44
–52
(2010). http://dx.doi.org/10.1002/jbio.200910080 JBOIBX 1864-063X Google Scholar
Z. Z. Maoet al.,
“Influence of alcohols on the optical clearing effect of skin in vitro,”
J. Biomed. Opt., 13
(2), 021104
(2008). http://dx.doi.org/10.1117/1.2892684 JBOPFO 1083-3668 Google Scholar
V. V. Tuchin,
“Optical clearing of tissues and blood using the immersion method,”
J. Phys. D Appl. Phys., 38
(15), 2497
–2518
(2005). http://dx.doi.org/10.1088/0022-3727/38/15/001 JPAPBE 0022-3727 Google Scholar
T. T. Yuet al.,
“Quantitative analysis of dehydration in porcine skin for assessing mechanism of optical clearing,”
J. Biomed. Opt., 16
(9), 095002
(2011). http://dx.doi.org/10.1117/1.3621515 JBOPFO 1083-3668 Google Scholar
Z. W. Zhiet al.,
“Improve optical clearing of skin in vitro with propylene glycol as a penetration enhancer,”
J. Innov. Opt. Health Sci., 2
(3), 269
–278
(2009). http://dx.doi.org/10.1142/S1793545809000590 Google Scholar
W. R. Leeet al.,
“Transdermal drug delivery enhanced and controlled by erbium:YAG laser: a comparative study of lipophilic and hydrophilic drugs,”
J. Contr. Release, 75
(1–2), 155
–166
(2001). http://dx.doi.org/10.1016/S0168-3659(01)00391-1 JCREEC 0168-3659 Google Scholar
S. Watanabe,
“Basics of laser application to dermatology,”
Arch. Dermatol. Res., 300
(Suppl. 1), S21
–S30
(2008). http://dx.doi.org/10.1007/s00403-007-0801-6 ADREDL 0340-3696 Google Scholar
D. L. Lloyd-SmithK. Mendelssohn,
“Tolerance limits to radiant heat,”
Brit. Med. J., 1
(4559), 975
–978
(1948). http://dx.doi.org/10.1136/bmj.1.4559.975 BMJOAE 0007-1447 Google Scholar
Y. Davidet al.,
“Heat pain thresholds: normative data and repeatability,”
Pain, 60
(3), 329
–332
(1995). http://dx.doi.org/10.1016/0304-3959(94)00132-X PAINDB 0304-3959 Google Scholar
O. Lademannet al.,
“Application of a plasma-jet for skin antisepsis: analysis of the thermal action of the plasma by laser scanning microscopy,”
Laser Phys. Lett., 7
(6), 458
–462
(2010). http://dx.doi.org/10.1002/lapl.v7:6 1612-2011 Google Scholar
J. W. Leeet al.,
“Microsecond thermal ablation of skin for transdermal drug delivery,”
J. Contr. Release, 154
(1), 58
–68
(2011). http://dx.doi.org/10.1016/j.jconrel.2011.05.003 JCREEC 0168-3659 Google Scholar
F. Rinaldi,
“Laser: a review,”
Clin. Dermatol., 26
(6), 590
–601
(2008). http://dx.doi.org/10.1016/j.clindermatol.2007.09.014 0738-081X Google Scholar
M. C. Pierceet al.,
“Advances in optical coherence tomography imaging for dermatology,”
J. Invest. Dermatol., 123
(3), 458
–463
(2004). http://dx.doi.org/10.1111/jid.2004.123.issue-3 JIDEAE 0022-202X Google Scholar
B. Yuet al.,
“In vitro visualization and quantification of oleic acid induced changes in transdermal transport using two-photon fluorescence microscopy,”
J. Invest. Dermatol., 117
(1), 16
–25
(2001). http://dx.doi.org/10.1046/j.0022-202x.2001.01353.x JIDEAE 0022-202X Google Scholar
S. J. LinS. H. JeeC. Y. Dong,
“Multiphoton microscopy: a new paradigm in dermatological imaging,”
Eur. J. Dermatol., 17
(5), 361
–366
(2007). http://dx.doi.org/10.1684/ejd.2007.0232 EJDEE4 1167-1122 Google Scholar
Y. Sunet al.,
“Investigating mechanisms of collagen thermal denaturation by high resolution second-harmonic generation imaging,”
Biophys. J., 91
(7), 2620
–2625
(2006). http://dx.doi.org/10.1529/biophysj.106.085902 BIOJAU 0006-3495 Google Scholar
|