|
|
1.IntroductionVascular remodeling and angiogenesis are important processes in the pathogenesis of many lung diseases such as asthma, chronic obstructive pulmonary disease, and lung cancer.1 To date, structural information on vascular remodeling in the lung has been primarily investigated in ex vivo surgical and postmortem specimens which cannot be used to measure dynamic vasculature parameters such as blood flow speed and directionality. Also, methods that require tissue removal do not permit longitudinal studies to follow vascular changes due to disease progression or response to treatment. Real-time minimally invasive techniques for mapping the three-dimensional (3-D) vascular network in the airway wall over time may provide new clinical tools for detecting and monitoring lung diseases. Current methods for the visualization of airway vasculature in vivo include computed tomography angiography and Doppler ultrasound. However, these methods lack the resolution and sensitivity to detect small vessels. Optical coherence tomography (OCT),2 has proven to have sufficient resolution for examining tissue morphology and microstructure. Fiber-optic probes have demonstrated in vivo endoscopic structural OCT imaging of the lungs.3–5 Several techniques based on OCT for visualizing vascular networks have been presented in the literature including several variants of Doppler OCT imaging,6,7 and structural methods such as speckle variance OCT,8 and speckle decorrelation.9 The vast majority of in vivo vascular detection techniques have been developed for geometries where access to the region of interest is external to the organism using large-scale galvanometer scanners. Fiber probe-based OCT vascular imaging is considerably more difficult than its galvo-scanned analog because artifacts caused by subject motion are compounded by the additional motion of the fiber probes and their scanning mechanisms. Nonetheless, two-dimensional (2-D) vascular detection inside internal organs using fiber optic based probes using color Doppler optical coherence tomography (CDOCT) has been demonstrated.10 Here we present the first 3-D CDOCT-generated vascular maps of in vivo human airways. In addition to structural information on the tissue morphology, it is hoped that inclusion of vascular maps from CDOCT imaging will provide additional diagnostic capabilities for lung diseases. 2.Methods and MaterialsA 50.4 kHz swept source laser (SSOCT-1310, Axsun Technologies Inc., Billerica, Massachusetts) with a polarized output power of 20 mW centered at wavelength and a bandwidth of (full width half maximum) was coupled 90% sample/10% reference into a single-mode Mach–Zehnder OCT interferometer. The sample arm was connected using a fiber-optic rotary joint (MJP-SAPB, Princetel Inc., Pennington, New Jersey) to a 0.9 mm diameter, rotationally driven, side-looking fiber optic probe (C7 Dragonfly Imaging Catheter, St. Jude Medical Inc., St. Paul, Minnesota). Light exits the probe at approximately 64 deg relative to the tip of the fiber optic probe (i.e., slightly forward looking). For in vivo lung imaging, the open-ended probe was inserted into a closed-ended 1.5 mm diameter sheath to prevent direct probe-patient contact and was guided to the imaging location using a bronchoscope. The probe was driven using a custom built motor capable of rotational speeds up to 100 Hz and pullbacks up to 50 mm in length. In this study, the probe was spun at speeds of 6.25 Hz (8064 A-lines/frame) and 12.5 Hz (4032 A-lines/frame). The interference signal was detected using a pair of 75 MHz balanced detectors (PDB420C, Thorlabs-Inc., Newton, New Jersey) using a polarization diversity detection scheme. A high speed digitizer (ATS9350, Alazar Technologies Inc., Pointe-Claire, Quebec) in “k-clock” acquisition mode collected the data. The data was processed to provide live-streaming 2-D structural OCT and CDOCT imaging using custom written data acquisition software. This study was approved by the Research Ethics Board of the University of British Columbia and the British Columbia Cancer Agency. All subjects gave written, informed consent. The data processing steps to produce a single CDOCT image frame, based on previously reported algorithms7 are briefly described. The data is collected in the polar domain (, ) to generate the structural OCT image and raw CDOCT images using the Kasai velocity estimator: where is the Doppler frequency shift of the pixel (, ), is the A-scan frequency, and and are the real and imaginary parts of the Fourier-transformed polar pixel (, ). A high degree of oversampling in the -direction is required for reliable CDOCT processing for slower flow velocties deeper into the airway wall because fanning out of the A-lines in our rotational scanning geometry means that adjacent pixels get farther apart at large radii.11 To improve accuracy, the calculation in Eq. (1) is averaged over pixels in the radial scan direction, and pixels in the azimuthal scan direction. For our data sets, we chose , and as a compromise between color Doppler SNR (which increases with higher values of and ) and spatial resolution (which decreases with higher values of and ). The mean velocity of the pixel (, ) is , where is the center wavelength, is the tissue refractive index, and is the Doppler angle. Assuming that blood vessels lie parallel to the axis of the airway (i.e., ) and , our nonaliased Doppler velocity measurement range is . To reduce Doppler noise, a copy of the structural OCT image is first smoothed using an by rectangular kernel. The Doppler image is then masked to include only pixels for which the SNR’s of the corresponding smoothed structural OCT image pixels are greater than 2.0. There may be artifact-based Doppler signals following the preceding processing steps that are present due to a combination of three factors: (1) involuntary motion of the subject, (2) in-plane (, ) vibrational motion of the rotary probe, and/or (3) out-of-plane () pullback motion of the probe. These artifacts are largely removed using a bulk tissue motion correction algorithm that first creates a Doppler velocity histogram for the ’th A-line, from to adjacent A-lines in the -dimension (in our case ), and specified ROI in the -dimension. Then, the Doppler velocity of the peak in is subtracted from all Doppler velocities in the ’th A-line. This process is repeated for all A-lines in each frame. The CDOCT and structural OCT images are transformed into Cartesian coordinates to generate cross-sectional views of the airway. Finally, to display the location of the flow regions with respect to the structural image, velocities are masked out prior to overlay on top of the structural image. Raw data sets were reprocessed into movies using MATLAB (Mathworks, Natick, Massachusetts) and 3-D rendered using Amira (Visualization Sciences Group, Burlington, Massachusetts).3.Results and DiscussionFigure 1 shows structural OCT and CDOCT imaging of a 15 mm long airway segment from a 68-year-old male subject. These images were collected with 8064 A-lines/frame () and pullback (). Figure 1(a) shows the 3-D rendering of the structural OCT data with a representative 2-D slice shown in Fig. 1(b). Figure 1(c) and 1(d) show the same structural information as Fig. 1(a) and 1(b), respectively, with the CDOCT imaging information overlaid. The color scale for the CDOCT overlay is taken from color Doppler ultrasound convention that dictates that flow towards the probe is shown in shades of red, while flow away from the probe is shown in shades of blue. As light exits the probe slightly forward looking, the color map shows distal-to-proximal blood flow in shades of red, and proximal-to-distal blood flow in shades of blue. A movie of the structural OCT image side by side with the CDOCT overlay of this airway from distal to proximal is presented in Video 1. Figure 1(b) shows a round structure approximately 1 mm in diameter at the 12 o’clock position. The CDOCT image [Fig. 1(d)] clearly shows this structure contains flow, and thus indicates the structure to be a blood vessel that runs alongside the airway. In this case, identifying the architecture of this large vessel using CDOCT imaging alone can be complicated by the fact that the flow within the vessel is pulsatile, and causes the vessel to “blink” on and off in the CDOCT overlay image during the 3-D pullback (Video 1). However, once identified as vasculature using CDOCT imaging, the feature in the structural OCT movie can be used to map the entire vessel. The complete vessel is readily visible as a dark shadow in the 3-D rendering of the airway and is outlined with a dashed red line in Fig. 1(a). This example demonstrates the necessity of using both structural OCT and CDOCT imaging modalities to identify and map vasculature of this nature. The Doppler signal from the vessel is predominantly blue indicating blood flow proximal to distal along the airway. Quantification of velocity in this case requires Doppler phase unwrapping approaches as Doppler aliasing is readily apparent in Video 1. Fig. 1A 15-mm-long segment of airway from a 68-year-old male subject oriented distal to proximal left to right. (a) Three-dimensional (3-D) rendered structural optical coherence tomography (OCT) volume and (c) color Doppler optical coherence tomography (CDOCT) overlay onto the structural OCT volume of the OCT 3-D-pullback data. (b) Two-dimensional (2-D) cross-sectional structural OCT image and (d) CDOCT overlay on the structural OCT image taken from the locations indicated in (a, c). The scale bars in (b, d) are 0.5 mm. The CDOCT scale bar shown in (d) spans . A 2-D structural OCT and CDOCT overlay movie of this airway from distal to proximal is presented in Video 1 (MPG, 5.3 MB) [URL: http://dx.doi.org/10.1117/10.1117/1.JBO.18.5.050501.1]. 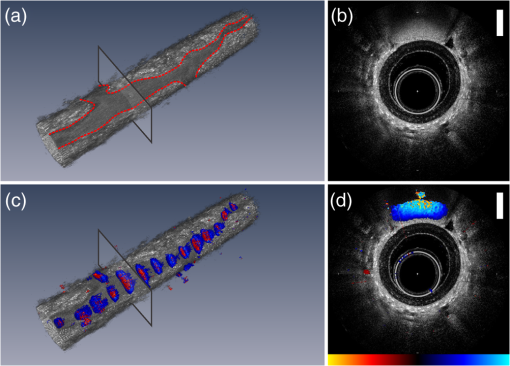 Figure 2 shows structural OCT and CDOCT imaging of an 8 mm long airway segment from a 52-year-old male subject with subfigures presented as in Fig. 1 with the corresponding movie shown in Video 2. These images were collected with 4032 A-scans/frame () and pullback speed (). In the 3-D rendering shown in Fig. 2(c), a larger, deeper blood vessel has been cropped to reveal the underlying smaller, approximately 80 μm diameter vessel shown. This vessel follows the airway and is joined by another vessel of similar size near the proximal end. The blue color of vessel in CDOCT indicates that flow in this vessel is also proximal to distal along the airway. In this case, the small vessel is continuously visualized by CDOCT in Video 2 as it does not exhibit the same magnitude of pulsatility as the larger vessel seen in F. 1. Unlike the previous airway, there is no obvious feature in the structural OCT images that corresponds to the 80-μm diameter blood vessel. However, CDOCT unequivocally identifies and maps this blood vessel along the entire length of the airway. Fig. 2An 8-mm-long segment of airway from a 52-year-old male subject oriented distal to proximal left to right. Subfigures are presented as in Fig. 1. A 2-D structural OCT and CDOCT overlay movie of this airway from distal to proximal is presented in Video 2 (MPG, 8.6 MB) [URL: http://dx.doi.org/10.1117/10.1117/1.JBO.18.5.050501.2]. 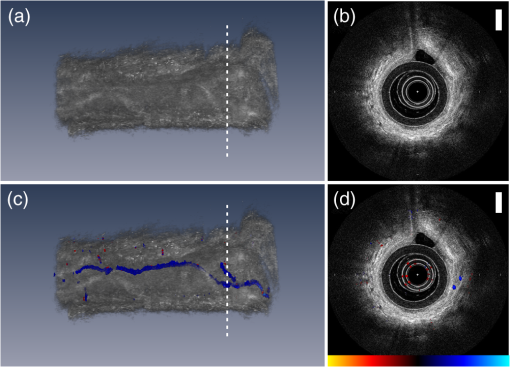 Comparing the CDOCT imaging quality between the two airways presented, Video 1 (8064 A-lines/frame) shows fewer radial artifacts due to laser phase instability than Video 2 (4032 A-lines/frame). However, the faster frame rate in Video 2 (12.5 fps) compared to Video 1 (6.25 fps) should mitigate subject motion artifacts more effectively and allows for finer imaging resolution along the airway axis. In comparison to other OCT vasculature detection modalities, CDOCT is ideally suited for endoscope-guided vasculature detection in the lung. As this implementation of CDOCT only relies on intraframe data processing to extract vasculature, it is less sensitive to ever-present subject and probe motion that can cripple techniques that require frame-to-frame image stability. Rotating fiber optic probes can also be plagued by nonuniform rotational distortion that can complicate inter-frame algorithms even in subject-motion-free environments. 4.SummaryThis study demonstrates for the first time 3-D imaging of in vivo lung vasculature using fiber-optic CDOCT. For diseases where vascular remodeling and angiogenesis play central roles, 3-D mapping of the lung vasculature may provide important insight into disease detection and diagnosis, following disease progression/regression, and interventional monitoring. In combination with morphological information gleaned from structural OCT imaging, vascular imaging is expected to increase the diagnostic capabilities of lung OCT imaging. AcknowledgmentsWe thank Rosa Lopez Lisbona, Hamid Pahlevaninezhad, Myles McKinnon, Chulho Hyun, and Barry Vuong for technical assistance and helpful discussions. This work was supported by the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, the Michael Smith Foundation for Health Research, the Canada Research Chairs and the Canada Foundation for Innovation. ReferencesN. F. Voelkelet al.,
“Angiogenesis in chronic lung disease,”
Chest J., 131
(3), 874
–879
(2007). http://dx.doi.org/10.1378/chest.06-2453 CHETBF 0012-3692 Google Scholar
D. Huanget al.,
“Optical coherence tomography,”
Science, 254
(5035), 1178
–1181
(1991). http://dx.doi.org/10.1126/science.1957169 SCIEAS 0036-8075 Google Scholar
M. Tsuboiet al.,
“Optical coherence tomography in the diagnosis of bronchial lesions,”
Lung Cancer, 49
(3), 387
–394
(2005). http://dx.doi.org/10.1016/j.lungcan.2005.04.007 0169-5002 Google Scholar
S. Lamet al.,
“In vivo optical coherence tomography imaging of preinvasive bronchial lesions,”
Clin. Cancer Res., 14
(7), 2006
–2011
(2008). http://dx.doi.org/10.1158/1078-0432.CCR-07-4418 CCREF4 1078-0432 Google Scholar
L. P. Haririet al.,
“Volumetric optical frequency domain imaging of pulmonary pathology with precise correlation to histopathology,”
Chest J., 143
(1), 64
–74
(2013). CHETBF 0012-3692 Google Scholar
J. A. Izattet al.,
“In vivo bidirectional color Doppler flow imaging of picoliter blood volumes using optical coherence tomography,”
Opt. Lett., 22
(18), 1439
–1441
(1997). http://dx.doi.org/10.1364/OL.22.001439 OPLEDP 0146-9592 Google Scholar
V. X. D. Yanget al.,
“High speed, wide velocity dynamic range Doppler optical coherence tomography (Part I): system design, signal processing, and performance,”
Opt. Express, 11
(7), 794
–809
(2003). http://dx.doi.org/10.1364/OE.11.000794 OPEXFF 1094-4087 Google Scholar
A. Mariampillaiet al.,
“Speckle variance detection of microvasculature using swept-source optical coherence tomography,”
Opt. Lett., 33
(13), 1530
–1532
(2008). http://dx.doi.org/10.1364/OL.33.001530 OPLEDP 0146-9592 Google Scholar
X. Liuet al.,
“Quantitative transverse flow measurement using optical coherence tomography speckle decorrelation analysis,”
Opt. Lett., 38
(5), 805
–807
(2013). http://dx.doi.org/10.1364/OL.38.000805 OPLEDP 0146-9592 Google Scholar
B. A. Standishet al.,
“Doppler optical coherence tomography monitoring of microvascular tissue response during photodynamic therapy in an animal model of Barrett’s esophagus,”
Gastrointest. Endosc., 66
(2), 326
–333
(2007). http://dx.doi.org/10.1016/j.gie.2007.02.040 GAENBQ 0016-5107 Google Scholar
C. Sunet al.,
“In vivo feasibility of endovascular Doppler optical coherence tomography,”
Biomed. Opt. Express, 3
(10), 2600
–2610
(2012). http://dx.doi.org/10.1364/BOE.3.002600 BOEICL 2156-7085 Google Scholar
|

