|
|
1.Introduction1.1.Current Laser Lithotripsy TechniquesApproximately 10% of the U.S. population will suffer from urinary stone disease during their lifetime.1 To treat severe urinary stone disease, an endoscopic approach is commonly used with an optical fiber, coupled to a holmium:YAG (holmium) laser, inserted into the working channel of an ureteroscope, which stretches from the urethra to the kidney or to the stone’s location. The holmium laser has become the gold standard laser lithotripter in recent years due, in part, to its low cost, relatively high pulse energies, and versatility to rapidly ablate both soft and hard urologic tissues. However, one limitation of the holmium laser is its relatively large and multimode beam waist, which limits optimal power coupling into only optical fibers with core diameters greater than .2,3 Due to space limitations in the flexible ureteroscope’s -mm inner diameter working channel, smaller fibers are preferred for improved irrigation and higher flexibility in the upper urinary tract.4–6 However, coupling the holmium laser’s multimode beam with smaller fibers (-μm core diameter) risks overfilling of the input fiber core, launching into the cladding, and damaging the fiber. Damage in the form of fiber tip degradation and “burn-back” also occur at the distal tip, where stone fragmentation occurs frequently resulting in the disposal of the fiber after each procedure. 1.2.Thulium Fiber Laser LithotripsyThe thulium fiber laser (TFL) () has recently been studied in the laboratory as an alternative laser lithotripter to holmium ()7–14 with the main advantage of TFL being the ability to optimally couple into smaller-diameter fibers. The TFL also has a higher absorption coefficient () and shorter optical penetration depth in water, compared with holmium (), which translates into an approximately four times lower ablation threshold.11,15,16 This property allows TFL tissue ablation at pulse energies significantly lower than with holmium. The diode-pumped TFL can also be electronically modulated to operate at nearly any pulse length or pulse configuration, unlike the flashlamp-pumped holmium laser. Customized micropulse trains, or pulse packets, have been reported to increase ablation rates during lithotripsy.12 Potential remaining obstacles for clinical acceptance of the TFL include reducing laser cost and demonstrating TFL’s versatility for other urological procedures as well. The main advantage of the TFL for this study is its near single mode or Gaussian spatial beam profile generated by the 18-μm core diameter fiber lasing medium compared with the multimode holmium beam (-μm diameter).2,8 The TFL spatial beam profile improves coupling and transmission of laser power through small-core fibers for lithotripsy, allowing the use of fiber core diameters .8 This reduction in fiber cross-section in turn provides less hindering of ureteroscope deflection and increased saline irrigation rates through the single working channel,6,10 which could potentially translate into reduced procedure times, reduced probability of ureteroscope damage, and improved patient safety. The TFL beam profile allows small-core trunk fiber and proximal fiber tip longevity; however, the smaller diameter distal fiber tips have been shown to mechanically degrade and suffer from burn-back more rapidly than the larger diameter fiber tips.10,17 1.3.Tapered Distal Fiber TipsThe TFL’s Gaussian beam profile allows improved coupling into small fiber core diameters essentially eliminating proximal fiber tip damage.8 By reversing the typical orientation of the tapered fiber, and using the increased taper and larger core at the output end, the distal tip is more robust and resistant to damage during the lithotripsy procedure.10 The benefits of a small core trunk fiber, including increased irrigation and flexibility, are combined with those of a large core distal tip for robustness. A steep tapered fiber with a large distal fiber tip can be extruded from the tip of the ureteroscope into contact with the stone for ablation, while also allowing improved irrigation since only the small diameter trunk fiber remains in the working channel. However, damage and burn-back may still occur at the large distal tip, and the entire fiber must be replaced during or after surgical procedures. Also, the tapered joint is delicate and susceptible to fracture during handling. 1.4.Detachable Distal Fiber TipsWith no proximal fiber tip damage, only the distal fiber tip surface degrades or experiences overall fiber burn-back during stone fragmentation. A low-profile ( outer diameter), twist-locking, detachable distal fiber tip interface for TFL lithotripsy was tested ex vivo on urinary stones, demonstrating similar stone ablation rates to the tapered distal fiber tip.14 The largest ablation rates were observed when using pulse rates ; however, these higher pulse rates also contributed to the fastest distal fiber tip degradation. For urologists desiring faster TFL lithotripsy procedures, the incorporation of detachable distal fiber tips allows quick replacement of damaged tips without worrying about the laser-to-trunk fiber connection. The main disadvantage of using detachable fiber tips is their manufacturing complexity. 1.5.Motivation for Hollow Fiber TipsRecently, distal fiber tip degradation during TFL lithotripsy was concluded primarily to be the result of superheating of water at the fiber tip/stone interface, and not the impact of stone fragments.14 It was hypothesized that when the distal fiber tip is in contact with the stone, and operating at a high pulse rate, the confined vapor bubble cannot collapse between the consecutive pulses. In this instance, the fiber tip may experience excessive melting temperatures (), which leads to fiber tip degradation. This effect can be observed during stone ablation by a visibly bright flash or sparking when the fiber tip comes in contact with the stone. It was postulated that fiber melting temperatures may also occur in stone contact during holmium laser lithotripsy because of the high pulse energy, leading to fiber degradation. Several ways to reduce heating of the fiber tip are to: decrease pulse rate, decrease pulse energy, increase fiber tip diameter, and/or increase the fiber distance to the stone. Decreasing the pulse rate is a viable option; however, urologists would prefer faster procedures by sacrificing fiber tip quality. Urologists currently can solve this problem by increasing the fiber core diameter; however, small diameter fibers are desirable due to increased flexibility and irrigation in the single working channel of the ureteroscope. With a bare fiber, the stone-to-fiber distance is nearly impossible to control due to the stone motion and retropulsion during the procedure, and ablation stallout has been observed beyond due to absorption of the TFL energy by the intervening water layer.10 In this study, we explore placing a hollow steel tube over a recessed and fixed fiber tip to precisely control the fiber-to-stone distance for TFL lithotripsy. Hollow fiber tips may reduce or eliminate fiber tip degradation in small diameter fibers, while still maintaining acceptable stone ablation rates for both TFL and holmium lithotripsy. 2.Materials and Methods2.1.Thulium Fiber LaserA 100-W, continuous-wave TFL (Model TLR 110-1908, IPG Photonics, Inc., Oxford, MA) with a center wavelength of 1908 nm was used in these studies. An 80-mm FL plano-convex calcium fluoride lens (LA5458, Thorlabs, Newton, NJ) was used to focus the 5.5-mm diameter collimated fiber laser beam down to a spot diameter of approximately 75 μm () for coupling into the 150-μm core fiber (FIP150165195, Polymicro, Phoenix, AZ). The laser was modulated with a function generator (Model DS345, Stanford Research Systems, Sunnyvale, CA) to produce a pulse energy of , pulse duration of 500 μs, and pulse rate of 150 Hz for all lithotripsy studies. This pulse rate was chosen based on previous studies that showed significant distal fiber tip degradation when using pulse rates greater than .14 2.2.Hollow Fiber TipsA low-OH silica trunk fiber (Polymicro) with a core and cladding diameter of 150 and 165 μm, respectively, and a length of 2 m with maximum numerical aperture (NA) of 0.22 was used. The polyimide fiber buffer (195-μm outer diameter) at the distal tip was removed up to 10 to 15 mm. Stainless steel hypodermic tubing (B000FMWK6A, Amazon Supply, Seattle, WA) with inner and outer diameters of 178 and 356 μm, respectively, cut to a length of 10 mm was used for each hollow tip. To clean the cut and free burs from the inner hole, a small drill bit was used to countersink the end holes of the tube. A micromanipulator, under magnification, was used to recess the fiber in the tube to the specified depth. A small amount of super glue was then placed on the back end of the tube to fix the tube and the fiber together. A photograph of the hollow fiber tip tested is shown in Fig. 1. Fig. 1Photograph of -μm outer diameter, 10-mm-long stainless steel tube glued to distal tip of 150-μm core diameter trunk fiber. 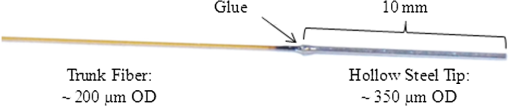 The ideal distal fiber tip recession distance was unknown, so various distances were tested including 100 μm, which was less than the fiber diameter; 1 mm, which was the experimental ablation stallout distance for a bare fiber; and 2 mm. The six scenarios tested (bare fiber and recessed fibers to 100, 250, 500, 1000, and 2000 μm) are shown in Table 1. Before testing, it was hypothesized that the vapor bubble could not collapse back into the front of the hollow tip after the initial pulse, possibly allowing longer fiber–stone separations than with a bare fiber due to reduced attenuation. The output NAs of the fibers were measured at in air, corresponding to a maximum divergence angle of in water, as illustrated in Table 1. However, the inner surface of the steel hypodermic tube was measured with a scanning electron microscope (Raith 150, Raith GmbH, Dortmund, Germany) and appeared to have surface features larger than the wavelength of (Fig. 2), and therefore they did not behave as an efficient reflective surface. Therefore, longer fiber recession distances could show reduced performance due to the poor wave-guiding ability of the hypodermic tubing. Table 1Scale representation of bare fiber and five hollow fiber tips tested as a function of fiber recession distance.
NOTE: The length of steel tubes used was 10 mm. The maximum divergence angle was 2 deg. The 2-mm recessed fiber was believed to lose significant laser energy due to the poor wave-guiding ability of the steel tubes. NA, not applicable. Fig. 2SEM image of cut stainless steel hypodermic tubes, countersunk with a drill bit. The drilled surface can be seen on top of magnified image at right, with the unmanipulated, relatively rough, hypodermic inner surface underneath. The hypodermic’s inner surface was not polished and therefore limited the wave-guiding ability at deeper fiber recession depths. 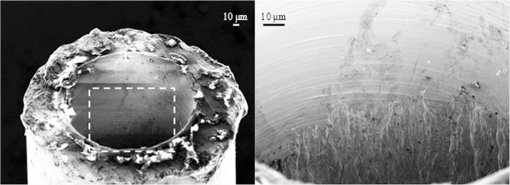 2.3.Stone Ablation StudyHuman calcium oxalate monohydrate (COM) urinary stone samples with purity were obtained from a stone analysis laboratory (LabCorp, Oklahoma City, OK). The stone samples ranged in diameter from 8 to 15 mm and in mass from 150 to 500 mg with an average mass of about 250 mg. The initial dry stone mass was recorded with an analytical balance (AB54-S, Mettler-Toledo, Switzerland) before securing the stone in place with a clamp, and then submerging it in a saline bath. The laser radiation was delivered through the hollow fiber tips and bare fiber to the stone surface in contact mode. The fiber was held manually, and a scanning motion over the stone surface under gentle force was used during laser irradiation to keep the fiber in contact with the stone’s surface. A total of 9000 pulses were delivered to each stone sample, equivalent to stone ablation for 1 min at 150 Hz. The stones were then dried in an oven at 70°C for before the final dry mass measurements were conducted. Optical transmission in air for each fiber was tested at a pulse rate of 10 Hz. Operating the hollow fiber tips at 150 Hz in air resulted in the stainless steel tube becoming red hot, permanently damaging the inner surface of the steel tips. However, this effect was never observed when operating in saline or in water at pulse rates up to 200 Hz. 2.4.Fiber Tip Burn-Back and DegradationAfter stone ablation, a separate 125-μm OD fiber was placed into the distal opening of the hollow fiber tips, and the inserted distance was measured. The overall fiber burn-back distance was calculated by comparing this measurement with the original depth before stone ablation. The bare fiber was marked at a distance down the fiber trunk, and burn-back was measured using photography before and after stone ablation. A microscope was also used to image the distal fiber tip surfaces for degradation with white light illumination on the proximal fiber tips. 2.5.Flow Velocimetry StudyPolymer microspheres (Duke Standards 4000 Series, Thermo Fisher Scientific, Waltham, MA) with diameters ranging from 30 to 50 μm, for visible light, and a density of were suspended in a water-filled Petri dish, which was illuminated from the side by a fiber optic lamp. The hollow-tip fibers were inserted into the dish, the laser energy was delivered into the water, and videos of particle flow were recorded under magnification of . The recorded particle flow was used to map the water flow for each fiber tip. Velocities of the microspheres along the optical axis were observed qualitatively and used to compare potential retropulsion effects that would be present during surgery. 3.Results3.1.Stone AblationTable 2 shows the mean stone ablation rates for the five hollow tips tested and the bare fiber. The ablation rates measured were comparable in magnitude to previous studies.11 The mean ablation rate appeared to decrease slightly with increasing fiber recession. A Student -test showed no statistical difference between stone ablation rates for the bare fiber and the fibers recessed 100 and 250 μm () or between 500 and 1000 μm (). However, there was a statistical difference between the two groups (). The 2-mm recessed fiber showed very low ablation rates believed to be a result of the poor wave-guiding ability of the hypodermic tubing, and therefore lower optical transmission at the output end of the hollow tip. The higher standard deviation for the bare fiber stone ablation rates may be due to the inconsistent tip geometry from burn-back or that the silica fiber tip is less visible in saline, and therefore was not in good contact with the stone as frequently as the hollow steel fiber tips. Table 2Calcium oxalate monohydrate (COM) stone ablation rates and fiber burn-back/degradation for the hollow fiber tips as a function of fiber recession distance.
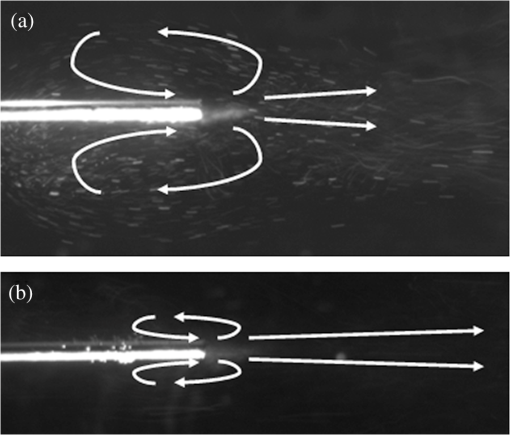
Note: Ablation rates were calculated from mass loss after 9000 pulses delivered through 150-μm core fibers at pulse energy of 34 mJ, pulse rate of 150 Hz, and 500-μs pulse duration (N≥5). Burn-back measurements, N≥10. 3.2.Fiber Burn-Back and Tip DegradationOnly the bare fiber showed measurable fiber burn-back (Table 2). Flashing or sparking was observed during stone ablation with all fibers. However, the frequency decreased with increasing fiber recession distance, and it is unclear whether it originated from the stone or the fiber. The measured recession distances of the hollow-tip fibers were unchanged after 10 min of COM stone ablation at 150 Hz. Microscopic imaging of the fiber tips provided additional information about the fiber tip surface degradation (Fig. 3). The 100- and 250-μm recessed tips displayed similar surface degradation to that of the bare fiber minus the overall fiber burn-back. At 500 μm, a flat surface was still apparent; however, a blistered or possibly roughened by dusting effect was observed, indicating a partial phase change of the silica fiber. The fibers recessed 1 and 2 mm were the only fibers to still show a polished surface in most areas. The unpolished areas were mostly debris, which could not be wiped off while the fiber was fixed inside the hollow steel tip. The blurriness observed in the images could be attributed to the limited NA when imaging deep into the hollow tips with a high NA microscope. Fig. 3Microscopic images of the hollow fiber tips compared with the bare fiber after 10 min of laser irradiation in contact with calcium oxalate monohydrate (COM) stones at 150 Hz (90,000 total pulses) with -mJ pulse energy. Decreasing fiber tip degradation was observed as the fiber recession distance was increased. The debris observed on the 1- and 2-mm fiber tips were remnants after cleaning with compressed air, and were believed to be fused to the surface. Focusing down within the deeper hollow fiber tips limited the resolution of images taken.  A correlation was observed between the stone ablation rates and the fiber tip degradation, similar to the previous detachable fiber tip study.14 The stone ablation rates were divided into two statistically significant groups, which correlate with whether the fiber tip showed significant or minimal surface degradation. 3.3.Flow VelocimetryParticle velocities along the optical axis were observed at the beginning of laser operation, because after a few seconds, a circulating vortex was created by the limited volume of the Petri dish. Figure 4 shows representative images of the particle flow for fiber recession distances of 100 and 1000 μm. Water flow increased in the forward direction with increasing fiber recession depth. The cavitation bubble is believed to be represented by the cloudy cone-like shape at the fiber tip. 4.Discussion4.1.Hollow Fiber TipsA simple and inexpensive approach for improving the durability of optical fibers for laser lithotripsy was demonstrated and tested on human COM urinary stones ex vivo. However, there were a few minor limitations to this preliminary design that may be addressed prior to use in the clinic. For example, the length of the hollow steel tips, which are rigid, could be reduced from to to improve the overall flexibility of the fiber design for the insertion through a flexible ureteroscope. More customized inner and outer diameter steel tips could allow improved mounting to the fiber buffer instead of the cladding, possibly increasing fiber robustness. Also, the super glue used to secure the hollow tip to the fiber may easily be replaced by a biocompatible, heat-resistant adhesive. 4.2.Stone AblationUse of hollow fiber tips instead of a bare fiber did not show a significant change in mean stone ablation rate, which decreased only slightly with increasing fiber recession. Statistical analysis showed no difference between the bare fiber and a fiber recessed 250 μm and only a drop to approximately two-thirds ablation rate for a fiber recession of 1 mm. Therefore, the stone ablation rates achieved by operating a 1-mm recessed hollow fiber tip at 150 Hz were comparable with operating a bare fiber at 100 Hz,11 but with the added benefit of no fiber tip degradation. The decrease in stone ablation rates for 500 and 1000 μm could be attributed to reduced pulse energy delivered to the stone due to the diverging fiber output reflecting off the inner surface of the stainless steel tube, which is unpolished (Fig. 2). Table 1 shows a diagram of the maximum divergence angle () for each hollow fiber tip. Since the experimental fibers were not perfectly centered in the tube, it was assumed that both the 500-μm and 1-mm recessed fibers lost some energy into the steel tube; however, this loss was not as significant as losses with the 2-mm recessed fiber. Some energy loss in the deeper fibers could also be attributed to increased absorption in the intervening water collapsing into the hollow tip, as the deeper fibers attempted to maintain a larger vapor region within the steel tubing. This effect may explain the negligible decrease in transmission measured in air for the deeper fiber tips. Future hollow-tip studies using polished inner tubing may show improved performance for longer recession distances (e.g., 2 mm), and possibly bring the ablation rates for 500- and 1000-μm recessed fibers closer to the bare fiber rate. 4.3.Fiber Tip Burn-Back and DegradationThe main advantage of using the hollow fiber tip design was the minimal overall burn-back of the fiber tip. Superficial fiber tip surface degradation was observed on fibers down to a recession depth of 250 μm, and the fiber at 1 mm still showed a polished surface after 90,000 pulses at 150 Hz. However, no recessed fibers lost their cladding or suffered from burn-back. Similar to the tapered fiber tip degradation observed in previous studies,10,14 the outer rim of the steel tube provided sufficient stone offset distance to prevent further burn-back. The minimal degradation for fibers recessed 500 μm and beyond was not believed to be a result of reduced stone ablation rates. The 1-mm recessed hollow fiber tip showed a factor of 4 improvement in stone ablation rate over previously studied bare fibers that suffered from fiber burn-back.10 4.4.Retropulsion and Flow VelocimetryLasers emitting at the wavelengths that are highly absorbed by water are known to create cavitation bubbles, rapidly expanding and collapsing vapor bubbles, on the timescale of microseconds in water.18 The cavitation bubbles provide a conduit for the subsequent laser energy to reach the stone with little absorption. However, the cavitation bubbles also contribute to stone retropulsion, which is a well studied but undesirable side effect that pushes the stone away from the fiber tip during lithotripsy.19–24 The bubbles are larger than the fiber diameter and can extend from the fiber tip up to depending on pulse rate and energy. The mechanical shockwaves created by the cavitation bubbles are not a major contributor to stone fragmentation at the 500-μs pulse duration used in this study, because the ablation mechanism is predominantly photothermal.25 Retropulsion studies were previously performed using plaster-of-Paris stone phantoms by measuring the displacement of the stone phantom from the fiber tip after laser irradiation in a submerged horizontal trough.11 A similar setup was used for attempting to measure stone retropulsion using hollow fiber tips. However, within the initial laser pulses () at 150 Hz, the stone phantoms experienced a significant force and were displaced at large and inconsistent distances () from the hollow fiber tip. These preliminary observations implied that retropulsion was greater for the hollow fiber tips than the bare fiber tips. For comparison, a separate flow velocimetry study was performed for each hollow fiber tip relating to retropulsion trends. Water flow surrounding the fiber tip, caused by exploding cavitation bubbles, was previously studied.13 Using a bare fiber placed in a water bath containing microspheres as visible scatterers, significant water flow was witnessed in the forward direction along the optical path. This flow increased with increasing pulse rate and energy. Weaker secondary water vortices were observed in the reverse direction and allowed pulling of the stone when placing the fiber tangent to the polar region of the stone instead of normal to the equator. In this study, similar observations were used to examine retropulsion forces from hollow fiber tips. Figure 4 shows a comparison between a 100- and 1000-μm recessed fiber. Microsphere speeds in front of the fiber were too great to quantify with our imaging system. Only qualitative observation of increasing velocity could be stated for increasing fiber recession depth and pulse rate. It was unclear whether the bare fiber created more forward water flow than the 100-μm recessed fiber; however, increased water flow was observed when increasing the recessed distance to 1 mm. 4.5.Specialty Fiber TipsTable 3 shows a comparison of different properties for the hollow fiber tip and other specialty tips previously tested during TFL lithotripsy. A potential disadvantage to using hollow fiber tips over other fiber types is the observed effect of increased forward water flow, which may correlate with increased stone retropulsion. An increase in particle velocity was observed with an increase in fiber recession depth. One possible cause of this effect may be that the cavitation bubble does not collapse back inside the hollow tip after the initial pulse, and that this region remains a vapor pocket during laser operation. This vapor exerts a pressure which increases in the forward direction as the pocket increases along the same axis. Table 3Comparison of small and large core optical fibers8 as well as previously studied tapered,10 detachable,14 and hollow fiber tips for use in thulium fiber laser (TFL) lithotripsy. 4.6.Hollow-Tip Fiber Integration with Stone BasketStone baskets are currently used to assist during lithotripsy procedures by expanding at the stone site, and then collapsing around the stone for removal. For laser lithotripsy, combining the laser operation with grasping and stabilization of the stone in a basket has been performed.26,27 For conventional holmium laser lithotripsy procedures, the fibers typically have core and overall diameters greater than 200 and 400 μm, respectively. Stone baskets are highly flexible, but when combined with a large-core-diameter laser fiber, endoscope flexibility and irrigation through the working channel is reduced. Independent fiber manipulation with respect to the stone basket is often needed due to fiber fragility and degradation when ablating stabilized stones. These fibers are typically labeled as disposable due to proximal and distal fiber tip damage experienced during the procedure or due to liabilities associated with re-sterilization. Figure 5 shows a stone basket (Boston Scientific, Natick, MA) with outer diameter of 1.9 Fr () placed side-by-side with a hollow fiber tip (195-μm trunk outer diameter). Due to the TFL’s ability to couple into small diameter fibers with a hollow fiber tip for minimal fiber degradation, integration of a dedicated fiber with a stone basket may be possible while still maintaining a relatively small footprint within the ureteroscope working channel’s limited dimensions (-mm diameter), potentially allowing unhindered operation in hard-to-reach areas like the lower pole of the kidney. The hollow fiber tips do not suffer significant burn-back like standard fibers, and therefore independent fiber manipulation may not be necessary, possibly reducing the instrument’s overall cross-section. The stone basket may also collapse, as the stone diameter shrinks due to ablation and fragmentation, constantly maintaining the stone in contact with the hollow fiber tip. Once a minimal stone size is reached, the urologist could then remove the stone from the body within the basket. Fig. 5Representation of potential hollow fiber tip integration with stone basket. The 1.9 Fr () outer diameter stone basket was positioned above a hollow fiber tip (-μm outer diameter, 150-μm core diameter) in different configurations: collapsed basket (a), open basket (b), and stone ablation (c). With minimal to no fiber tip burn-back, the hollow fiber tip could be directly integrated within a stone basket at a fixed position, allowing simultaneous stone stabilization and ablation within a single combined instrument with potentially greater flexibility than the current basket integration methods. Scale in millimeters. 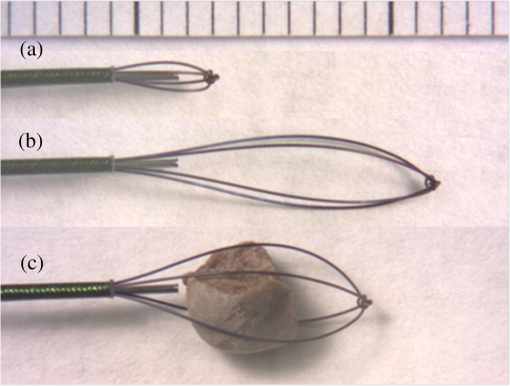 5.ConclusionsThis study introduces a simple, compact, robust, and inexpensive hollow fiber tip design for use during TFL lithotripsy. A hollow steel tube placed over a recessed and fixed small-core fiber tip significantly reduced or eliminated distal fiber tip damage, while maintaining acceptable stone ablation rates. A potential disadvantage to using hollow fiber tips is the observed effect of increased forward water flow or retropulsion; however, hollow-tip fibers could potentially be integrated into a stone basket to minimize stone retropulsion during the procedure. Hollow fiber tips could also potentially be used to improve distal fiber tip longevity during holmium:YAG laser lithotripsy as well. AcknowledgmentsThe authors thank Arash Darafsheh and Kenneth Allen from Dr. Vasily Astratov’s Mesophotonics Lab at UNC Charlotte as well as Doctor Lou Deguzman for assistance with the scanning electron microscope. Richard Blackmon was supported by a National Science Foundation Graduate Fellowship. ReferencesJ. Y. ClarkI. M. ThompsonS. A. Optenberg,
“Economic impact of urolithiasis in the United States,”
J. Urol., 154
(6), 2020
–2024
(1995). http://dx.doi.org/10.1016/S0022-5347(01)66680-1 JOURAA 0022-5347 Google Scholar
S. Griffin,
“Fiber optics for destroying kidney stones,”
Biophoton. Int., 11
(4), 44
–47
(2004). Google Scholar
A. J. Markset al.,
“Holmium:yttrium-aluminum-garnet lithotripsy proximal fiber failures from laser and fiber mismatch,”
Urology, 71
(6), 1049
–1051
(2008). http://dx.doi.org/10.1016/j.urology.2007.10.060 0090-4295 Google Scholar
B. E. Knudsenet al.,
“Performance and safety of holmium:YAG laser optical fibers,”
J. Endourol., 19
(9), 1092
–1097
(2005). http://dx.doi.org/10.1089/end.2005.19.1092 JENDE3 0892-7790 Google Scholar
A. C. MuesJ. M. TeichmanB. E. Knudsen,
“Evaluation of 24 holmium:YAG laser optical fibers for flexible ureteroscopy,”
Urology, 182
(1), 348
–354
(2009). http://dx.doi.org/10.1016/j.juro.2009.02.112 0090-4295 Google Scholar
T. A. Khemeeset al.,
“Evaluation of a new 240-μm single-use holmium:YAG optical fiber for flexible ureteroscopy,”
J. Endourol., 27
(4), 475
–479
(2013). http://dx.doi.org/10.1089/end.2012.0513 JENDE3 0892-7790 Google Scholar
N. M. Fried,
“Thulium fiber laser lithotripsy: an in vitro analysis of stone fragmentation using a modulated 110-watt Thulium fiber laser at 1.94 micrometers,”
Lasers Surg. Med., 37
(1), 53
–58
(2005). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
N. J. ScottC. M. CilipN. M. Fried,
“Thulium fiber laser ablation of urinary stones through small-core optical fibers,”
IEEE J. Sel. Top. Quantum. Electron., 15
(2), 435
–440
(2009). http://dx.doi.org/10.1109/JSTQE.2008.2012133 IJSQEN 1077-260X Google Scholar
R. L. BlackmonP. B. IrbyN. M. Fried,
“Holmium:YAG () versus thulium fiber () laser lithotripsy,”
Lasers Surg. Med., 42
(3), 232
–236
(2010). http://dx.doi.org/10.1002/lsm.v42:3 LSMEDI 0196-8092 Google Scholar
R. L. BlackmonP. B. IrbyN. M. Fried,
“Thulium fiber laser lithotripsy using tapered fibers,”
Lasers Surg. Med., 42
(1), 45
–50
(2010). http://dx.doi.org/10.1002/lsm.v42:1 LSMEDI 0196-8092 Google Scholar
R. L. BlackmonP. B. IrbyN. M. Fried,
“Comparison of holmium:YAG and thulium fiber laser lithotripsy: ablation thresholds, ablation rates, and retropulsion effects,”
J. Biomed. Opt., 16
(7), 071403
(2011). http://dx.doi.org/10.1117/1.3564884 JBOPFO 1083-3668 Google Scholar
R. L. BlackmonP. B. IrbyN. M. Fried,
“Enhanced thulium fiber laser lithotripsy using micro-pulse train modulation,”
J. Biomed. Opt., 17
(2), 028002
(2012). http://dx.doi.org/10.1117/1.JBO.17.2.028002 JBOPFO 1083-3668 Google Scholar
R. L. Blackmonet al.,
“Fiber-optic manipulation of urinary stone phantoms using holmium:YAG and thulium fiber lasers,”
J. Biomed. Opt., 18
(2), 028001
(2013). http://dx.doi.org/10.1117/1.JBO.18.2.028001 JBOPFO 1083-3668 Google Scholar
T. C. Hutchenset al.,
“Detachable fiber optic tips for use in thulium fiber laser lithotripsy,”
J. Biomed. Opt., 18
(3), 038001
(2013). http://dx.doi.org/10.1117/1.JBO.18.3.038001 JBOPFO 1083-3668 Google Scholar
G. M. HaleM. R. Querry,
“Optical-constants of water in 200-nm to 200-μm wavelength region,”
Appl. Opt., 12
(3), 555
–563
(1973). http://dx.doi.org/10.1364/AO.12.000555 APOPAI 0003-6935 Google Scholar
K. T. Schomackeret al.,
“ laser ablation of tissue: effect of wavelength on ablation threshold and thermal damage,”
Lasers Surg. Med., 11
(2), 141
–151
(1991). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
A. C. MuesJ. M. TeichmanB. E. Knudsen,
“Quantification of holmium:yttrium aluminum garnet optical tip degradation,”
J. Endourol., 23
(9), 1425
–1428
(2009). http://dx.doi.org/10.1089/end.2009.0384 JENDE3 0892-7790 Google Scholar
M. Frenzet al.,
“Starting mechanisms and dynamics of bubble formation induced by a Ho:Yttrium aluminum garnet laser in water,”
J. Appl. Phys., 84
(11), 5905
–5912
(1998). http://dx.doi.org/10.1063/1.368906 JAPIAU 0021-8979 Google Scholar
M. D. Whiteet al.,
“Evaluation of retropulsion caused by holmium:YAG laser with various power settings and fibers,”
J. Endourol., 12
(2), 183
–186
(1998). http://dx.doi.org/10.1089/end.1998.12.183 JENDE3 0892-7790 Google Scholar
H. Leeet al.,
“Stone retropulsion during holmium:YAG lithotripsy,”
J. Urol., 169
(3), 881
–885
(2003). http://dx.doi.org/10.1097/01.ju.0000046367.49923.c6 JOURAA 0022-5347 Google Scholar
D. S. Finleyet al.,
“Effect of holmium:YAG laser pulse width on lithotripsy retropulsion in vitro,”
J. Endourol., 19
(8), 1041
–1044
(2005). http://dx.doi.org/10.1089/end.2005.19.1041 JENDE3 0892-7790 Google Scholar
C. G. Marguetet al.,
“In vitro comparison of stone retropulsion and fragmentation of the frequency doubled, double pulse Nd:YAG laser and the holmium:YAG laser,”
J. Urol., 173
(5), 1797
–1800
(2005). http://dx.doi.org/10.1097/01.ju.0000154341.08206.69 JOURAA 0022-5347 Google Scholar
H. W. Kanget al.,
“Dependence of calculus retropulsion on pulse duration during Ho:YAG laser lithotripsy,”
Lasers Surg. Med., 38
(8), 762
–772
(2006). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
P. KalraN. LeD. Bagley,
“Effect of pulse width on object movement in vitro using Ho:YAG laser,”
J. Endourol., 21
(2), 228
–231
(2007). http://dx.doi.org/10.1089/end.2005.1130 JENDE3 0892-7790 Google Scholar
G. J. Vassaret al.,
“Holmium:YAG lithotripsy: photothermal mechanism,”
J. Endourol., 13
(3), 181
–190
(1999). http://dx.doi.org/10.1089/end.1999.13.181 JENDE3 0892-7790 Google Scholar
A. G. Hofstetteret al.,
“Laser in urological surgery,”
Laser Lithotripsy of Ureteral Stones, 131
–136 Springer, Berlin, Heidelberg
(1995). Google Scholar
S. S. Kesleret al.,
“Use of the escape nitinol stone retrieval basket facilitates fragmentation and extraction of ureteral and renal calculi: a pilot study,”
J. Endourol., 22
(6), 1213
–1217
(2008). http://dx.doi.org/10.1089/end.2008.0070 JENDE3 0892-7790 Google Scholar
|