|
|
1.IntroductionNanostructures such as semiconductor quantum dots (QDs) emerged in the fields of biology and medicine as promising imaging and therapeutic agents.1–6 Lately, it was proposed that QDs could be used in the photodynamic therapy (PDT) of cancer.5,6 Unique optical properties of QDs such as size-tunable photoluminescence (PL) spectrum, high PL quantum yield and long lifetime as well as high extinction coefficient and broad absorption spectrum make them advantageous energy donors for conventional photosensitizers (PS) used in PDT.3–5,7 Moreover, due to extremely large two-photon (TP) absorption cross-section of QDs8–10 it becomes possible to use TP excitation in a QD (donor)–PS (acceptor) system for indirect excitation of PS, which usually have a low TP absorption cross-section.11–14 TP excitation is usually provided within the near infrared (NIR) spectral region, which coincides with the tissue optical window. NIR irradiation can penetrate deeper into the tissue compared to visible light, allowing more profound examination and treatment of tumor. In addition, since TP excitation is confined within the focal volume of laser beam, selective activation of PS is achieved, which effectively enhances spatial resolution and produces less damage to surrounding healthy tissue.15 To our knowledge, there are only a few studies to date focusing on TP excitation of QD–PS systems,16–18 which show that TP excited QDs can efficiently donate energy to electrostatically16,17 or covalently18 attached PS. However, covalent coupling of QD–PS complexes requires additional chemical procedures which are not always feasible, while QD–PS complexes formed via electrostatic interaction might be less stable in biological media. In our study, for the first time, we have exploited the hydrophobic interaction to design QD–PS complex. Furthermore, we have used biocompatible QDs with lipid-based coating, which not only guarantees biocompatibility and stability of QDs for future applications in biological medium, but also serve as binding sites for amphiphilic PS ensuring its close localization to QD core and subsequently efficient Forster resonance energy transfer (FRET). As a PS, chlorin () was chosen. It is a typical light-activated drug for PDT with a high singlet oxygen generation quantum yield. However, a small TP absorption cross-section limits its application in TP PDT. We have already shown, using single-photon (SP) excitation, that lipid-coated CdSe/ZnS QD form a stable, noncovalent complex with PS .19,20 molecules penetrate into the lipid coating of QD and localize close enough to QD shell/core for the FRET to occur. Here, we demonstrate that TP excited QD can work as efficient energy donors for as well. 2.Materials and Methodstetrasulfonic acid was purchased from Frontier Scientific Inc. (USA). CdSe/ZnS QDs (, core diameter—,21 hydrodynamic diameter—) coated by phospholipid layer with PEG and terminal carboxyl groups were obtained from eBioscience Inc. (USA). All materials were used without further purification. was dissolved in a small amount of 0.2 M NaOH solution and further diluted with a phosphate buffer (PB) (pH 7) to concentrations varying from 0.02 to 0.3 μM. Stock solution of QDs (10 μM) was diluted with PB to 0.02 μM working concentration. solutions were made by adding 5 μL of concentrated solution to the already prepared 0.02 μM solution of QDs, obtaining molar ratios from up to . Dilution effect for solution after addition of was encountered by adding 5 μL of PB to the control QD solution. SP absorption spectra were recorded with Cary 50 UV–Vis spectrophotometer (Varian Inc., Palo Alto, California). SP excited PL spectra were measured with Cary Eclipse spectrophotometer (Varian Inc., USA). TP excitation of samples was produced by laser system PHAROS (Light Conversion, Lithuania) operating at 1030 nm wavelength with pulse duration of 280 fs and 1 kHz repetition rate. The average laser power of focused beam entering the sample was approximately 200 mW (pulse energy 200 μJ; peak pulse power 0.7 GW). TP induced PL signal was collected at a 90-deg angle and guided to spectrophotometer by an optical fiber. All experiments were performed at room temperature (RT). TP absorption cross-sections of samples were calculated by a comparative method22 using Rhodamine 6G TP absorption cross-section as a reference [ Goeppert-Mayer (GM) in methanol].23 SP and TP induced PL intensities of samples were measured at their corresponding PL band maxima and respectively. In both cases . The SP extinction coefficient at the excitation wavelength of the samples , ( ) was evaluated from the absorption spectra. The Rhodamine 6G SP and TP induced PL intensities at their corresponding band maxima and , respectively, and SP extinction coefficient were evaluated in the same way as for investigated samples. TP absorption cross-section can then be calculated as follows: By this method, neither the parameters of the excitation light (pulse energy, pulse duration, spatial, and temporal intensity distribution), nor the parameters of the detection setup (detector characteristics and refractive indexes of solvents), nor PL quantum yield need to be known. The efficiency of FRET between QDs and molecules was calculated from the decrease of QD (donors) PL in the presence of molecules (acceptors): where and correspond to the intensities of donor PL intensity in the absence and in the presence of acceptor, respectively.3.Results and DiscussionSP absorption and PL spectra of pure and QDs aqueous solutions are shown in Fig. 1. Absorption spectrum of consists of the intensive Soret band at 405 nm and less intensive bands, with the most intensive in the red spectral region at 654 nm. In aqueous solution the maximum of fluorescence band is at 660 nm. Absorbance of QDs gradually decreases from UV to the red spectral region, ending with the last excitonic band at 615 nm. QDs have an intensive, narrow, and symmetrical PL band with a peak at 627 nm. It can be clearly seen that the PL band of QDs overlaps with the absorption spectrum of (Fig. 1). This satisfies the main requirement for FRET between QDs and to occur. Our previous study showed that addition of to QD aqueous solution results in stable complex formation, driven by the hydrophobic interaction between nonpolar moiety of and lipids of QD coating.19,20 Here, PL spectra after SP excitation of QDs solution mixed with different amounts of are shown in Fig. 2(a). Localization of molecules in the hydrophobic interior of the lipid part of QDs results in shift of fluorescence band from 660 to 670 nm. Analogous red shift of fluorescence maximum to 670 nm has been reported when molecules were incorporated into lipid bilayer.24,25 An increasing concentration significantly quenches PL intensity of QDs, while the fluorescence intensity of bound molecules at 670 nm simultaneously increases [Figs. 2(a) and 3]. A significant increase in fluorescence intensity of is observed up to the molar ratio of however, further rise in concentration leads only to negligible changes in fluorescence intensity [Figs. 2(a) and 3(b)]. Meanwhile, the PL intensity of QDs noticeably decreases even at higher concentrations of () [Figs. 2(a) and 3(a)]. This might be explained by the self-quenching of bound molecules on the surface of QDs at relatively high concentrations of . Moreover, the fluorescence intensity of pure under SP (at 515 nm) excitation is much lower than that bound to QDs [Fig. 2(a)], as can be clearly seen in inset of Fig. 3(b), where the intensity of pure fluorescence maximum (660 nm) is shown against the concentration. In addition, in our previous experiments we have shown that the fluorescence excitation spectra measured at 670 nm, contain a significant contribution of QDs spectrum.19 Also, the average PL decay time of QDs decreases upon complex formation.19 All spectral features listed above indicate FRET occurring between QDs and bound molecules. The efficiency of FRET in complex at molar ratios of and was found to be 45% and 82%, respectively. Similar PL measurements for pure QDs, , and aqueous solutions at different molar ratios were conducted using TP excitation at 1030 nm [Fig. 2(b)]. The intensive PL band of QDs observed at 627 nm confirms that QDs can be effectively excited using TP excitation. QDs are known to have a large TP absorption cross-section, which ranges from 75 to 47,000 GM, depending on their chemical structure, size, and excitation wavelength.9 Here, TP absorption cross-section for QDs was calculated according to Eq. (1) ; ; ; ; ; and found to be 3325 GM, which is in agreement with the previous reports.8–10 Fig. 1Normalized SP absorption and PL spectra of pure QDs and aqueous solutions (PB pH 7). The arrow indicates a wavelength used for SP at 515 nm and corresponding TP at 1030 nm excitation of the samples. 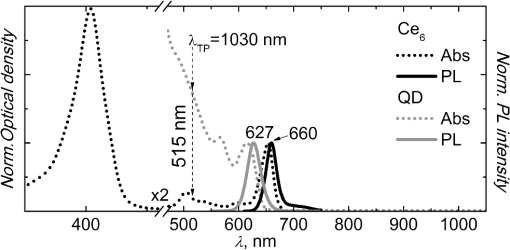 Fig. 2PL spectra of pure QDs (0.02 μM), (0.1 μM), and mixed aqueous solutions at different molar ratios ( to ) under excitation of (a) SP 515 nm and (b) TP 1030 nm. Spectra were normalized to the PL peak of QDs at 627 nm. 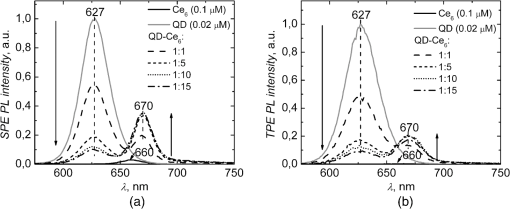 Fig. 3Changes in the PL intensities of solutions upon increasing molar ratio from to under SP and TP excitation (a) at QD maximum at 627 nm (values are normalized to the initial intensity of QDs without ) and (b) at bound maximum at 670 nm. Inset shows the intensity changes of pure solution at fluorescence maximum at 660 nm by increasing the concentration of from 0.02 to 0.3 μM under SP and TP excitation wavelength. 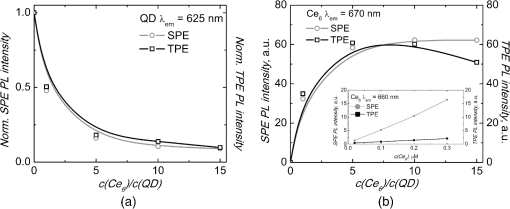 Meanwhile, only a negligible emission signal can be observed for pure solution under TP excitation, since TP absorption cross-section is significantly lower (13 GM ). For organic dyes and porphyrin type molecules TP absorption cross-section are usually in the order of tens of GM units or less.11–14,22,23 Thus, effective application of such organic molecules in TP PDT is limited. Higher TP absorption cross-section for QDs is obtained at shorter wavelengths as 800 nm.10 However, 1030 nm wavelength light is more suitable for FRET investigation in complex, since only QD is excited via TP excitation. Upon TP excitation similar spectral changes in mixed solutions are observed as in the case of SP excitation [Figs. 2(b) and 3]. In mixed solution PL intensity of QDs significantly decreases compared to pure QDs and an intensive fluorescence peak at 670 nm appears. No significant differences in PL changes of QDs between SP and TP excitation were observed suggesting that energy transfer pathway to bound molecules is independent on the excitation way [Fig. 3(a)]. Fluorescence intensity of at 670 nm, under TP excitation at 1030 nm of solution increases until molar ratio of , and begins to decrease for higher concentrations of [Fig. 3(b)]. This suggests that up to five molecules per single QD can localize within the lipid part of QDs coating without loss in fluorescence intensity. Further increase in concentration negatively acts on PL of both the QDs and , without any enhancements on energy transfer efficiency or PL intensity. Such decrease was not observed under SP excitation at 515 nm most probably because it is compensated for by the fluorescence signal from direct excitation of free . However, when SP excitation at 465 nm is used, where absorption of is minimal, a similar decrease can be observed (data not shown). As mentioned previously, it can be explained by fluorescence self-quenching of bound molecules due to their possible aggregation on the surface of QDs at higher concentrations. Under TP excitation at 1030 nm, the fluorescence of can be selectively observed only due to FRET from TP excited QDs to bound molecules. FRET efficiency under TP excitation from QD to in complex at molar ratio of was found to be 49% and increased to the maximum 82% at molar ratio . FRET efficiencies under TP excitation coincide with those obtained under SP excitation. It can be assumed that TP excitation can also be used to effectively excite other amphiphilic PS that can hydrophobically interact with the lipid coating of QDs.20 It is noteworthy to mention that FRET efficiency values were obtained at RT; however, during the biomedical applications (experiments in cell cultures and experimental animals) complexes will be exposed to higher temperatures. The increase of the temperature to 50°C causes decrease in FRET efficiency approximately by 10% (data not shown). It can be considered as negligible and complex can be used for biomedical applications. 4.ConclusionsFor the first time, we demonstrated that complex self-assembled due to the hydrophobic interaction between nonpolar moiety of molecules and lipids of QDs coating can be effectively excited via TP irradiation at 1030 nm, which spectrally coincides with the transparency window of biological tissue. Indirect excitation of by energy transfer from TP excited QDs, overcomes difficulties in direct TP excitation of , thus allowing one to utilize therapeutic action of under TP irradiation. The highest efficiency of FRET within complex under TP excitation reached up to 82% when multiple molecules are bound to QD, which is significantly higher than that obtained by other similar QD–PS systems to date.16–18 High FRET efficiency within complex exploits full PDT potential of at relatively small concentrations of PS that is very important when concerning dark toxicity and clearance issues of complex. These promising results constitute a first step toward the application of such type QD–PS complexes alongside TP irradiation in PDT. AcknowledgmentsThis study was supported by the Research Council of Lithuania Grant No. MIP-095/2011, the EU and Norwegian Grant “Multifunctional nanoparticles for specific noninvasive early diagnostics and treatment of cancer” No. 2004-LT0036-IP-1. Jurga Valanciunaite was supported by the project “Postdoctoral Fellowship Implementation in Lithuania” funded by European Union Structural Fund. Artiom Skripka was supported by the Lithuanian Science Council Student Research Fellowship Award. ReferencesX. Michaletet al.,
“Quantum dots for live cells, in vivo imaging, and diagnostics,”
Science, 307
(5709), 538
–544
(2005). http://dx.doi.org/10.1126/science.1104274 JBBOAJ 1110-7251 Google Scholar
I. L. Medintzet al.,
“Quantum dot bioconjugates for imaging, labelling and sensing,”
Nat. Mater., 4
(6), 435
–446
(2005). http://dx.doi.org/10.1038/nmat1390 NMAACR 1476-1122 Google Scholar
P. Juzenaset al.,
“Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer,”
Adv. Drug Delivery Rev., 60
(15), 1600
–1614
(2008). http://dx.doi.org/10.1016/j.addr.2008.08.004 ADDREP 0169-409X Google Scholar
E. YaghiniA. M. SeifalianA. J. MacRobert,
“Quantum dots and their potential biomedical applications in photosensitization for photodynamic therapy,”
Nanomedicine, 4
(3), 353
–363
(2009). http://dx.doi.org/10.2217/nnm.09.9 1743-5889 Google Scholar
A. C. S. SamiaX. B. ChenC. Burda,
“Semiconductor quantum dots for photodynamic therapy,”
J. Am. Chem. Soc., 125
(51), 15736
–15737
(2003). http://dx.doi.org/10.1021/ja0386905 JACSAT 0002-7863 Google Scholar
R. Bakalovaet al.,
“Quantum dot anti-CD conjugates: are they potential photosensitizers or potentiators of classical photosensitizing agents in photodynamic therapy of cancer?,”
Nano Lett., 4
(9), 1567
–1573
(2004). http://dx.doi.org/10.1021/nl049627w NALEFD 1530-6984 Google Scholar
J. M. Tsayet al.,
“Singlet oxygen production by peptide-coated quantum dot-photosensitizer conjugates,”
J. Am. Chem. Soc., 129
(21), 6865
–6871
(2007). http://dx.doi.org/10.1021/ja070713i JACSAT 0002-7863 Google Scholar
D. R. Larsonet al.,
“Water-soluble quantum dots for multiphoton fluorescence imaging in vivo,”
Science, 300
(5624), 1434
–1436
(2003). http://dx.doi.org/10.1126/science.1083780 SCIEAS 0036-8075 Google Scholar
S. C. Puet al.,
“The empirical correlation between size and two-photon absorption cross section of CdSe and CdTe quantum dots,”
Small, 2
(11), 1308
–1313
(2006). http://dx.doi.org/10.1002/(ISSN)1613-6829 1613-6829 Google Scholar
L. M. Maestroet al.,
“Nanoparticles for highly efficient multiphoton fluorescence bioimaging,”
Opt. Express, 18
(23), 23544
–23553
(2010). http://dx.doi.org/10.1364/OE.18.023544 OPEXFF 1094-4087 Google Scholar
M. A. AlbotaC. XuW. W. Webb,
“Two-photon fluorescence excitation cross sections of biomolecular probes from 690 to 960 nm,”
Appl. Opt., 37
(31), 7352
–7356
(1998). http://dx.doi.org/10.1364/AO.37.007352 APOPAI 0003-6935 Google Scholar
B. A. Reinhardtet al.,
“Highly active two-photon dyes: design, synthesis, and characterization toward application,”
Chem. Mater., 10
(7), 1863
–1874
(1998). http://dx.doi.org/10.1021/cm980036e CMATEX 0897-4756 Google Scholar
C. XuW. W. Webb,
“Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm,”
J. Opt. Soc. Am. B, 13
(3), 481
–491
(1996). http://dx.doi.org/10.1364/JOSAB.13.000481 JOBPDE 0740-3224 Google Scholar
P. Chenet al.,
“Two-photon excitation of chlorin-e(6)-C-15 monomethyl ester for photodynamic therapy,”
Proc. SPIE, 5630 209
–217
(2005). http://dx.doi.org/10.1117/12.575941 PSISDG 0277-786X Google Scholar
A. M. SmithM. C. ManciniS. M. Nie,
“Bioimaging second window for in vivo imaging,”
Nat. Nanotechnol., 4
(11), 710
–711
(2009). http://dx.doi.org/10.1038/nnano.2009.326 1748-3387 Google Scholar
S. DayalC. Burda,
“Semiconductor quantum dots as two-photon sensitizers,”
J. Am. Chem. Soc., 130
(10), 2890
–2891
(2008). http://dx.doi.org/10.1021/ja0781285 JACSAT 0002-7863 Google Scholar
Y. N. Wenet al.,
“Activation of porphyrin photosensitizers by semiconductor quantum dots via two-photon excitation,”
Appl. Phys. Lett., 95
(14), 143702
(2009). http://dx.doi.org/10.1063/1.3243979 APPLAB 0003-6951 Google Scholar
Z. D. Qiet al.,
“Biocompatible CdSe quantum dot-based photosensitizer under two-photon excitation for photodynamic therapy,”
J. Mater. Chem., 21
(8), 2455
–2458
(2011). http://dx.doi.org/10.1039/c0jm03229h JMACEP 0959-9428 Google Scholar
J. Valanciunaiteet al.,
“Complex of water-soluble CdSe/ZnS quantum dots and chlorin e(6): interaction and FRET,”
Proc. SPIE, 7376 737607
(2010). http://dx.doi.org/10.1117/12.871524 Google Scholar
J. Valanciunaiteet al.,
“Spectroscopic study of non-covalent complex formation between different porphyrin analogues and quantum dots with lipid-based coating,”
Chemija, 22
(4), 181
–187
(2011). CHMJES 0235-7216 Google Scholar
T. L. Jenningset al.,
“Reactive semiconductor nanocrystals for chemoselective biolabeling and multiplexed analysis,”
ACS Nano, 5
(7), 5579
–5593
(2011). http://dx.doi.org/10.1021/nn201050g 1936-0851 Google Scholar
M. Drobizhevet al.,
“Extremely strong near-IR two-photon absorption in conjugated porphyrin dimers: quantitative description with three-essential-states model,”
J. Phys. Chem. B, 109
(15), 7223
–7236
(2005). http://dx.doi.org/10.1021/jp044261x JPCBFK 1520-6106 Google Scholar
N. S. MakarovM. DrobizhevA. Rebane,
“Two-photon absorption standards in the 550–1600 nm excitation wavelength range,”
Opt. Express, 16
(6), 4029
–4047
(2008). http://dx.doi.org/10.1364/OE.16.004029 OPEXFF 1094-4087 Google Scholar
A. A. Frolovet al.,
“Chlorin e6-liposome interaction—investigation by the methods of fluorescence spectroscopy and inductive resonance energy-transfer,”
J. Photochem. Photobiol. B, 7
(1), 43
–56
(1990). http://dx.doi.org/10.1016/1011-1344(90)85142-J JPPBEG 1011-1344 Google Scholar
H. Mojzisovaet al.,
“The pH-dependent distribution of the photosensitizer chlorin e6 among plasma proteins and membranes: a physico-chemical approach,”
Biochim. Biophys. Acta, 1768
(2), 366
–374
(2007). http://dx.doi.org/10.1016/j.bbamem.2006.10.009 BBACAQ 0006-3002 Google Scholar
|

