|
|
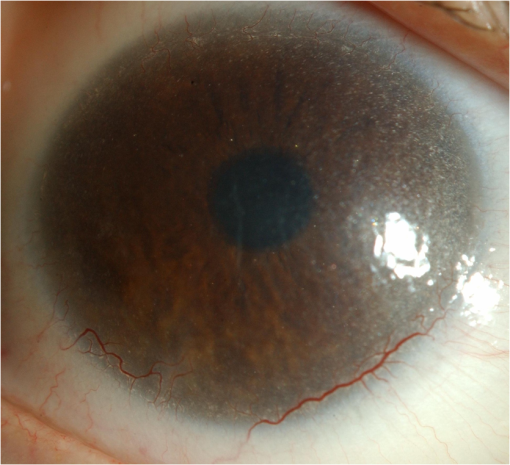
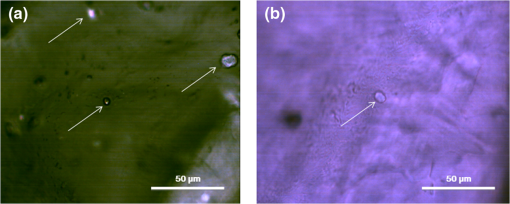
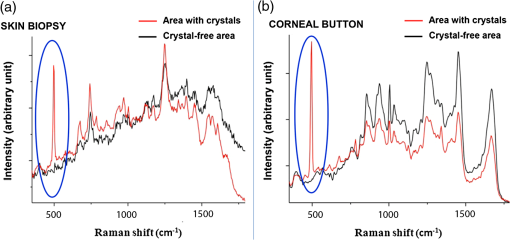
1.IntroductionNephropathic cystinosis (NC) is a rare autosomal recessive storage disease characterized by lysosomal accumulation of cystine crystals throughout the body, particularly in blood cells, the cornea, skin, kidneys, the central nervous system and muscles.1 Diagnosis and follow-up of NC are based on the demonstration and quantification of cystine crystal deposition. The concentration of cystine is normally assessed in blood cells, which is the gold standard for diagnosis.1 However, the presence of crystals has also been investigated in the cornea,2 the skin,3 and in other tissues such as kidney1 and intestinal mucosa.4 Thanks to in vivo reflectance confocal microscopy (IVCM), the cornea and skin crystals have recently become of greater interest because of the possibility of noninvasive identification and quantification in two easily accessible locations.2,3 While IVCM provides high definition images of the crystals, but its optical principle does not help characterize their chemical composition and therefore cannot provide diagnostic certainty, with Raman spectroscopy (RS) coupled with a microscope, the chemical signature of particles can be determined in a biological sample.5 We combined IVCM and ex vivo RS for the examination of skin and cornea samples from a patient with NC to confirm the cystine composition of crystals. We present both optical devices and discuss their potential value in the diagnosis and follow-up of metabolic disorders. 2.Materials and MethodsWe examined a 36-year-old female patient with NC with mostly renal and corneal involvement and without clinically evident dermatological lesions except for skin xerosis. Forearm and eyelid skin, corneas (Fig. 1) and bulbar conjunctiva were analyzed by IVCM using a multilaser (488, 658, and 785 nm) Vivascope 1500 and a handheld monolaser Vivascope 3000 (Lucid Inc., New York, MAVIG GmbH, Munich, Germany). For the multilaser IVCM, we used an adapter between the tip and the ocular surface to reduce the diameter of the current tip made for the skin, while preserving its optic qualities (Fig. 2). Fig. 2Vivascope adaptor for the ocular surface. Adapted tip (arrow) for the ophthalmic application of Vivascope 1500.  Ex vivo confocal RS (LabRAM ARAMIS, Horiba Jobin-Yvon, France) was later performed on two 5 mm punch biopsies taken from the forearm and on a corneal button retrieved during a penetrating keratoplasty. Both were immediately frozen in liquid nitrogen. The cornea was analyzed without further processing, while the skin biopsies were cut into 20 μm thick horizontal slices to make the crystals easier to identify in this uneven and nontransparent tissue. Randomly selected refringent crystals present in these tissues were scanned with RS (HeNe laser with a 150 μm pinhole) and identified by the optical microscope coupled to the RS. The control tissue consisted of randomly selected sites of crystal-free corneal stroma and skin dermis of the same patient. 3.ResultsMultilaser and monolaser IVCM showed hyper-reflective bodies in the dermal layer of forearm and eyelid skin, in corneal stroma, and in bulbar conjunctiva (Fig. 3). Skin and conjunctiva particles were round or oval in shape and approximately 10 μm in diameter, while corneal particles were spindle-shaped, approximately 50 μm long and 10 μm thick. All wavelengths provided similar images. Crystals were slightly autofluorescent in the cornea at 785 nm. Fig. 3In vivo confocal microscopy aspect of cystine crystals. Cystine crystals appear as roundish hyper-reflective bodies in the (a) eyelid, (b) forearm skin and (c) conjunctiva, but as spindle-shaped hyper-reflective bodies in the (d) cornea. 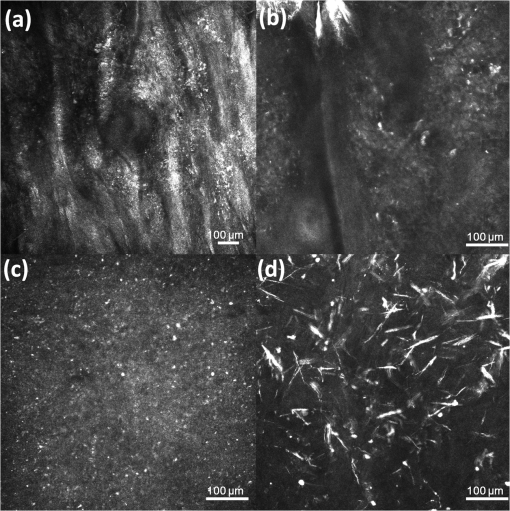 Raman spectra from the corneal stroma and the skin dermis overlapped in different sites showing a spectrum of collagen.6 Raman spectra obtained targeting refringent crystals (Fig. 4) found the optical profile of cystine6 with vibrational fingerprints at wave numbers of 499, 542, 675, 785, 870, 960, 1340, 1383, and (Fig. 5). The Raman spectrum of cystine was not modified by rotating the tissue samples or examining the samples after leaving them in the open air without fixative for 6 h. The Raman shift pic at was very high and was easily identified in the spectrum of the crystals, but was never found in the crystal-free corneal stroma and skin dermis of the same patient. 4.DiscussionWe considered NC as a prototype storage disease characterized by the accumulation of materials with a perfectly well defined chemical composition in easily accessible tissues. Thanks to the IVCM and RS combination, we were able to exploit IVCM to characterize the deposits morphologically and RS to identify them chemically. IVCM, an emerging technique that allows in vivo noninvasive high-resolution histomorphological analysis of skin, mucosa and ocular surface, has shown its usefulness in clinical practice and research,7–11 and has recently been used to identify the presence of crystals in the cornea and skin of NC patients.2,3,12 However, the identification of crystals in the cornea may not be sufficient for a diagnosis, since crystals within the cornea can also be identified by IVCM in other conditions, such as tyrosinemia, Schnyder dystrophy, Bietti crystalline dystrophy, multiple myelomas, and infectious crystalline keratopathy.13–16 In the skin, IVCM identification of crystals appears to be more specific for NC,3 but as yet it is not possible to state that such crystals are found only in NC, because no IVCM studies have been conducted in other storage diseases in the skin. The identification of crystals in both skin and eye samples suggest a systemic disease with material deposition in different tissues and could provide additional evidence for a diagnosis of NC. The use of IVCM has also been suggested to quantify crystal deposition in order to monitor disease progression.3 Also of note is that until now, IVCM of the skin and ocular surface had been performed with IVCM devices specifically designed for the use in dermatology and ophthalmology, respectively, whereas we used, for the first time, a skin-specific IVCM to examine both localizations concurrently. The Vivascope 3000 is a handheld laser scanning confocal microscope equipped with a class I 830 nm laser that can easily be used to acquire image stacks of the ocular surface. Thanks to our specific adaptation of an ophthalmology stand, the multilaser Vivascope 1500 can now also be used to examine the ocular surface using class I 658 and 785 nm lasers. All wavelengths provided similar images, but curiously, crystals were slightly autofluorescent in the cornea at 785 nm. IVCM provides valuable morphological data, but, and this is its greatest limitation, it does not identify the chemical nature of the crystals. Conversely, RS is an optical technique that measures the vibrational motions of molecules to provide specific information on the molecular composition of a sample.17 There are several potential applications of RS in different biomedical disciplines, such as studies on DNA, proteins, and cancer, as well as in dermatology18 and ophthalmology.17 This technique has also been recently used to analyze the correlation between chemical information and histological structures.19 RS lends itself particularly well to the study of cystine because this sulphur-containing amino acid presents a characteristic vibrational frequency of the S–S bond at that makes it easy to recognize.6 Additional signals of cystine Raman spectrum are intense bands at wave numbers of 542, 613, 678, 785, 873, 967, 1341, 1385, and .6 In our examinations, the amplitude of Raman shift peak at was very high and was easily identified in the spectrum, allowing a rapid identification of cystine. All other cystine characteristic bands have also been recognized in the spectra from the crystals of the cornea and of the skin, but due to their lower intensities, they were visible only with careful reading of the spectra. Characteristically, the same cystine signature was found in different crystals of both the skin and the cornea, demonstrating that exactly the same material is deposited in both tissues. The crystal-free corneal stroma and skin dermis of the same patient were used as control tissue and never presented the Raman shift peak at . After rotating the tissue samples and therefore changing the inclination of the faces of the crystals within, the Raman spectrum did not change, demonstrating that the spectrum of cystine was not influenced by the orientation of the crystals. We also showed that the Raman examination was a reproducible technique to analyze samples with different degrees of hydration, and cystine was found in the same samples left in the open air without any fixative for 6 h. RS appears to be a precise tool to assess material accumulation in ex vivo samples and has also been recently adapted for in vivo applications,20–22 but it still has several limitations, namely the need to be handled by highly qualified personnel because the type and energy of the laser, pinhole size, width of the observed spectrum, and possible application of any filters may vary according to the samples to be analyzed, and the difficulty in locating the target point that has to be analyzed in a heterogeneous sample. RS can be coupled with an optical microscope to scan the sample and target the area of interest; however, as tissue cannot be stained with the usual histological dyes because they would alter the Raman spectrum, it is sometimes difficult to distinguish the different structures under the microscope. In order to overcome these difficulties, Raman microspectroscopy has recently been combined with IVCM for both imaging and chemical analysis of the skin at cellular level.23 Our data suggests that in the future, cystinosis could be diagnosed by means of a noninvasive technique that combines the use of IVCM to identify the crystals and in vivo RS to confirm their composition. In conclusion, this is the first observation of a cutaneous and corneal cystine deposition made with a single IVCM suitable for both skin and eye, which will facilitate experience sharing between dermatologists and ophthalmologists, and it is the first RS characterization of these deposits previously identified by IVCM. Cystine is easily recognized by RS producing a characteristic Raman shift at . In the future, the diagnosis of NC could be based on a noninvasive skin and cornea examination by IVCM coupled with in vivo RS to identify cystine crystals with certainty. As it provides an optical signature of the chemical nature of the observed constituents, RS also opens promising new perspectives in other storage diseases. AcknowledgmentsWe are very grateful to Gianfranca Gessa Shepheard and Luisa Galbusera for the English revision of the text. ReferencesM. J. Wilmeret al.,
“Cystinosis: practical tools for diagnosis and treatment,”
Pediatr. Nephrol., 26
(2), 205
–215
(2011). http://dx.doi.org/10.1007/s00467-010-1627-6 PENED3 0931-041X Google Scholar
A. Labbéet al.,
“In vivo confocal microscopy and anterior segment optical coherence tomography analysis of the cornea in nephropathic cystinosis,”
Ophthalmology, 116
(5), 870
–876
(2009). http://dx.doi.org/10.1016/j.ophtha.2008.11.021 OPANEW 0743-751X Google Scholar
C. Chiavériniet al.,
“In vivo reflectance confocal microscopy of the skin: a noninvasive means of assessing body cystine accumulation in infantile cystinosis,”
J. Am. Acad. Dermatol., 68
(4), e111
–e116
(2013). http://dx.doi.org/10.1016/j.jaad.2011.08.010 JAADDB 0190-9622 Google Scholar
R. DohilA. CarriggR. Newbury,
“A potential new method to estimate tissue cystine content in nephropathic cystinosis,”
J. Pediatr., 161
(3), 531
–535
(2012). http://dx.doi.org/10.1016/j.jpeds.2012.03.011 ZEKIAS 0044-2917 Google Scholar
J. J. Chuiet al.,
“Bluebottle envenomation-induced crystalline keratopathy,”
Cornea, 30
(7), 835
–837
(2011). http://dx.doi.org/10.1097/ICO.0b013e318203cfdb CORNDB 0277-3740 Google Scholar
G. Zhuet al.,
“Raman spectra of amino acids and their aqueous solutions,”
Spectrochim. Acta A Mol. Biomol. Spectrosc., 78
(3), 1187
–1195
(2011). http://dx.doi.org/10.1016/j.saa.2010.12.079 SAMCAS 1386-1425 Google Scholar
M. UlrichS. Lange-Asschenfeldt,
“In vivo confocal microscopy in dermatology: from research to clinical application,”
J. Biomed. Opt., 18
(6), 61212
(2013). http://dx.doi.org/10.1117/1.JBO.18.6.061212 JBOPFO 1083-3668 Google Scholar
A. Nwaneshiuduet al.,
“Introduction to confocal microscopy,”
J. Invest. Dermatol., 132
(12), e3
(2012). http://dx.doi.org/10.1038/jid.2012.429 JIDEAE 0022-202X Google Scholar
H. Y. KangP. BahadoranJ.-P. Ortonne,
“Reflectance confocal microscopy for pigmentary disorders,”
Exp. Dermatol., 19
(3), 233
–239
(2010). http://dx.doi.org/10.1111/exd.2010.19.issue-3 EXDEEY 0906-6705 Google Scholar
S. González,
“Confocal reflectance microscopy in dermatology: promise and reality of non-invasive diagnosis and monitoring,”
Actas Dermosifiliogr., 100
(Suppl. 2), 59
–69
(2009). http://dx.doi.org/10.1016/S0001-7310(09)73380-0 ADSIAZ 0001-7310 Google Scholar
R. L. NiedererC. N. J. McGhee,
“Clinical in vivo confocal microscopy of the human cornea in health and disease,”
Prog. Retin. Eye Res., 29
(1), 30
–58
(2010). http://dx.doi.org/10.1016/j.preteyeres.2009.11.001 PRTRES 1350-9462 Google Scholar
C. N. GrupchevaS. E. OrmondeC. McGhee,
“In vivo confocal microscopy of the cornea in nephropathic cystinosis,”
Arch. Ophthalmol., 120
(12), 1742
–1745
(2002). AROPAW 0003-9950 Google Scholar
J. E. Sutphinet al.,
“Evaluation of infectious crystalline keratitis with confocal microscopy in a case series,”
Cornea, 16
(1), 21
–26
(1997). http://dx.doi.org/10.1097/00003226-199701000-00005 CORNDB 0277-3740 Google Scholar
N. HoubenB. Foets,
“Confocal microscopy in multiple myeloma associated crystalline keratopathy: case report,”
Bull. Soc. Belge Ophtalmol.,
(300), 13
–17
(2006). Google Scholar
P.-P. Schauwvliegheet al.,
“Confocal microscopy of corneal crystals in a patient with hereditary tyrosinemia type I, treated with NTBC,”
Cornea, 32
(1), 91
–94
(2013). http://dx.doi.org/10.1097/ICO.0b013e318243e474 CORNDB 0277-3740 Google Scholar
L. Totoet al.,
“Spectral domain optical coherence tomography and in vivo confocal microscopy imaging of a case of Bietti’s crystalline dystrophy,”
Clin. Exp. Optom., 96
(1), 39
–45
(2013). http://dx.doi.org/10.1111/j.1444-0938.2012.00784.x 0816-4622 Google Scholar
C.-C. LinM.-T. KuoH.-C. Chang,
“Review: Raman spectroscopy—a novel tool for noninvasive analysis of ocular surface fluid,”
J. Med. Biol. Eng., 30
(6), 343
–354
(2010). http://dx.doi.org/10.5405/jmbe.846 IYSEAK 0021-3292 Google Scholar
M. Försteret al.,
“Confocal Raman microspectroscopy of the skin,”
Eur. J. Dermatol., 21
(6), 851
–863
(2011). http://dx.doi.org/10.1684/ejd.2011.1494 EJDEE4 1167-1122 Google Scholar
M. Pudlaset al.,
“Raman spectroscopy: a noninvasive analysis tool for the discrimination of human skin cells,”
Tissue Eng. Part C Meth., 17
(10), 1027
–1040
(2011). http://dx.doi.org/10.1089/ten.tec.2011.0082 1937-3341 Google Scholar
N. J. BauerF. HendrikseW. F. March,
“In vivo confocal Raman spectroscopy of the human cornea,”
Cornea, 18
(4), 483
–488
(1999). http://dx.doi.org/10.1097/00003226-199907000-00015 CORNDB 0277-3740 Google Scholar
P. D. A. Pudneyet al.,
“A new in vivo Raman probe for enhanced applicability to the body,”
Appl. Spectrosc., 66
(8), 882
–891
(2012). http://dx.doi.org/10.1366/12-06640 APSPA4 0003-7028 Google Scholar
C. Brouilletteet al.,
“Raman spectroscopy using 1550 nm (retina-safe) laser excitation,”
Appl. Spectrosc., 65
(5), 561
–563
(2011). http://dx.doi.org/10.1366/11-06232 APSPA4 0003-7028 Google Scholar
C. A. Patilet al.,
“A handheld laser scanning confocal reflectance imaging-confocal Raman microspectroscopy system,”
Biomed. Opt. Express, 3
(3), 488
–502
(2012). http://dx.doi.org/10.1364/BOE.3.000488 BOEICL 2156-7085 Google Scholar
|