|
|
1.IntroductionNear-infrared spectroscopy (NIRS) is a noninvasive method for monitoring regional tissue oxygenation () from a reflectance-type sensor attached to the skin. NIR light penetrates the tissue and is absorbed by hemoglobin and other chromophores.1 NIRS has been used to provide information about blood flow matching to oxygen requirements and possible ischemia or hyperemia.2 Despite numerous publications regarding the use of this technology in various settings, such as intensive care or cardiac surgery, and awareness of its potential benefits, NIRS has not yet become a widespread standard monitoring device in clinical practice.1,2 This is perhaps because the imprecision of the early devices was such that clinicians questioned the readiness of these devices for routine care.3–5 Various NIRS devices are available, but they use different techniques of measurement. Some of these devices have claims for use in neonates as trend monitors, e.g., the INVOS 5100C (Covidien, Colorado), and others claim to measure absolute tissue oxygen saturation () e.g., FORE-SIGHT (CAS Medical Systems, Connecticut). This study focused on these two commercially available tissue oximeters: FORE-SIGHT and INVOS 5100C. The INVOS monitor is intended to generate changes in oximetry values and enables the recording of trends; it utilizes a light emitting diode (LED)–based sensor which uses two wavelengths (730 and 810 nm) to measure changes in . The FORE-SIGHT monitor is intended to generate absolute oximetry values; it utilizes four wavelengths of laser light (690, 780, 805, and 850 nm) to determine . The aim of this observational study was to compare the simultaneous values of cerebral measured with the INVOS 5100C and FORE-SIGHT oximeters in term and preterm newborns. The repeatability of the measurement was also assessed for both devices. These data were used to compare the performance of the two devices. 2.MethodsPatients were enrolled from newborns treated at the Department of Neonatology, Poznan University of Medical Sciences. The study protocol was approved by the Bioethical Committee and informed parental consent was obtained for each patient. Thirty newborn babies were enrolled in the study: 15 term (median 38 weeks of gestation, ranges 37 to 40 weeks) and 15 preterm (median 35 weeks of gestation, ranges 32 to 36 weeks). In the term group, the median birth weight was 3380 g (ranges 2920 to 4120 g). In the preterm group, the median birth weight was 2375 g (ranges 1200 to 3130 g). During data acquisition, all enrolled patients were in a stable clinical condition as evidenced by pulse oximetry values within normal ranges, i.e., pulse rate and oxygen saturation measured by pulse oximetry (). All babies studied were breathing spontaneously without the need for supplemental oxygen and none suffered from anemia. Each newborn underwent an echocardiographic study and a head ultrasound study. Congenital heart defects, hemodynamically significant patent ductus arteriosus, anomalies of central nervous system, severe intraventricular hemorrhage (grades III and IV), and respiratory distress, were exclusion criteria. NIRS measurements were performed on the first and third day of life. Values of were recorded simultaneously, using the FORE-SIGHT (CAS Medical Systems, Connecticut) and INVOS 5100C (Covidien, Colorado) devices. Both devices are cleared for use in neonates by the United States Food and Drug Administration. One sensor of each oximeter was placed on the right and the other on the left side of the baby’s forehead. The smallest available sensors were used: for the FORE-SIGHT monitor, it was the sensor identified by the manufacturer as the “small size sensor”; for the INVOS monitor, it was the “neonatal cerebral sensor.” The distance between sensors was about 3 to 4 cm. Before the start of data acquisition, sensors were checked for possible light interference. This was done by recording signals when only the FORE-SIGHT sensor was active, then when only the INVOS sensor was active, and finally when both sensors were active. numerical values and their plots on monitors were observed for rapid changes associated with simultaneous operation. If there had been evidence of optical crosstalk, the distance between sensors would have been increased; however, this was not necessary in any of the studied patients. The recording was divided into two parts. First, 5 min for stabilization of the signal and then 3 h of recording. Subsequently, the FORE-SIGHT and INVOS sensor positions were switched to the contralateral side and a further 3 h of recording was performed. Preductal saturation () was concurrently measured by pulse oximetry (Nellcor N-600x, Covidien Nellcor Puritan Bennett, Colorado). Thirty minutes of stable signal recording (e.g., calm baby, with no procedures performed, without changes of position the head and supine position of the body, without desaturations , or without symptoms of respiratory distress) was chosen for analysis. The maximum sampling rate for each device was used for acquisition of values (approximately 6 s for the INVOS device and 2 s for the FORE-SIGHT device, which are the device settings fixed by the manufacturers). Statistical analysis was performed using the Bland–Altman method (MedCalc software ver. 12.3.0.0, Mariakerke, Belgium).6 Mean values during recorded periods were analyzed as single points of measurement. The analysis consisted of two parts: (1) the assessment of agreement between mean values that were measured simultaneously by the INVOS and FORE-SIGHT oximeters and (2) the assessment of repeatability of measurements before and after the change of sensor positions for each device. Both monitors were also observed for possible adverse effects associated with their use. 3.ResultsIn both the term and preterm groups, the mean values of cerebral recorded by the INVOS and FORE-SIGHT devices were similar, with differences not exceeding 3.5%. However, higher standard deviations were found for the INVOS in both patient groups (Table 1). Table 1Cerebral tissue oxygen saturation values recorded in full term and premature newborns.
Note: StO2 INV 1 1: values recorded with the INVOS device on the first day of life before sensor positions were changed; StO2 INV 1 2: values recorded with the INVOS device on the first day of life after sensor positions were changed; StO2 INV 3 1: values recorded with the INVOS device on the third day of life before sensor positions were changed; StO2 INV 3 2: values recorded with the INVOS device on the third day of life after sensor positions were changed; StO2 FS 1 1: values recorded with the FORE-SIGHT device on the first day of life before sensor positions were changed; StO2 FS 1 2: values recorded with the FORE-SIGHT device on the first day of life after sensor positions were changed; StO2 FS 3 1: values recorded with the FORE-SIGHT device on the third day of life before sensor positions were changed; StO2 FS 3 2: values recorded with the FORE-SIGHT device on the third day of life after sensor positions were changed. SD, standard deviation. Using the Bland–Altman method, the limits of agreement (LOA) for values recorded simultaneously by the INVOS and FORE-SIGHT devices were 8.3% to 11.8% for full term babies and 7% to 11.8% for preterm babies. Values of bias ranged from 0.5% to 3.5% for full term newborns and from to 2.7% for preterm newborns (Fig. 1). Fig. 1Bland–Altman plots showing the agreement between regional cerebral tissue oxygenation () values of full term (upper plot) and preterm (lower plot) newborns recorded simultaneously using INVOS and FORE-SIGHT devices on the first day of life before the change in position of oximeter sensors. INVOS 1 1: values recorded for INVOS on the first day of life before the change in position of oximeter sensors; FS 1 1: values recorded using FORE-SIGHT on the first day of life before the change in position of oximeter sensors. 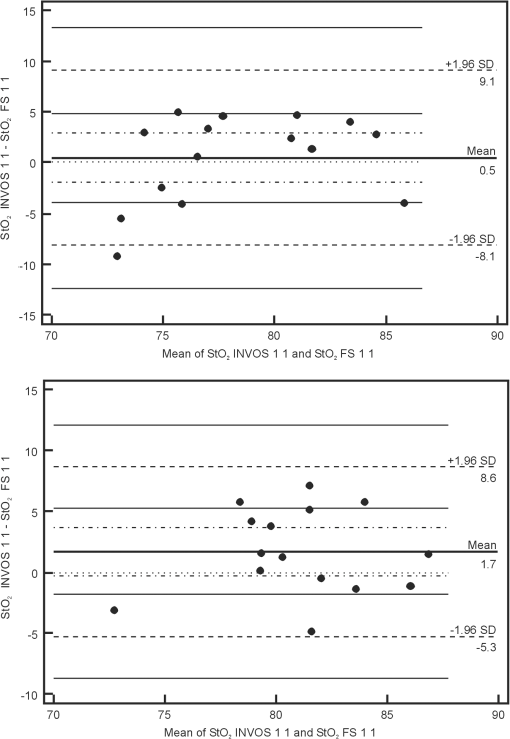 The LOA for values recorded before and after the change of NIRS sensor position for the INVOS device was 9.1% to 11.2% for full term babies and between 9.6% and 11% for preterm babies. Values of bias for the INVOS device ranged from to 0.7% in the full term babies and from to 1.4% for the preterm group (Fig. 2). Fig. 2Bland–Altman plots showing the agreement between regional cerebral tissue oxygenation () values of full term (upper plot) and preterm (lower plot) newborns recorded before and after the change in position of oximeter sensors using INVOS device on the first day of life. INVOS 1 1: values recorded for INVOS on the first day of life before the change in position of oximeter sensors; INVOS 1 2: values recorded using INVOS on the first day of life after the change in position of oximeter sensors. 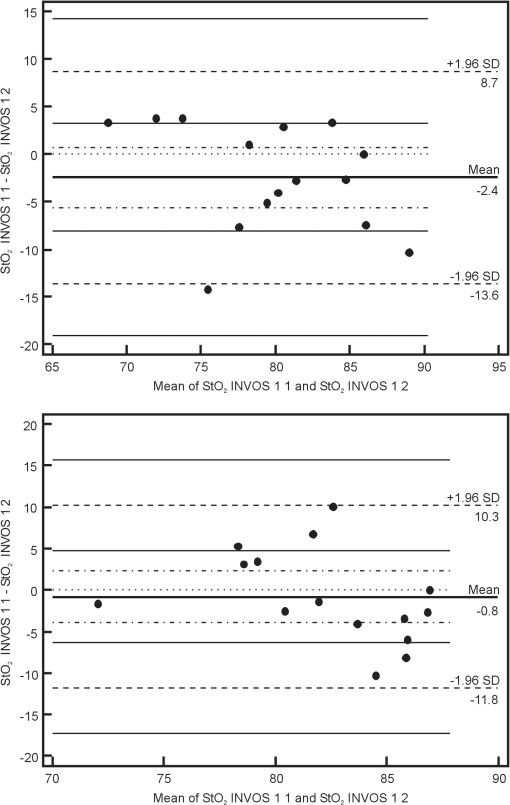 The LOA for values before and after the change of NIRS sensor position for the FORE-SIGHT device were 5.5% to 7.3% in the full term group and 8.4% to 9% in the preterm group. Values of bias for the FORE-SIGHT device ranged from to for the full term group and from to 0.2% for the preterm group (Fig. 3). Fig. 3Bland–Altman plots showing the agreement between regional cerebral tissue oxygenation () values of full term (upper plot) and preterm (lower plot) newborns recorded before and after the change in position of oximeter sensors using FORE-SIGHT device on the first day of life. FS 1 1: values recorded using FORE-SIGHT on the first day of life before the change in position of oximeter sensors; FS 1 2: values recorded using FORE-SIGHT on the first day of life after the change in position of oximeter sensors. 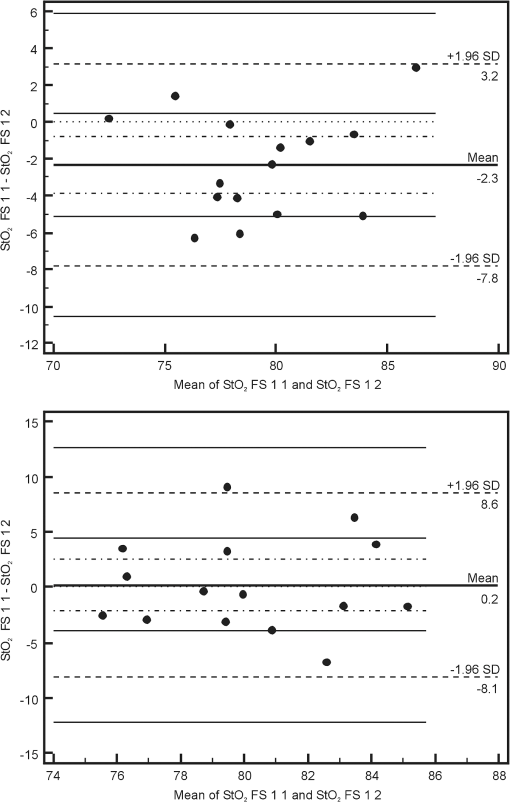 There were no adverse effects associated with the use of either device during the study. 4.DiscussionComparison of values recorded simultaneously by the INVOS and FORE-SIGHT devices in this neonatal population, revealed reasonable agreement with a bias up to 3.5%, showing that average measurements were close. LOA were up to 11.8%, which is probably acceptable for clinical use. To our knowledge, this is the first time that a direct comparison between these two technologies has been made in newborn babies. However, precision of the measurement needed for clinical purposes remains controversial. It is not strictly defined and the results of available studies are not easily comparable. Different authors have studied this issue with various study protocols: e.g., variable recording periods, different approach to sensor placement, and different patients. Various statistical methods were used in different papers.6–8 Comparisons were also made between different types and models of devices. In some studies comparing INVOS and NIRO oximeters their performance was found to be similar (with LOA of 14.7%).8,9 Other reports described significant disparities between cerebral values (with mean differences of 10%) and unacceptable LOA (15.6%).7,10,11 In a study assessing the influence of INVOS 4100 sensor location on the measurements it was found that LOA between at the two different sensor locations on the forehead ranged from 10.7% to 12.1% with bias of 2.3% to 2.7%, which was considered to be unacceptable disagreement.4 Interestingly, using an INVOS monitor, Wijbenga et al.12 reported up to 18% difference in between different brain regions in the same patient. In neonates, precision of measurement has been reported to increase with increasing tissue homogeneity.13 Based on the previous studies, it seems that the issue of acceptable agreement between various NIRS devices has not been clearly defined and different authors accept different ranges of LOA. Perhaps acceptable precision for tissue oximetry should be similar to that required for pulse oximetry so interventions could be finely managed. The approach to the sensor placement and data acquisition varied between studies.8,14 In our study, lack of interference was verified before each set of data acquisition so simultaneously recorded values were reliable (with emitters and detectors orientation similar to previous studies).12 In a study by Moerman et al., INVOS oximeter was found to interfere with FORE-SIGHT, increasing the variability of the signal. However, induced variability was small and the interference did not influence mean values.15 Analysis of our data revealed a larger standard deviation for values obtained using the INVOS oximeter than the FORE-SIGHT device, suggesting greater variability for the INVOS device. Similar observations were reported in the studies comparing the accuracy of the FORE-SIGHT and INVOS devices in adults.15,16 In a study comparing NIRO 300 and INVOS 5100, the reproducibility of cerebral measurements was found to be similar.7 Good reproducibility was described for NIRO 300 in neonates;17 however, in other studies that utilized the same device, the reproducibility was found to be poor.18,19 After switching the sensor position to the opposite side of the forehead, we found better repeatability for the FORE-SIGHT monitor. This might be explained by the different software algorithms but since complete algorithms have not been published, this issue remains unclear. Differences in wavelengths utilized by INVOS and FORE-SIGHT monitors might also influence measurements and were proposed as a possible reason of larger range of INVOS by Moerman et al.15 Other possible reasons of differences between measured by the compared NIRS oximeters include an unequal penetration depth due to different light intensity, sensitivity of light detector, different spacing between emitter and detectors, and variable sensitivity to extracranial tissue contamination.15,20 Closer agreement of measurements using the FORE-SIGHT oximeter as compared with the INVOS oximeter was also described in a study on healthy adults when values from five different oximeters were compared with the simultaneous weighted invasive jugular bulb CO-oximetry and arterial oxygen saturation measurements.21 In a study by MacLeod et al., differences between and CO-oximetry values in some subjects exposed to hypoxia were quite high for the INVOS “giving the appearance that cerebral oxygenation was falling more rapidly than it really was”.16 In theory, this might be important in the intensive care of preterm infants, who might be unintentionally exposed to hyperoxia in response to apparently low values of .22 The NIRS devices tested in the study use continuous wave (CW) method, which measures changes in light absorption by recording the changes in the intensity of light both transilluminated through or reflected by photon scattering in tissue. A spatially resolved (SR) NIRS device measures intensity changes at multiple light sources and detector spacings. The SR device uses the gradients of the optical signal from this multidistance measurement to quantify the NIRS parameters. The INVOS device uses two detectors placed 30 and 40 mm from the LEDs to measure the relative regional value of .9,23,24 The FORE-SIGHT small sensor has a light source composed of a fiber optic light guided from the monitor with a prism and a light detector at a fixed distance of 25 mm from the light source. Unlike the pediatric and adult size FORE-SIGHT sensors, there is no scalp detector for sampling extracranial tissue, so the SR method is not applied. The laser intensity for each wavelength is monitored and the detector samples the strength (i.e., attenuation) of each wavelength upon exiting the skin–sensor interface at the assumed path length ( or ). The characteristic absorbance () for each wavelength in relation to oxy- and deoxy-hemoglobin is known and differs widely over the range of infrared and red light used by FORE-SIGHT. These data are entered into wavelength iterations of a modified Beer–Lambert equation (), where is the hemoglobin concentration. Thus, a wavelength resolved CW methodology is used to derive the oxygen saturation of hemoglobin in the tissue under the sensor. A proprietary algorithm in combination with a large database of direct blood oximetry from neonatal systemic arterial and brain venous specimens is used to calculate the absolute value of cerebral .25 The FORE-SIGHT algorithm is supposed to provide improved measurement accuracy thanks to “compensation for wavelength dependent absolute quantification, scattering losses, and by accounting for interference from other background light absorbers.”26 An advantage of tissue oximeters that give absolute values rather than monitoring relative change is that there is no need for baseline calibration.27 NIRS manufacturers apply empirical calibration coefficients based upon clinical and optical phantom data. NIRS tissue oxygen saturation values are then related to the calibration reference as determined from the weighted venous and arterial oxygen saturations ( for INVOS and for FORE-SIGHT). There are some limitations to the present study. All enrolled newborns were in a stable clinical condition with their within normal limits and, unlike studies performed on healthy adults, our patients were not exposed to hypoxic or hyperoxic episodes. Monitoring babies with cardiopulmonary instability and with a known history of desaturations might have been a useful addition to our study, possibly providing more information about the oximeters’ performance. Our findings may also contribute to the development of a definition of the range of normal values for stable term and preterm newborns, given the context of our stated exclusion criteria. In summary, absolute oximeter values using the FORE-SIGHT device and relative oximeter values using the INVOS device seem to be comparable in stable newborn babies, suggesting that these devices can be used interchangeably and data can be compared. However, the FORE-SIGHT monitor showed less variability of recorded values and closer agreement of measurements between values recorded before and after changing the NIRS sensor position. More studies are needed to understand the clinical significance of these findings. AcknowledgmentsThe authors would like to thank Robert Kopotic for his support regarding technical aspects of NIRS measurements. ReferencesA. J. WolfbergA. J. du Plessis,
“Near-infrared spectroscopy in the fetus and neonate,”
Clin. Perinatol., 33
(3), 707
–728
(2006). http://dx.doi.org/10.1016/j.clp.2006.06.010 CLPEDL 0095-5108 Google Scholar
G. GreisenT. LeungM. Wolf,
“Has the time come to use near-infrared spectroscopy as a routine clinical tool in preterm infants undergoing intensive care?,”
Philos. Trans. R. Soc., A, 369
(1955), 4440
–4451
(2011). http://dx.doi.org/10.1098/rsta.2011.0261 PTRMAD 1364-503X Google Scholar
J. S. SoulA. J. du Plessis,
“New technologies in pediatric neurology: near-infrared spectroscopy,”
Semin. Pediatr. Neurol., 6
(2), 101
–110
(1999). http://dx.doi.org/10.1016/S1071-9091(99)80036-9 1071-9091 Google Scholar
K. Kishiet al.,
“Influence of patient variables and sensor location on regional cerebral oxygen saturation measured by INVOS 4100 near-infrared spectrophotometers,”
J. Neurosurg. Anesthesiol., 15
(4), 302
–306
(2003). http://dx.doi.org/10.1097/00008506-200310000-00002 JNANEV 0898-4921 Google Scholar
S. E. Nicklinet al.,
“The light still shines, but not that brightly? The current status of perinatal near infrared spectroscopy,”
Arch. Dis. Child. Fetal Neonatal Ed., 88
(8476), F263
–F268
(2003). http://dx.doi.org/10.1136/fn.88.4.F263 1359-2998 Google Scholar
J. M. BlandD. G. Altman,
“Statistical methods for assessing agreement between two methods of clinical measurement,”
Lancet, 327
(8476), 307
–310
(1986). http://dx.doi.org/10.1016/S0140-6736(86)90837-8 LANCAO 0140-6736 Google Scholar
M. Pocivalniket al.,
“Regional tissue oxygen saturation: comparability and reproducibility of different devices,”
J. Biomed. Opt., 16
(5), 057004
(2011). http://dx.doi.org/10.1117/1.3575647 JBOPFO 1083-3668 Google Scholar
H. Choet al.,
“Comparison of two commercially available near-infrared spectroscopy instruments for cerebral oximetry. Technical note,”
J. Neurosurg., 93
(2), 351
–354
(2000). http://dx.doi.org/10.3171/jns.2000.93.2.0351 JONSAC 0022-3085 Google Scholar
M. Thavasothyet al.,
“Comparison of cerebral oxygenation as measured by the NIRO 300 and the INVOS 5100 near-infrared spectrophotometers,”
Anaesthesia, 57
(10), 999
–1006
(2002). http://dx.doi.org/10.1046/j.1365-2044.2002.02826.x ANASAB 1365-2044 Google Scholar
A. Dullenkopfet al.,
“Measurement of cerebral oxygenation state in anaesthetized children using the INVOS 5100 cerebral oximeter,”
Paediatr. Anaesth., 13 384
–391
(2003). Google Scholar
R. E. Gagnonet al.,
“Comparison of two spatially resolved NIRS oxygenation indices,”
J. Clin. Monit. Comput., 17
(7–8), 385
–391
(2002). http://dx.doi.org/10.1023/A:1026274124837 1387-1307 Google Scholar
R. G. WijbengaP. M. LemmersF. van Bel,
“Cerebral oxygenation during the first days of life in preterm and term neonates: differences between different brain regions,”
Pediatr. Res., 70
(4), 389
–394
(2011). http://dx.doi.org/10.1203/PDR.0b013e31822a36db PEREBL 0031-3998 Google Scholar
S. J. Arriet al.,
“Precision of cerebral oxygenation and hemoglobin concentration measurements in neonates measured by near-infrared spectroscopy,”
J. Biomed. Opt., 16
(4), 047005
(2011). http://dx.doi.org/10.1117/1.3570303 JBOPFO 1083-3668 Google Scholar
K. Yoshitaniet al.,
“A comparison of the INVOS 4100 and the NIRO 300 near-infrared spectrophotometers,”
Anesth. Analg., 94
(3), 586
–590
(2002). http://dx.doi.org/10.1097/00000539-200203000-00020 AACRAT 0003-2999 Google Scholar
A. Moermanet al.,
“Relation between mixed venous oxygen saturation and cerebral oxygen saturation measured by absolute and relative near-infrared spectroscopy during off-pump coronary artery bypass grafting,”
Br. J. Anaesth., 110
(2), 258
–265
(2013). http://dx.doi.org/10.1093/bja/aes375 BJANAD 0007-0912 Google Scholar
D. B. MacLeodK. IkedaC. Vacchiano,
“Absolute and trending accuracy of Fore-sight and Invos cerebral oximeters in healthy volunteers,”
in Proc. Annual Meeting of the American Society of Anesthesiologists,
A298
(2009). Google Scholar
J. Menkeet al.,
“Reproducibility of cerebral near infrared spectroscopy in neonates,”
Biol. Neonate, 83
(1), 6
–11
(2003). http://dx.doi.org/10.1159/000067006 BNEOBV 0006-3126 Google Scholar
A. Dullenkopfet al.,
“Reproducibility of cerebral oxygenation measurement in neonates and infants in the clinical setting using the NIRO 300 oximeter,”
Pediatr. Crit. Care Med., 6
(3), 344
–347
(2005). http://dx.doi.org/10.1097/01.PCC.0000161282.69283.75 1529-7535 Google Scholar
L. C. SorensenG. Greisen,
“Precision of measurement of cerebral tissue oxygenation index using near-infrared spectroscopy in preterm neonates,”
J. Biomed. Opt., 11
(5), 054005
(2006). http://dx.doi.org/10.1117/1.2357730 JBOPFO 1083-3668 Google Scholar
S. N. DavieH. P. Grocott,
“Impact of extracranial contamination on regional cerebral oxygen saturation: a comparison of three cerebral oximetry technologies,”
Anesthesiology, 116
(4), 834
–840
(2012). http://dx.doi.org/10.1097/ALN.0b013e31824c00d7 ANESAV 0003-3022 Google Scholar
P. E. Bickleret al.,
“Performance of 5 cerebral oximeters during hypoxia in healthy volunteers,”
in Proc. Annual Meeting of the American Society of Anesthesiologists,
LBT07
(2011). Google Scholar
W. BaertsP. M. LemmersF. van Bel,
“Cerebral oxygenation and oxygen extraction in the preterm infant during desaturation: effects of increasing to assist recovery,”
Neonatology, 99
(1), 65
–72
(2011). http://dx.doi.org/10.1159/000302717 NEONCC 1661-7800 Google Scholar
M. Kimet al.,
“Estimation of jugular venous saturation from cerebral oximetry of arterial saturation during isocapnic hypoxia,”
J. Clin. Monit., 16
(3), 191
–199
(2001). http://dx.doi.org/10.1023/A:1009940031063 JCMOEH 0748-1977 Google Scholar
G. W. Fischer,
“Recent advances in application of cerebral oximetry in adult cardiovascular surgery,”
Semin. Cardiothorac. Vasc. Anesth., 12
(1), 60
–69
(2008). http://dx.doi.org/10.1177/1089253208316443 1089-2532 Google Scholar
K. Rais-BahramiO. RiveraB. L. Short,
“Validation of a noninvasive neonatal optical cerebral oximeter in veno-venous ECMO patients with a cephalad catheter,”
J. Perinatol. , 26
(10), 628
–635
(2006). http://dx.doi.org/10.1038/sj.jp.7211573 JOPEEI 0743-8346 Google Scholar
(
(2013) http://www.perfusion.com/cgi-bin/absolutenm/articlefiles/chen2008/chen2008.pdf May ). 2013). Google Scholar
C. ZaouterE. Arbeid,
“Influence of ambient light on cerebral oximeters,”
Br. J. Anaesth., 105
(6), 873
–874
(2010). http://dx.doi.org/10.1093/bja/aeq330 Google Scholar
|
|||||||||||||||||||||||||||||||||||||||||||||||||

