|
|
|
Tissue oxygenation is a major factor influencing the success or failure of radiation therapy.1 Thus, techniques for measuring tumor partial pressure of oxygen () during the daily fractions of radiation therapy given to patients could be extremely useful for tuning treatment conditions and monitoring therapeutic outcome. However, most methods to measure in tissue require invasive instruments2 and thus are difficult to repeat daily and are sensitive to the high microscopic heterogeneity of in tumors.3 An alternative, noninvasive imaging approach using oxygen-dependent quenching of phosphorescence has been shown to provide accurate images of in tumor tissues in planar4 and tomographic5 imaging geometries. The phosphorescence lifetime of these probes is a robust and sensitive indicator of in the environment. Applying this noninvasive approach during radiation therapy could enable monitoring of tissue oxygenation during the entire course of treatment, allowing assessment and dose optimization throughout the regimen. The occurrence of Čerenkov radiation in tissue during external beam radiotherapy (EBRT) has been demonstrated in several recent papers.6–7 Measuring this emission, as well as secondary luminescence of a fluorophore or phosphor excited by the Čerenkov radiation, i.e., Čerenkov radiation excited luminescence (CREL), has potential for monitoring a variety of functional parameters during radiation therapy. Previously, we demonstrated that phosphorescence of a near-infrared (NIR) oxygen-sensitive probe [platinum(II)-G4, PtG4]7,8 can be excited by Čerenkov emission and measured EBRT in tissue phantoms7 in a point-probe geometry. Coupling this measurement approach with a tomographic imaging modality would facilitate volumetric assessment of tissue oxygenation and provide more accurate quantification of these parameters in deep tissues. In this letter, we investigate the ability to recover tomographic images of CREL-induced phosphorescence yield in a variety of phantom configurations and oxygenations states, and examine the feasibility of CREL tomography to recover images of from time-resolved phosphorescence measurements. The experimental configuration is shown in Fig. 1(a). The external beam irradiator was a Varian Clinic 2100CD linear accelerator (LINAC). In this study, the LINAC was set to irradiate the phantom with a 4-cm-square gamma photon beam with energy of 6 MV and a dose rate of . A 60-mm-diameter cylindrical tissue-simulating phantom was positioned on the treatment bed 100 cm from the LINAC head. The phantom contained a 20-mm-diameter cylindrical anomaly region to simulate tumor tissue. Both the background and anomaly were filled with 1% intralipid emulsion in water to mimic tissue properties for both external beam radiation and optical photon propagation. In addition to the water/intralipid solution, PtG4, the oxygen-sensitive phosphor, was added to the background and anomaly regions of the phantom. Oxygen quenches the phosphorescence emission and lengthens the phosphorescence lifetime of this probe in a well-defined way, making PtG4 a reliable reporter of . Four combinations of PtG4 contrast and oxygenation states were used, as tabulated in Fig. 2. Oxygenation in the anomaly was controlled using the glucose/glucose oxidase/catalase system. Reference values for the PtG4 phosphorescence lifetime in deoxygenated and oxygenated environments were measured independently using a frequency-domain phosphorometer.9 Fig. 1(a) Diagram of the measurement system consisting of a linear accelerator, radiation/optical tissue phantom, and an optical fiber that couples light from the phantom to a spectrometer with a gated ICCD synchronized to the radiation bursts of the LINAC. (b) Top view of the phantom geometry. (c) A two-dimensional cross-section of the Čerenkov field modeled using Geant4-based architecture for machine-oriented simulations (GAMOS) and used as the excitation field for phosphorescence yield image reconstruction. 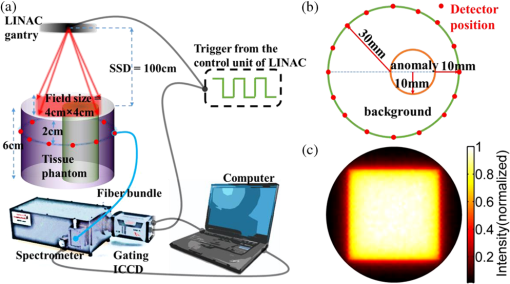 Fig. 2Images of phosphorescence yield from CREL tomography and associated contrast-to-background values for four PtG4 phantom configurations.  Optical measurements were acquired with a gated Princeton Instruments PI-MAX3 ICCD attached to an Acton Research Insight spectrograph positioned outside the treatment room. The ICCD was cooled to and the intensifier set to the maximum gain. Light emitted from the phantom was coupled to the spectrometer through a fiber bundle composed of nineteen 200-μm silica fibers. Using a gated ICCD enables the system to take advantage of the pulsed operation of LINACs. Radiation from the LINAC is delivered in 3.23 μs bursts at a repetition rate of 200 Hz. By synchronizing the ICCD with a trigger signal from the LINAC, as described previously,7,10 the intensity of CREL at different time points after each radiation burst can be measured. This effectively eliminates the contaminating excitation signal, i.e., the Čerenkov emission itself, and facilitates the measurement of phosphorescence lifetime. In this study, optical measurements were acquired after the radiation pulses at time delays between 0 and 200 μs in 4 μs increments, with a gate width of 500 μs. To improve signal-to-noise, 100 spectra were acquired at each time delay and summed up. Background measurements were also acquired and subtracted from the spectra. To produce a full tomographic data set, this process was repeated (sequentially) for 16 positions of the fiber bundle around the boundary of the phantom as represented in Fig. 1(a) and 1(b). Once processed, all spectra were reduced to a single intensity value suitable for image reconstruction by integrating around the PtG4 phosphorescence peak . Reconstructing images of phosphorescence yield requires an estimate of the optical excitation field, which in this case is the Čerenkov field. Accurate modeling of the Čerenkov field can be accomplished by employing established tools from radiation oncology. In this study, GAMOS (Geant4-based Architecture for Medicine-Oriented Simulations)11was used to simulate a 6 MV gamma photon beam, irradiating a area with voxel size . A total of 107 primary particles were launched, and the Čerenkov photons generated were scored.7 A two-dimensional slice of the field, corresponding to the plane of the optical detectors, was extracted and adopted as the excitation field for image reconstruction. The excitation field and processed optical data from the CREL measurements were combined to recover images of phosphorescence yield using the fluorescence toolbox in NIRFAST,12,13 a finite element-based software package for diffusion-based NIR image reconstruction. Images were recovered using a 60-mm-diameter mesh with 1785 nodes, homogeneous absorption () and scattering () properties at the excitation (, ) and emission (, ) wavelengths, and a refractive index . To demonstrate external beam-induced CREL tomography of PtG4, reconstructions were performed in the four phantom configurations using a time delay of zero with respect to the falling edge of the radiation burst, which is simply the integrated phosphorescence decay after the pulse. Images of phosphorescence yield and the associated recovered contrast values, calculated as the mean phosphorescence yield in the known area of interest divided by the same in the known background area, are presented in Fig. 2. These results closely follow expected trends, with the deoxygenated region producing the highest phosphorescence yields and contrasts. Particularly encouraging are the images for phantoms with a constant concentration of PtG4 in both the background and tumor region. While clear contrast in phosphorescence yield was observed when the phantom contained an anoxic tumor region, a nearly homogenous distribution was obtained when both regions were aerated. This demonstrates the strong sensitivity of PtG4 CREL tomography to , and insensitivity to phosphor concentration. To examine the feasibility of using this imaging approach for CREL-based tomography during radiation therapy, time-resolved phosphorescence decay images were analyzed for the phantom with a constant concentration of PtG4 but with an aerated background and anoxic tumor region, as may be encountered in vivo. This was accomplished by reconstructing images for each time delay (from 0 to 200 μs in 4 μs increments) and fitting each image pixel over time to a single exponential [Eq. (1)]: where is the decay initial intensity, is the intensity measured for different time points, and is the phosphorescence lifetime. Figure 3(a) shows the phosphorescence decay curves for pixels in the anoxic and aerated regions, labeled Anomaly and Background, respectively. The full image of phosphorescence lifetime is shown in Fig. 3(b). From this image, an image of can be recovered directly using the Stern-Volmer equation, which relates lifetime and . where is phosphorescence lifetime, is the lifetime in the absence of oxygen (, and is the oxygen quenching constant.Fig. 3(a) Phosphorescence decays calculated as the average values in the aerated anomaly and anoxic background. (b) Recovered lifetime distribution. (c) distribution converted from the lifetime distribution using the Stern-Volmer model. 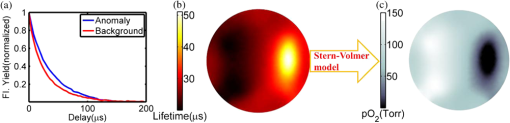 The resulting image is shown in Fig. 3(c). It is noteworthy that the recovered values of in the aerated (150 Torr approximately) and anoxic regions (0 Torr approximately) are very close to the true values measured with the reference probe (reported in Fig. 2). Furthermore, images of and lifetime provide much higher contrasts than those of phosphorescence yield alone, suggesting that the time-dependent approach is more sensitive and quantitative than imaging based on intensity alone. These results suggest that CREL-based oxygen tomography is feasible and that incorporating time-domain analysis can provide an accurate, robust, and quantitative imaging paradigm. The delivery of megavoltage radiotherapy is well controlled and extensively planned for each subject and Čerenkov radiation emission will be emitted from targeted cancer tissues where the charged primary or secondary particles deposit most of their energy. Thus, CREL will often be excited in rather deep tissue regions. The emission peaks of the oxygen-sensitive phosphors are well above 750 nm and thus propagate readily through most tissues. Standard diffuse optical tomography (DOT) systems commonly measure light at these wavelengths through 10 cm of tissue without requiring invasive placement of the optical detectors. As in all forms of optical NIR tomography, tissue scattering and absorption limit depth resolution of the disclosed method. However, because of the relatively slow time scale of triplet emission, phosphorescence lifetime imaging is less affected by scattering and absorption14 than, e.g., fluorescence lifetime imaging, allowing for more accurate spatial reconstructions. The image quality of the tomograms is consistent with typical DOT images, which are generally of low-spatial resolution. However, the prevailing trend in DOT is the implementation of spatial priors from conventional imaging modalities. Since all patients undergoing EBRT also have extensive co-registered CT scans used for treatment planning, one could envision using these data to guide the recovery of . While total acquisition times reported here were rather long (about 25 s per position), the instrument and experimental procedure used in this proof-of-concept study were not optimized for fast acquisition. Small-diameter fibers and single-channel spectroscopic detection were all unnecessary constraints limiting acquisition speed. Large-diameter fiber bundles and coupled to gated wide-area avalanche photodiodes or photomultiplier tubes could improve light collection efficiency by over 1500-fold without loss of image quality. Additional improvements in signal intensity would obtain using more optimized oxygen-sensitive probes at higher concentrations, as have been studied previously.8 The ability to image distribution during radiotherapy, as demonstrated here, would provide unprecedented information about the tumor micro-environment. This may have a significant impact in radiotherapy research programs, such as in the development of adjuvant and synergistic therapies, and in planning and tailoring clinical treatment regimens. AcknowledgmentsThis work was supported by NIH grants R01CA120368 (BWP), R01CA109558 (BWP) and Department of Defense award W81XWH-09-1-0661 (SCD). SAV acknowledges support of the grant from the Penn Comprehensive Neuroscience Center. ReferencesP. VaupelA. MayerM. Hockel,
“Impact of hemoglobin levels on tumor oxygenation: the higher, the better?,”
Strahlentherapie Und Onkologie, 182
(2), 63
–71
(2006). http://dx.doi.org/10.1007/s00066-006-1543-7 STONE4 0179-7158 Google Scholar
M. Nordsmarket al.,
“Measurements of hypoxia using pimonidazole and polarographic oxygen-sensitive electrodes in human cervix carcinomas,”
Radiother. Oncol., 67
(1), 35
–44
(2003). http://dx.doi.org/10.1016/S0167-8140(03)00010-0 RAONDT 0167-8140 Google Scholar
S. M. EvansC. J. Koch,
“Prognostic significance of tumor oxygenation in humans,”
Cancer Lett., 195
(1), 1
–16
(2003). http://dx.doi.org/10.1016/S0304-3835(03)00012-0 CALEDQ 0304-3835 Google Scholar
D. F. WilsonG. J. Cerniglia,
“Localization of tumors and evaluation of their state of oxygenation by phosphorescence imaging,”
Cancer Res., 52
(14), 3988
–3993
(1992). CNREA8 0008-5472 Google Scholar
S. V. AprelevaD. F. WilsonS. A. Vinogradov,
“Tomographic imaging of oxygen by phosphorescence lifetime,”
Appl. Opt., 45
(33), 8547
–8559
(2006). http://dx.doi.org/10.1364/AO.45.008547 APOPAI 0003-6935 Google Scholar
J. Axelssonet al.,
“Cerenkov emission induced by external beam radiation stimulates molecular fluorescence,”
Med. Phys., 38
(7), 4127
–4132
(2011). http://dx.doi.org/10.1118/1.3592646 MPHYA6 0094-2405 Google Scholar
R. Zhanget al.,
“Cerenkov radiation emission and excited luminescence (CREL) sensitivity during external beam radiation therapy: Monte Carlo and tissue oxygenation phantom studies,”
Biomed. Opt. Express, 3
(10), 2381
–2394
(2012). http://dx.doi.org/10.1364/BOE.3.002381 BOEICL 2156-7085 Google Scholar
T. V. Esipovaet al.,
“Two new “protected” oxyphors for biological oximetry: properties and application in tumor imaging,”
Anal. Chem., 83
(22), 8756
–8765
(2011). http://dx.doi.org/10.1021/ac2022234 ANCHAM 0003-2700 Google Scholar
S. A. Vinogradovet al.,
“Frequency domain instrument for measuring phosphorescence lifetime distributions in heterogeneous samples,”
Rev. Sci. Instrum., 72
(8), 3396
–3406
(2001). http://dx.doi.org/10.1063/1.1386634 RSINAK 0034-6748 Google Scholar
A. K. Glaseret al.,
“Time-gated Cherenkov emission spectroscopy from linear accelerator irradiation of tissue phantoms,”
Opt. Lett., 37
(7), 1193
–1195
(2012). http://dx.doi.org/10.1364/OL.37.001193 OPLEDP 0146-9592 Google Scholar
P. ArceP. R. MendesJ. I. Lagares,
“GAMOS: an Easy and Flexible Framework for Geant4 Simulations,”
3162
–3168
(2008). Google Scholar
S. C. Daviset al.,
“Image-guided diffuse optical fluorescence tomography implemented with Laplacian-type regularization,”
Opt. Express, 15
(7), 4066
–4082
(2007). http://dx.doi.org/10.1364/OE.15.004066 OPEXFF 1094-4087 Google Scholar
H. Dehghaniet al.,
“Near infrared optical tomography using NIRFAST: Algorithm for numerical model and image reconstruction,”
Commun. Numer. Methods Eng., 25
(6), 711
–732
(2009). http://dx.doi.org/10.1002/cnm.v25:6 CANMER 0748-8025 Google Scholar
S. V. AprelevaS. A. Vinogradov,
“Influence of optical heterogeneities on reconstruction of spatial phosphorescence lifetime distributions,”
Opt. Lett., 33
(8), 782
–784
(2008). http://dx.doi.org/10.1364/OL.33.000782 OPLEDP 0146-9592 Google Scholar
|

