|
|
1.IntroductionFirst developed in 1986, optical trapping has become a viable method to analyze cells in a basic science setting.1–6 In particular, there have been many studies conducted that used optical tweezers to study sperm biology.7–12 One of these studies used fluorescent probes to analyze changes in sperm physiology and showed that laser trapping for less than 2 min resulted in a temperature rise of 1°C for every 100 mW of optical power while the sperm’s pH and genetic stability seem to be undisturbed.13 For experimental trapping of longer than 2 min, the sperm’s physiological vitality had a tendency to diminish, and many of the sperm died.13 The studies mentioned have mostly considered the physiological effects of optical tweezers on sperm function, but not the long-term effects of laser radiation on fertilization and subsequent development. The two research groups who have attempted to test the effects of optical tweezers on fertilization and embryogenesis have had limited success due to the difficulty in trapping sperm and handling gametes.14–16 Specifically in 1995, Enginsu saw that only four out of 22 (18%) murine oocyte fertilization attempts passed the two-cell stage, and of those, only two passed into the blastocyst stage. In 1996, Clement-Sengewald determined that only 3.8% of bovine oocyte fertilization attempts yielded two pronuclei and a sperm tail within the cytoplasm after 20 h compared with 50% in controls. Clement-Sengewald mentioned “difficulties in catching sperm” and “prolonged exposure times to room temperature” as potential causes of the low fertilization rate. Enginsu reaffirmed these difficulties by mentioning that experimental conditions caused “an increased amount of time spent finding a spermatozoon in the droplet” as well as having to “increase the energy to maximum to be able to move and insert the mouse spermatozoa.” To overcome these challenges, we have developed a custom microfluidic chamber using advances in soft lithography17,18 combined with an automated sperm tracking and trapping laser microscopy system. Specifically, this system isolates the sperm and eggs into separate chambers using microfluidics. When ready, the automated track and trap system then traps and moves sperm directly to the egg. In order to optimize experiment and instrument parameters, a simple model organism was chosen, the purple sea urchin, Strongylocentrotus purpuratus. This organism has gametes that are similar in size to mammalian gametes, but are much easier to obtain.19 2.Materials and Methods2.1.Microfluidic Chamber FabricationA multilayer microfluidic chamber was designed and optimized in Autocad (Autodesk, San Rafael, California) (Fig. 1). The chamber consists of channels with two heights of 20 and 150 μm. Sperm and eggs are loaded into separate 150-μm high channels. The sperm and egg channels are connected by 20-μm high channels. Sea urchin eggs (80-μm diameter) cannot fit through the channel while sperm can readily pass. Using optical trapping, sperm from the sperm-loading channel can be moved to the eggs in the egg-loading channel (Fig. 1). Fig. 1Top view of the microfluidic chamber. Eggs flow from the egg inlet toward the shared outlet through 150-μm-height channels (black). Eggs reach smaller 20-μm-height channels (gray) that are impassable and are immobilized. Sperm are flowed from the sperm inlet to the shared outlet and can be moved to eggs using optical trapping. 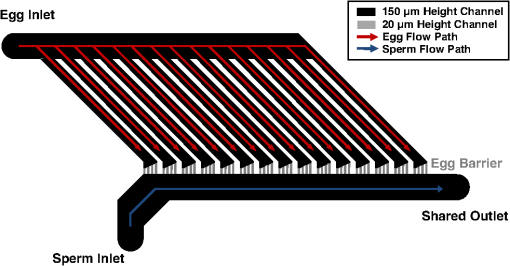 Microfluidic designs were sent to a cleanroom for master mold fabrication (Stanford Microfluidics Foundry, Stanford, California). Mold fabrication was done using standard SU-8 photolithography.17,18,20 Upon obtaining the mold, microfluidic chambers were cast using Sylgard 184 silicone. First, 100 g of Sylgard 184 silicone elastomeric base was mixed with 10 g of Sylgard 184 silicone elastomeric curing agent (Dow Corning, Midland, Michigan). The mixture was then placed into a vacuum desiccator to remove air bubbles. In a different vacuum desiccator, 2 mL of trichloromethylsilane was evaporated onto the master mold wafers for 20 min (Sigma-Aldrich, Saint Louis, Missouri). The elastomer mixture was poured onto the mold and left to cure on a hotplate at 80ºC for 20 min. After being cured, the elastomer was removed from the master mold and trimmed into individual chambers. A 20-gauge blunt-tipped needle (0.603-mm inner diameter, 0.908-mm outer diameter) (McMaster Carr, Elmhust, Illinois) was used to create input and output holes for the two inlets and shared outlet. The microfluidic chambers were subsequently mounted onto cover glasses (Thermo Fisher Scientific, Waltham, Massachusetts). 2.2.Sea Urchin Gamete PreparationS. purpuratus gametes were obtained at the Scripps Institute of Oceanography (La Jolla, California) by injecting 0.5 M KCl into the sea urchin perivisceral body cavities. The injection of KCl causes gonadal muscle contractions and near-complete shedding of gametes.21 Gametes were placed into 0.2-μm filtered seawater and transported to UCSD. Gametes were diluted to cells per mL filtered seawater. Filtered seawater was supplemented with 0.5% (w/v) polyvinylpyrrolidone (PVP40, Sigma-Aldrich) to reduce gamete adhesion in tubing. The microfluidic chamber’s shared outlet was connected to an automated computer-controlled syringe infusion/withdrawal pump (KD Scientific, Holliston, Massachusetts). The two inlets were individually connected to 10-mL valved holding vessels. Connections were made using silicone tubing (0.79-mm inner diameter; 2.4-mm outer diameter) and 20-gauge hypodermic steel tubing (0.603-mm inner diameter; 0.908-mm outer diameter). The chambers were temperature controlled to 16ºC using a thermoelectric stage cooler (CL-100, Harvard Apparatus, Holliston, Massachusetts). 2.3.Optical System SetupThe optical trapping system is built around a Zeiss Axiovert S100 inverted microscope that uses a 1.3 NA oil immersion phase III objective. The objective has a 49% transmission rate at 1064 nm that was measured using a modified double objective method.22,23 The laser source is a Spectra Physics BL-106C (1064 nm wavelength; 5 W average power, continuous wave) laser (Newport, Newport, California) coupled to the side port of the microscope and focused onto the specimen plane to create a single-point three-dimensional (3-D) gradient laser trap. The maximum laser power focused into the specimen plane is 510 mW. 3.Results3.1.Manipulation of Gametes Using Optical TrappingUsing the developed system, sperm and egg were easily isolated within the microfluidic chamber. The optical trap was used to transport sperm to the eggs through the 20-μm high connecting channels (Fig. 2, Video 1). Sperm were trapped and moved with 510 mW of focused laser power. The system was validated by moving 24 different sperm to unfertilized eggs. Fig. 2Movement of trapped sperm from sperm channel to egg channel. (1 to 3 s) Sperm are motile and swimming in a circle. (4 s) The optical trap immobilizes the sperm. (5 to 14 s) The sperm is moved next to the egg. (15 s) The sperm is released from the trap. (16 s) The sperm resumes swimming near the egg (Video 1, MPEG, 9.6 MB) [URL: http://dx.doi.org/10.1117/1.JBO.18.4.040501.1. 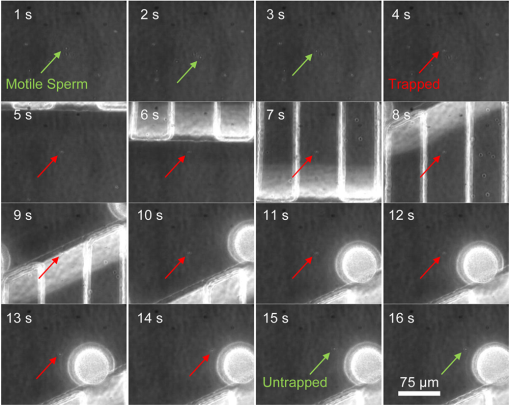 4.DiscussionA system has been developed that allows for the fluidic and optical manipulation of sperm and eggs. Previously we have developed a microfluidic system for the optical ablation of a single cell type, surf clam oocytes.20 In this current system, a novel design has been developed that manipulates two cell types of differing morphology and physiology (motile sea urchin sperm with head lengths of approximately 5 μm and immotile eggs with diameters of approximately 80 μm). Manipulating two cell types requires a design that supports the transport of the two cell types without mixing and unwanted fertilization. In the developed design, this was achieved where sperm and eggs can be easily loaded into two separate areas within a custom microfluidic chamber (Fig. 1). After loading, selected sperm can be moved to the eggs using an optical trap. This system has been validated with sea urchin gametes, which approximate the size of mammalian gametes. During the validation experiments, 24 different sperm were moved to unfertilized eggs. Of the 24 sperm moved to the egg, none of the sperm were able to achieve egg fertilization. Differences in physiology might account for the published fertilization success in mammalian species and the lack of success in sea urchins.14,15 One consideration is that the powers required to trap sea urchin sperm are much higher than those required to trap mammalian sperm. Clement-Sengewald used powers between 30 and 100 mW to trap bovine sperm. For all sea urchin sperm the maximum trap power (510 mW in the focus) was required, and only the weakest of sperm could be trapped. The weakest sperm selected may be the least likely to fertilize an egg. Also the 5.1°C heat shock upon trapping due the high powers used may be causing infertility.13 Another possible reason for sea urchin infertility is that sea urchin eggs require higher concentrations of sperm to be fertilized in vitro compared with mammalian species. To achieve 80% fertilization success, sea urchins require sperm concentrations of approximately , mice require approximately , and humans require approximately .24–26 Rothschild also noted high concentrations of sperm needed for sea urchin fertilization and suggested that sea urchins, which are externally fertilized, possibly need “sperm-sperm interactions of a physical nature” for fertilization.27 This idea supports single-sperm intracytoplasmic sperm injection in vitro fertilization experiments where fertilizations have been unsuccessful in sea urchins while successful in mammalian species.28–30 In summary, a system has been designed and fabricated that allows for the optical and fluidic manipulation of sperm and eggs. This system has potential uses for in vitro fertilization of mammalian eggs. Clement-Sengewald and Enginsu published work describing successful fertilization of mammalian eggs using optical trapping, but also reported difficulty due to handling the gametes. By adding microfluidics to isolate and manipulate gametes, higher throughput, and higher fertilization rates than published by Clement-Sengewald et al.14 and Enginsu et al.15 should be possible. AcknowledgmentsThe authors would like to thank Dr. Victor Vacquier, Dr. Amro Hamdoun, Dr. Gary Moy, and Joseph Campanale from the Scripps Institute of Oceanography for providing expertise and materials. This work was supported by funds from the Beckman Laser Institute Inc. Foundation awarded to MWB. CC would like to acknowledge support from a NDSEG fellowship and an NSF fellowship. ReferencesA. Ashkinet al.,
“Observation of a single-beam gradient force optical trap for dielectric particles,”
Opt. Lett., 11
(5), 288
(1986). http://dx.doi.org/10.1364/OL.11.000288 OPLEDP 0146-9592 Google Scholar
A. AshkinJ. M. Dziedzic,
“Optical trapping and manipulation of viruses and bacteria,”
Science, 235
(4795), 1517
–1520
(1987). http://dx.doi.org/10.1126/science.3547653 SCIEAS 0036-8075 Google Scholar
M. W. Bernset al.,
“Use of a laser-induced optical force trap to study chromosome movement on the mitotic spindle,”
4539
–4543
(1989). Google Scholar
H. Lianget al.,
“Directed movement of chromosome arms and fragments in mitotic newt lung cells using optical scissors and optical tweezers,”
Exp. Cell Res., 213
(1), 308
–312
(1994). http://dx.doi.org/10.1006/excr.1994.1203 ECREAL 0014-4827 Google Scholar
Y. Wanget al.,
“Visualizing the mechanical activation of Src,”
Nature, 434
(7036), 1040
–1045
(2005). http://dx.doi.org/10.1038/nature03469 NATUAS 0028-0836 Google Scholar
X. WeiB. J. TrombergM. D. Cahalan,
“Mapping the sensitivity of T cells with an optical trap: polarity and minimal number of receptors for signaling,”
8471
–8476
(1999). Google Scholar
E. Araujoet al.,
“Relative force of human epididymal sperm,”
Fertil. Steril., 62
(3), 585
–590
(1994). FESTAS 0015-0282 Google Scholar
Z. N. Dantaset al.,
“Effect of freezing on the relative escape force of sperm as measured by a laser optical trap,”
Fertil. Steril., 63
(1), 185
–188
(1995). FESTAS 0015-0282 Google Scholar
J. M. Nascimentoet al.,
“Analysis of sperm motility using optical tweezers,”
J. Biomed. Opt., 11
(4), 044001
(2006). http://dx.doi.org/10.1117/1.2337559 JBOPFO 1083-3668 Google Scholar
J. M. Nascimentoet al.,
“Use of laser tweezers to analyze sperm motility and mitochondrial membrane potential,”
J. Biomed. Opt., 13
(1), 014002
(2008). http://dx.doi.org/10.1117/1.2839051 JBOPFO 1083-3668 Google Scholar
Y. Tadiret al.,
“Force generated by human sperm correlated to velocity and determined using a laser generated optical trap,”
Fertil. Steril., 53
(5), 944
–947
(1990). FESTAS 0015-0282 Google Scholar
L. M. Westphalet al.,
“Exposure of human spermatozoa to the cumulus oophorus results in increased relative force as measured by a 760 nm laser optical trap,”
Hum. Reprod., 8
(7), 1083
–1086
(1993). 0268-1161 Google Scholar
Y. Liuet al.,
“Physiological monitoring of optically trapped cells: assessing the effects of confinement by 1064-nm laser tweezers using microfluorometry,”
Biophys. J., 71
(4), 2158
–2167
(1996). http://dx.doi.org/10.1016/S0006-3495(96)79417-1 BIOJAU 0006-3495 Google Scholar
A. Clement-Sengewaldet al.,
“Fertilization of bovine oocytes induced solely with combined laser microbeam and optical tweezers,”
J. Assist. Reprod. Genet., 13
(3), 259
–265
(1996). http://dx.doi.org/10.1007/BF02065947 JARGE4 1058-0468 Google Scholar
M. E. Enginsuet al.,
“Micromanipulation of mouse gametes with laser microbeam and optical tweezers,”
Hum. Reprod., 10
(7), 1761
–1764
(1995). 0268-1161 Google Scholar
K. SchützeA. Clement-SengewaldA. Ashkin,
“Zona drilling and sperm insertion with combined laser microbeam and optical tweezers,”
Fertil. Steril., 61
(4), 783
–786
(1994). FESTAS 0015-0282 Google Scholar
M. A. Ungeret al.,
“Monolithic microfabricated valves and pumps by multilayer soft lithography,”
Science, 288
(5463), 113
–116
(2000). http://dx.doi.org/10.1126/science.288.5463.113 SCIEAS 0036-8075 Google Scholar
Y. XiaG. M. Whitesides,
“Soft lithography,”
Angew. Chem. In. Ed. Engl., 37
(5), 550
–575
(1998). http://dx.doi.org/10.1002/(ISSN)1521-3773 ACIEAY 0570-0833 Google Scholar
A. Monroy,
“A centennial debt of developmental biology to the sea urchin,”
Biol. Bull., 171
(3), 509
–519
(1986). http://dx.doi.org/10.2307/1541620 BIBUBX 0006-3185 Google Scholar
C. Chandsawangbhuwanaet al.,
“High-throughput optofluidic system for the laser microsurgery of oocytes,”
J. Biomed. Opt., 17
(1), 015001
(2012). http://dx.doi.org/10.1117/1.JBO.17.1.015001 JBOPFO 1083-3668 Google Scholar
R. S. TuanC. W. Lo, Developmental Biology Protocols Volume II, Humana Press Inc., Totowa, NJ
(2000). Google Scholar
X. Konget al.,
“Comparative analysis of different laser systems to study cellular responses to DNA damage in mammalian cells,”
Nucleic Acids Res., 37
(9), e68
(2009). http://dx.doi.org/10.1093/nar/gkp221 NARHAD 0305-1048 Google Scholar
K. Koniget al.,
“Determination of motility forces of human spermatozoa using an 800 nm optical trap,”
Cell. Mol. Biol. (Noisy-le-grand), 42
(4), 501
–509
(1996). MCEBD4 0270-7306 Google Scholar
L. R. FraserL. M. Drury,
“The relationship between sperm concentration and fertilization in vitro of mouse eggs,”
Biol. Reprod., 13
(5), 513
–518
(1975). http://dx.doi.org/10.1095/biolreprod13.5.513 BIREBV 0006-3363 Google Scholar
H. Vogelet al.,
“Fertilization kinetics of sea urchin eggs,”
Math. Biosci., 58
(2), 189
–216
(1982). http://dx.doi.org/10.1016/0025-5564(82)90073-6 MABIAR 0025-5564 Google Scholar
D. P. Wolfet al.,
“Sperm concentration and the fertilization of human eggs in vitro,”
Biol. Reprod., 31
(4), 837
–848
(1984). http://dx.doi.org/10.1095/biolreprod31.4.837 BIREBV 0006-3363 Google Scholar
L. RothschildM. M. Swann,
“The fertilization reaction in the sea-urchin egg; the probability of a successful sperm-egg collision,”
J. Exp. Biol., 28 403
–416
(1951). JEBIAM 0022-0949 Google Scholar
Y. Hiramoto,
“Microinjection of the live spermatozoa into sea urchin eggs,”
Exp. Cell Res., 27
(3), 416
–426
(1962). http://dx.doi.org/10.1016/0014-4827(62)90006-X ECREAL 0014-4827 Google Scholar
Y. KimuraR. Yanagimachi,
“Development of normal mice from oocytes injected with secondary spermatocyte nuclei,”
Biol. Reprod., 53
(4), 855
–862
(1995). http://dx.doi.org/10.1095/biolreprod53.4.855 BIREBV 0006-3363 Google Scholar
G. Palermoet al.,
“Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte,”
Lancet, 340
(8810), 17
–18
(1992). http://dx.doi.org/10.1016/0140-6736(92)92425-F LANCAO 0140-6736 Google Scholar
|

