|
|
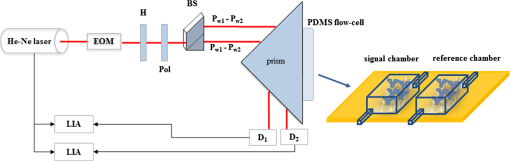
1.IntroductionPolyomavirus BK (BKV) is a nonenveloped DNA virus from the polyomaviridae family, which causes ubiquitous infection in early childhood and with seroprevalence in adults ranging from 60 to 100%.1–5 Although BKV infection is of no consequence to the immune-competent host, it establishes persistent latent infections and is capable of reactivating in immunosuppressed hosts.1,6 In contrast, renal transplant patients are treated with immunosuppressive therapy, which results in the reactivation of BKV.6–8 The reactivation of the latent virus that can impair cellular immunity enables sustained viral replication in urothelial cells, which potentially leads to the development of polyomavirus-associated nephropathy (PVAN).9 In addition, PVAN is now recognized as one of the major consequences associated with the infection of polyomaviruses.7,9–13 Currently, 1 to 10% of renal transplant recipients are diagnosed to have PVAN, leading to graft loss in 20 to 80% of patients.6,7,14–19 Since no established antiviral treatment is currently available and the immunosuppressed state is critical for renal transplant patients, a careful manipulation of immunosuppression to avoid rejection but early identification of BKV reactivation is probably the best option available for management at this time.6,7 Thus, over the last several years, many studies have shown that screening by monitoring of the viral load in urine is able to predict patients at risk for the development of PVAN.6,17,20 Therefore, a simple and rapid detection method for BKV monitoring is significantly important for clinical application. Recently, several methods for the detection of BKV infections have been reported and some of them were not ready for clinical application. A polymerase chain reaction (PCR)-based technique, which has been widely used in clinical treatments, is the most effective and prevalent method for screening and monitoring active BKV infection.1,15,17,20–24 Still, this method has some limitations because it is time-consuming, laborious, and expensive.7,9,25 Urine cytology is frequently employed as a screening test for active viral infection, although the sensitivity and specificity of decoy cell measurement is disputable.9,26–31 Loop-mediated isothermal amplification (LAMP)-based technique is another option. Even though LAMP is an effective and rapid method for amplification of nucleic acid, which typically occurs within 60 min under isothermal conditions, the complex primer design and standardization may limit its implementation in clinical settings.32,33 Besides, there are other ways to detect BKV infection such as mass spectrometry-based and electron microscopy-based methods. But still, the requirement of highly skilled operators and costly equipment emerge as the major drawbacks.9,25,34 Surface plasmon resonance (SPR) biosensor is an effective alternative for virus detection. The highly localized electromagnetic fields render biosensor sensitive to changes in the effective refractive index () of the dielectric medium near a metal film surface under the attenuated total reflection arrangement. Moreover, the capabilities for rapid, label-free, and real-time detection make it widely used to investigate biomolecular interactions.35–42 However, limits on the detection sensitivity of conventional SPR biosensors make them incapable of detecting small changes in the , particularly in the measurement of biomolecular interactions at ultralow concentrations.36 This evidence opens a fruitful area of research since SPR emanates as an outstanding platform for virus detection because virus could cause larger changes in .36,43–46 In this study, the developed dual-channel heterodyne-based SPR biosensor is utilized to rapidly detect BKV. Researches denoted that the limit of detection (LOD) of the biosensor for BKV detection is in urine, which is much lower than the threshold loads of a renal transplant patient who is probable to develop PVAN.7,17,20,34,47 Also, the assay time of the biosensor is known to be . It is noteworthy that this work may offer a great opportunity to develop an alternative PCR-free method enabling the detection of viral pathogens by incorporating an appropriate pathogen-specific antibody. 2.Material and Methods2.1.Clinical SamplesThis study was approved by the institutional review board of Chang Gung Memorial Hospital. Samples were taken from renal transplant recipients in the Department of Nephrology, Linkou Chang Gung Memorial Hospital, Taiwan, after their informed consent was obtained. A total of five patients were recruited in this study from July 2009 to June 2011. 2.2.MaterialsThe bare silver/gold chips (SPR chips) used in this study were produced by the semiconductor laboratory of Chang Gung University (Taoyuan, Taiwan). The immobilization buffer and amine coupling kit containing 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), and 1.0 M ethanolamine-HCl, pH 8.5 (ETH) was purchased from Biacore Inc. (Uppsala, Sweden). and were obtained from SensoPath Technologies (Bozeman, MT). All chemicals were used without further purification. The monoclonal BKV antibody (a-BKV) used as capture antibody and monoclonal nonspecific antibody used against influenza B virus were obtained from Abnova (Taipei, Taiwan) and Abcam (Cambridge, MA), respectively. The BKV (archetype strain) was purchased from American Type Culture Collection (CCL-137; Manassas, VA). Human proximal tubular cells, HK-2, were used for viral infection. The HK-2 cells were cultured in Dulbecco’s modified Eagle medium/Ham’s F12 (Life Technologies, Paisley, UK) supplemented with 5% fetal calf serum (Biological Industries Ltd, Cumbernauld, UK), 2 mM glutamine (Life Technologies Ltd), 20 mM Hepes buffer (Gibco BRL, Paisley, UK), hydrocortisone, insulin, transferring, and sodium selenite (Sigma Chemical Company Ltd, Poole, UK). The BKV copy number was determined at as described previously.48 2.3.Optical Setup of the Dual-Channel Heterodyne-Based SPR BiosensorThe optical setup of the dual-channel heterodyne-based SPR biosensor is illustrated in Fig. 1. The laser beam from a frequency-stabilized and linearly polarized He–Ne laser with wavelength of 632.8 nm was integrated with an electro-optic modulator driven at frequency . Afterward, the beam passes through a half-wave plate and a polarizer sequentially to produce two highly correlated -polarized waves (TM wave, and ) at different frequencies. The -polarized light was split into two parallel beams by passing it through a lateral displacement beam splitter and a homemade dual-channel SPR device consisting of two independent polydimethylsiloxane (PDMS) flow channels. Two lock-in amplifiers were used for simultaneously measuring the amplitudes of -heterodyne signals from the reference chamber and signal chamber, respectively, for BKV detection. 2.4.Preparation of SPR ChipThe SPR chip used in this study was BK7 glass slide coated with a laminated () metal layer. The SPR chips were cleaned with UV / Ozone Cleaning System (Novascan, Ames, IA) to purify the gold surface prior to self-assembled monolayers (SAMs) surface functionalization. Mixed SAMs of dithiols consisting of 90% and 10% , used as a diluent at a mixing ratio of , were assembled at the substrates (SPR chip), generating a poly(ethylene glycol) (PEG)-based binding matrix optimized for the formation of an antibody monolayer via an amine-coupling protocol. The capture antibody and the nonspecific antibody were covalently immobilized to the signal chamber and reference chamber of SPR chip, respectively, by utilizing the homemade dual-channel PDMS flow-cell. The volume of each chamber was . It must be noted that the capture antibodies were only immobilized in the signal chamber, and the reference chamber only immobilized nonspecific antibodies. The amine-coupling protocol was performed as follows:
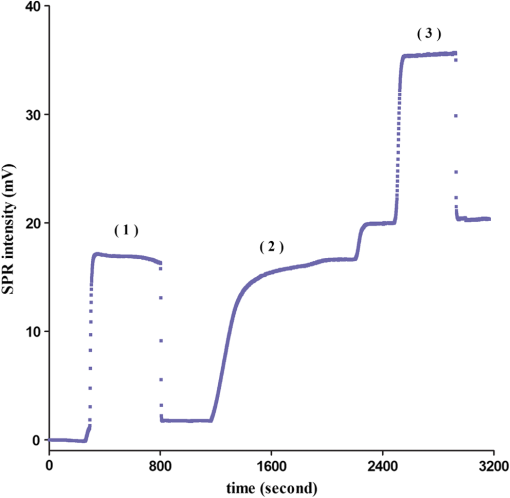
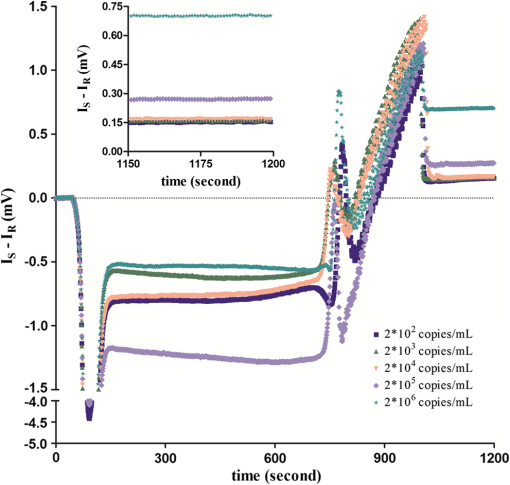
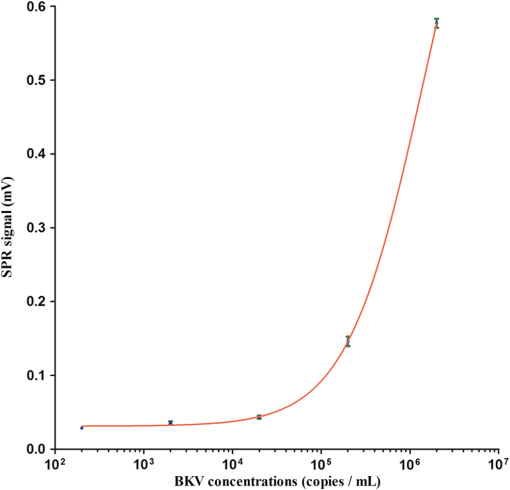
The covalent immobilization procedures of the capture antibody (a-BKV) were recorded by SPR intensity obtained by measuring the optical heterodyne signal of the reflected -polarized waves as shown in Fig. 2. The sensorgram describes (1) the NHS/EDC activation, (2) a-BKV () immobilizations, and (3) deactivation by ETH. 2.5.Measurement of BKV Using the Dual-Channel Heterodyne-Based SPR BiosensorThe original concentration of the BKV culture supernatant was and the supernatant was irradiated by UV for 30 min before the experiment. In the mock experiments, the BKV isolate was 10-fold serial diluted by urine to mimic the in vivo isolation of BKV from a patient. In addition, five urine samples including two PVAN-negative renal transplant patients and three PVAN-positive renal transplant patients were tested. All the analytes were simultaneously injected into both signal and reference chambers to interact with the capture antibody (a-BKV) and nonspecific antibody, respectively. 3.Results and Discussion3.1.Performance of the Dual-Channel Heterodyne-Based SPR Biosensor on Detecting BKV in UrineThe SPR chip was prepared using the method described above, and the original concentration of the UV-irradiated BKV culture supernatant before urine dilution was . In order to mimic the clinical situation, 10-fold serial dilutions of UV-irradiated BKV culture supernatant in 1 mL volume each, i.e., , , , , and , were spiked in urine (from a healthy adult) separately. After a-BKV and the nonspecific antibody were immobilized in the signal chamber and the reference chamber, respectively, each of the mimic solutions was injected into the signal and the reference chambers simultaneously to interact with the immobilized a-BKV and nonspecific antibody, respectively. Figure 3 shows the real-time SPR curve of interaction, where is described as the heterodyne signal measured from the signal chamber. It can be seen that the curve exhibits a strange behavior after the washing step. This results from a higher flow rate during the washing process in order to reduce assay time. Finally, the flow rate returned to normal. It is remarkable that the sensing surface has low nonspecific protein adsorption after phosphate-buffered saline (PBS) washing. Therefore, it is observable that PEG-based SAMs provide a low-fouling ability that prevents nonspecific adsorption on the SPR chip. The inset in Fig. 3 presents the last 50 data points of the experiment. However, there is no correlation between the measured and the concentration of the spiked BKV isolate over the range of to . It is allegedly because the real signal coming from the specific binding of BKV is covered by the signal of nonspecific binding. Fig. 3Binding processes of a-BKV interaction with different concentrations of BKV over the range to measured with single channel. Inset: zoom-in of the last 50 data points. 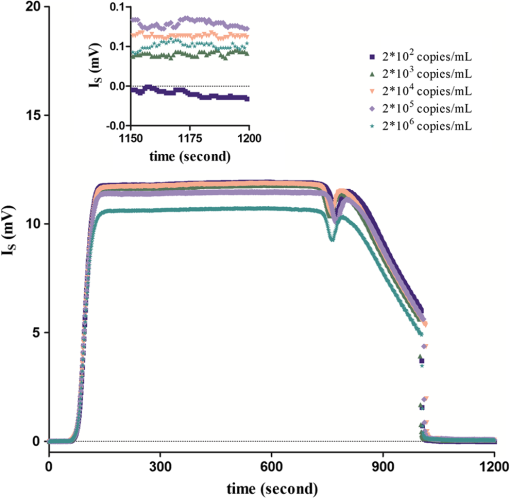 Accordingly, a differential method using a symmetrical reference channel is introduced to reduce the environmental variations and the nonspecific noise in order to promote the sensor sensitivity. The results are shown in Fig. 4, and in this experiment, the interaction was analyzed by subtracting from in which is described as the heterodyne signal measured from the reference chamber. After the mimic solution and PBS injections, an abnormal phenomenon took place, associated with the different lengths of the flow channels during the transport processes. Nonetheless, the kinetic information is not the focus here. Hence, the end-point measurements are presented in the inset of Fig. 4. The values of () seem to be able to differentiate the BKV concentrations. The correlation between the SPR signal and the concentration of the spiked BKV in urine over the range of to is depicted in Fig. 5. The SPR signal was acquired by subtracting the background level from the average of () over the last 50 data points of the experiment. The results were analyzed using a sigmoidal dose-response curve with variable slope, the so-called four-parameter logistic equation, found in GraphPad Prism software; the correlation coefficient () is 0.9999 and the error bar indicates one standard deviation in each measurement. Generally, the LOD is the concentration at which the signal corresponds to three times of the standard deviation positioned in the dose-response curve. Consequently, theoretical LOD of the dual-channel heterodyne-based SPR biosensor for BKV detection in urine was calculated to be from both experimental data and the fitting curve for this experimental design. This value is well below the threshold level of the urine BKV of the renal transplant patient who is about to develop PVAN; the reasonable threshold loads considered to be clinically significant are always determined at to in urine.7,17,20,34 3.2.BKV Detection in Clinical SampleTo prove that the dual-channel heterodyne-based SPR biosensor can be applied to detect clinical samples, urine from five renal transplant patients were checked by the developed biosensor. The results displayed in Fig. 6 reveal that the BKVs in the urine were successfully detected and PVAN-positive and PVAN-negative renal transplant patients were also clearly and promptly distinguished by this platform. Fig. 6Five urine samples detected by the dual-channel heterodyne-based SPR biosensor. SPR signals of the two PVAN negative were compared to those of the three PVAN positive renal transplant patients. 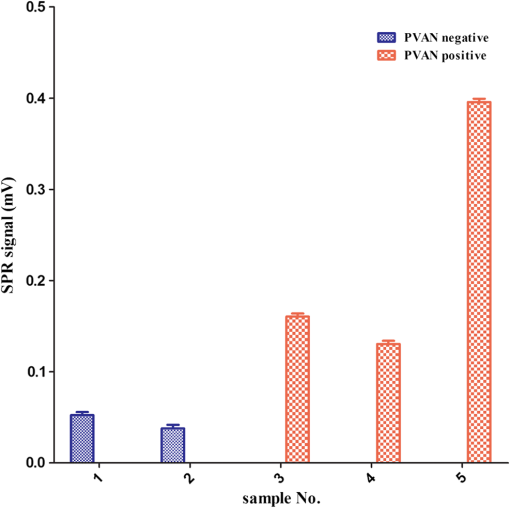 In renal transplant patients, the use of immunosuppressive therapy may trigger the BKV reactivation, leading to PVAN in which its malignant stage is likely to conduct an inevitable allograft failure.4,8,10 Based on this consideration, early identification and continuous monitoring of BKV reactivation and reduction of immunosuppressants are substantial to prevent the development of PVAN.7,9,11–13,17,20 Current guidelines recommend regular observation of BKV reactivation by the detection of infected urothelial cells in urine (decoy cells) or viral nucleic acid in urine or blood.9 On the contrary, the sensitivity and specificity of decoy cell measurement remain debated while PCR-based assays seem to be impractical owing to the needs of highly skilled operators and time-consuming processes.7,9 Several groups hitherto reported advanced detection techniques wherein the sensitivity of BKV detection in urine reached level. A comparison of the LOD of BKV measurement in urine of the various techniques is shown in Table 1. Although the dual-channel heterodyne-based SPR biosensor is not as sensitive as those advanced detection techniques, its simplicity, rapidity, and applicability do have valuable contributions in clinical continuous BKV monitoring. Table 1Comparison of the LOD of BKV measurement in urine obtained from various techniques.
In this study, the dual-channel heterodyne-based SPR biosensor could be employed to measure clinical urine samples where PEG-based low-fouling SAMs play an essential role. One of the main challenges for bioapplications of SPR biosensors is to maintain its high sensitivity in real-world complex media such as serum or urine due to the nonspecific adsorption on the sensing surface.39 This nonspecific adsorption is attributed to high background noise that aggravates the LOD of the SPR biosensors.39,42 Current studies point out that various low-fouling or nonfouling materials perform a great resistance to the nonspecific adsorption in SPR biosensor surface.39–42,49 PEG, a water-soluble, nontoxic, and nonimmunogenic polymer, is regarded as an effective protein-resistant material and has been frequently used in biosensors, although it does not exhibit the best antifouling capability compared with other advanced nonfouling materials such as zwitterionic polymer.49–54 Fortunately, the concentration of urine proteins is much lower than that of serum proteins.55 In consequence of the availability and cost-effectiveness, the PEG-based SAMs would be a great option to withstand the nonspecific adsorption in urine as illustrated in Fig. 3. 4.ConclusionsIn accordance with the differential method using a symmetrical reference channel integrated with the PEG-based low-fouling SAMs, we successfully showed that the developed dual-channel heterodyne-based SPR biosensor is applicable to rapid urinary BKV detection. The LOD of the biosensor for urinary BKV detection is estimated to be , which is much lower than the threshold loads of the renal transplant patient who is at the risk of developing PVAN. Even though the dual-channel heterodyne-based SPR biosensor sensitivity is not superior as compared to other detection techniques, yet, its simplicity, rapidity, and applicability have noteworthy contributions in continuous clinical BKV monitoring. Furthermore, when it was carried out in the detection of the urine samples from five renal transplant patients, our proposed SPR biosensor achieved a rapid determination of PVAN-positive and negative. This fact indicates that the developed dual-channel heterodyne-based SPR biosensor may be taken into account as a prospective biosensor in clinical applications in the future. AcknowledgmentsThis research was supported by the Linkou Chang Gung Memorial Hospital through the research grants (CMRPD2A0091 and CMRPG381062) and National Science Council, Taiwan (NSC101-2221-E-182-034-MY3 and NMRPD190372). The support from The Ministry of Education, Taiwan, through the Aiming for the Top University Plan (EMRPD1B0301) is also highly appreciated. ReferencesK. K. Iwakiet al.,
“Development of a real-time quantitative PCR assay for detection of a stable genomic region of BK virus,”
Virol. J., 7 295
(2010). http://dx.doi.org/10.1186/1743-422X-7-295 1743-422X Google Scholar
W. A. Knowles,
“Discovery and epidemiology of the human polyomaviruses BK virus (BKV) and JC virus (JCV),”
Adv. Exp. Med. Biol., 577 19
–45
(2006). http://dx.doi.org/10.1007/0-387-32957-9 AEMBAP 0065-2598 Google Scholar
A. LundstigJ. Dillner,
“Serological diagnosis of human polyomavirus infection,”
Adv. Exp. Med. Biol., 577 96
–101
(2006). http://dx.doi.org/10.1007/0-387-32957-9 AEMBAP 0065-2598 Google Scholar
M. Sadeghiet al.,
“Strong association of phenylalanine and tryptophan metabolites with activated cytomegalovirus infection in kidney transplant recipients,”
Hum. Immunol., 73
(2), 186
–192
(2012). http://dx.doi.org/10.1016/j.humimm.2011.11.002 HUIMDQ 0198-8859 Google Scholar
P. Randhawaet al.,
“A comparative study of BK and JC virus infections in organ transplant recipients,”
J. Med. Virol., 77
(2), 238
–243
(2005). http://dx.doi.org/10.1002/(ISSN)1096-9071 JMVIDB 1096-9071 Google Scholar
I. A. AghaD. C. Brennan,
“BK virus and current immunosuppressive therapy,”
Graft, 5 65
–72
(2002). http://dx.doi.org/10.1177/1522162802238464 1522-1628 Google Scholar
B. R. Bistaet al.,
“Development of a loop-mediated isothermal amplification assay for rapid detection of BK virus,”
J. Clin. Microbiol., 45
(5), 1581
–1587
(2007). http://dx.doi.org/10.1128/JCM.01024-06 JCMIDW 1070-633X Google Scholar
H.-H. Wanget al.,
“BK virus infection in association with posttransplant urothelial carcinoma,”
Transplant. Proc., 41
(1), 165
–166
(2009). http://dx.doi.org/10.1016/j.transproceed.2008.08.138 TRPPA8 0041-1345 Google Scholar
R. Konietznyet al.,
“Detection of BK virus in urine from renal transplant subjects by mass spectrometry,”
Clin. Proteomics, 4 1
–9
(2012). http://dx.doi.org/10.1186/1559-0275-9-4 69KQLA 1542-6416 Google Scholar
R. MarcÊn,
“Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection,”
Drugs, 69
(16), 2227
–2243
(2009). http://dx.doi.org/10.2165/11319260-000000000-00000 DRUGAY 0012-6667 Google Scholar
S. Hariharan,
“BK virus nephritis after renal transplantation,”
Kidney Int., 69
(4), 655
–662
(2006). http://dx.doi.org/10.1038/sj.ki.5000040 KDYIA5 0085-2538 Google Scholar
H. H. Hirsch,
“BK virus: opportunity makes a pathogen,”
Clin. Infect. Dis., 41
(3), 354
–360
(2005). http://dx.doi.org/10.1086/431488 CIDIEL 1058-4838 Google Scholar
H. H. HirschJ. Steiger,
“Polyomavirus BK,”
Lancet Infect. Dis., 3
(10), 611
–623
(2003). http://dx.doi.org/10.1016/S1473-3099(03)00770-9 1473-3099 Google Scholar
C. B. Drachenberget al.,
“Polyomavirus disease in renal transplantation: review of pathological findings and diagnostic methods,”
Hum. Pathol., 36
(12), 1245
–1255
(2005). http://dx.doi.org/10.1016/j.humpath.2005.08.009 HPCQA4 0046-8177 Google Scholar
H. H. Hirschet al.,
“Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations,”
Transplantation, 79
(10), 1277
–1286
(2005). http://dx.doi.org/10.1097/01.TP.0000156165.83160.09 TRPLAU 0041-1337 Google Scholar
V. NickeleitM. J. Mihatsch,
“Polyomavirus allograft nephropathy and concurrent acute rejection: a diagnostic and therapeutic challenge,”
Am. J. Transplant., 4
(5), 838
–839
(2004). http://dx.doi.org/10.1111/ajt.2004.4.issue-5 AJTMBR 1600-6135 Google Scholar
A. Vatset al.,
“Quantitative viral load monitoring and cidofovir therapy for the management of BK virus-associated nephropathy in children and adults,”
Transplantation, 75
(1), 105
–112
(2003). http://dx.doi.org/10.1097/00007890-200301150-00020 TRPLAU 0041-1337 Google Scholar
P. S. RandhawaA. J. Demetris,
“Nephropathy due to polyomavirus type BK,”
N. Engl. J. Med., 342
(18), 1309
–1315
(2000). http://dx.doi.org/10.1056/NEJM200005043421809 NEJMAG 0028-4793 Google Scholar
M. Ahujaet al.,
“Polyoma virus infection after renal transplantation: use of immunostaining as a guide to diagnosis,”
Transplantation, 71
(7), 896
–899
(2001). http://dx.doi.org/10.1097/00007890-200104150-00013 TRPLAU 0041-1337 Google Scholar
P. Randhawaet al.,
“Correlates of quantitative measurement of BK polyomavirus (BKV) DNA with clinical course of BKV infection in renal transplant patients,”
J. Clin. Microbiol., 42
(3), 1176
–1180
(2004). http://dx.doi.org/10.1128/JCM.42.3.1176-1180.2004 JCMIDW 1070-633X Google Scholar
G. V. Kaigalaet al.,
“Automated screening using microfluidic chip-based PCR and product detection to assess risk of BK virus-associated nephropathy in renal transplant recipients,”
Electrophoresis, 27
(19), 3753
–3763
(2006). http://dx.doi.org/10.1002/(ISSN)1522-2683 ELCTDN 0173-0835 Google Scholar
A. Behzadbehbahaniet al.,
“Detection of BK virus in urine by polymerase chain reaction: a comparison of DNA extraction methods,”
J. Virol. Methods, 67
(2), 161
–166
(1997). http://dx.doi.org/10.1016/S0166-0934(97)00101-8 JVMEDH 0166-0934 Google Scholar
S. Milleret al.,
“Analytical and clinical performance characteristics of the Simplexa BK virus quantitative PCR assay for the diagnosis of polyomavirus-associated nephropathy in renal transplant recipients using plasma and urine specimens,”
J. Clin. Virol., 55
(4), 310
–316
(2012). http://dx.doi.org/10.1016/j.jcv.2012.08.016 JCVIFB 1386-6532 Google Scholar
J. A. LeffertsN. D. HicksG. J. Tsongalis,
“Evaluation of a BK virus real-time PCR assay designed using novel bioinformatics technology,”
Exp. Mol. Pathol., 89
(2), 99
–102
(2010). http://dx.doi.org/10.1016/j.yexmp.2010.06.001 EXMPA6 0014-4800 Google Scholar
H. K. Singhet al.,
“Negative-staining electron microscopy of the urine for the detection of polyomavirus infections,”
Ultrastruct. Pathol., 30
(5), 329
–338
(2006). http://dx.doi.org/10.1080/01913120600932347 ULPAD3 1521-0758 Google Scholar
A. V. KahanD. V. ColemanL. G. Koss,
“Activation of human polyomavirus infection-detection by cytologic technics,”
Am. J. Clin. Pathol., 74
(3), 326
–332
(1980). AJCPAI 0002-9173 Google Scholar
C. B. Drachenberget al.,
“Human polyoma virus in renal allograft biopsies: morphological findings and correlation with urine cytology,”
Hum. Pathol., 30
(8), 970
–977
(1999). http://dx.doi.org/10.1016/S0046-8177(99)90252-6 HPCQA4 0046-8177 Google Scholar
R. R. ArthurK. V. Shah,
“Occurrence and significance of papovaviruses BK and JC in the urine,”
Prog. Med. Virol., 36 42
–61
(1989). PMVIA6 0079-645X Google Scholar
D. V. ColemanS. D. GardnerA. M. Field,
“Human polyomavirus infection in renal allograft recipients,”
Br. Med. J., 3
(5876), 371
–375
(1973). http://dx.doi.org/10.1136/bmj.3.5876.371 BMJOAE 0007-1447 Google Scholar
H. H. Hirschet al.,
“Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients,”
N. Engl. J. Med., 347
(7), 488
–496
(2002). http://dx.doi.org/10.1056/NEJMoa020439 NEJMAG 0028-4793 Google Scholar
P. RandhawaA. VatsR. Shapiro,
“Monitoring for polyomavirus BK and JC in urine: comparison of quantitative polymerase chain reaction with urine cytology,”
Transplantation, 79
(8), 984
–986
(2005). http://dx.doi.org/10.1097/01.TP.0000157573.90090.FD TRPLAU 0041-1337 Google Scholar
Y. MoriT. Notomi,
“Loop-mediated isothermal amplification (LAMP): a rapid, accurate, and cost-effective diagnostic method for infectious diseases,”
J. Infect. Chemother., 15
(2), 62
–69
(2009). http://dx.doi.org/10.1007/s10156-009-0669-9 JICHFN 1341-321X Google Scholar
R. Abdul-GhaniA. M. Al-MekhlafibP. Karanisc,
“Loop-mediated isothermal amplification (LAMP) for malarial parasites of humans: would it come to clinical reality as a point-of-care test?,”
Acta Trop., 122
(3), 233
–240
(2012). http://dx.doi.org/10.1016/j.actatropica.2012.02.004 ACTRAQ 0001-706X Google Scholar
C. Y. W. Tonget al.,
“Monitoring the progress of BK virus associated nephropathy in renal transplant recipients,”
Nephrol. Dial. Transplant., 19
(10), 2598
–2605
(2004). NDTREA Google Scholar
Y.-C. Liet al.,
“Sensitive detection of unlabeled oligonucleotides using a paired surface plasma waves biosensor,”
Biosens. Bioelectron., 35
(1), 342
–348
(2012). http://dx.doi.org/10.1016/j.bios.2012.03.014 BBIOE4 0956-5663 Google Scholar
L.-C. Suet al.,
“Rapid and highly sensitive method for influenza A (H1N1) virus detection,”
Anal. Chem., 84
(9), 3914
–3920
(2012). http://dx.doi.org/10.1021/ac3002947 ANCHAM 0003-2700 Google Scholar
L.-C. Suet al.,
“Detection of prostate-specific antigen with a paired surface plasma wave biosensor,”
Anal. Chem., 82
(9), 3714
–3718
(2010). http://dx.doi.org/10.1021/ac100071h ANCHAM 0003-2700 Google Scholar
Y.-C. Kuoet al.,
“Development of a surface plasmon resonance biosensor for real-time detection of osteogenic differentiation in live mesenchymal stem cells,”
PLOS One, 6
(7), e22382
(2011). http://dx.doi.org/10.1371/journal.pone.0022382 1932-6203 Google Scholar
M. PiliarikM. BockováJ. Homola,
“Surface plasmon resonance biosensor for parallelized detection of protein biomarkers in diluted blood plasma,”
Biosens. Bioelectron., 26
(4), 1656
–1661
(2010). http://dx.doi.org/10.1016/j.bios.2010.08.063 BBIOE4 0956-5663 Google Scholar
N. D. Braultet al.,
“Directly functionalizable surface platform for protein arrays in undiluted human blood plasma,”
Anal. Chem., 85
(3), 1447
–1453
(2013). http://dx.doi.org/10.1021/ac303462u ANCHAM 0003-2700 Google Scholar
H. Vaisocherovaet al.,
“Ultralow fouling and functionalizable surface chemistry based on a zwitterionic polymer enabling sensitive and specific protein detection in undiluted blood plasma,”
Anal. Chem., 80
(20), 7894
–7901
(2008). http://dx.doi.org/10.1021/ac8015888 ANCHAM 0003-2700 Google Scholar
C.-J. HuangY. LiS. Jiang,
“Zwitterionic polymer-based platform with two-layer architecture for ultra low fouling and high protein loading,”
Anal. Chem., 84
(7), 3440
–3445
(2012). http://dx.doi.org/10.1021/ac3003769 ANCHAM 0003-2700 Google Scholar
C. E. Nilssonet al.,
“A novel assay for influenza virus quantification using surface plasmon resonance,”
Vaccine, 28
(3), 759
–766
(2010). http://dx.doi.org/10.1016/j.vaccine.2009.10.070 VACCDE 0264-410X Google Scholar
A. K. Trillinget al.,
“The effect of uniform capture molecule orientation on biosensor sensitivity: dependence on analyte properties,”
Biosens. Bioelectron., 40
(1), 219
–226
(2013). http://dx.doi.org/10.1016/j.bios.2012.07.027 BBIOE4 0956-5663 Google Scholar
J. Huet al.,
“Development of a label-free and innovative approach based on surface plasmon resonance biosensor for on-site detection of infectious bursal disease virus (IBDV),”
Biosens. Bioelectron., 31
(1), 475
–479
(2012). http://dx.doi.org/10.1016/j.bios.2011.11.019 BBIOE4 0956-5663 Google Scholar
X. Houet al.,
“Real-time analysis of the interaction of a multiple-epitope peptide with antibodies against classical swine fever virus using surface plasmon resonance,”
J. Biotechnol., 161
(3), 221
–227
(2012). http://dx.doi.org/10.1016/j.jbiotec.2012.05.004 JBITD4 0168-1656 Google Scholar
H. H. HirschP. Randhawa,
“The AST infectious diseases community of practice. BK virus in solid organ transplant recipients,”
Am. J. Transplant., 9
(Suppl. 4), S136
–146
(2009). http://dx.doi.org/10.1111/ajt.2009.9.issue-s4 AJTMBR 1600-6135 Google Scholar
Y.-J. Liet al.,
“A suppressive effect of cyclosporine A on replication and noncoding control region activation of polyomavirus BK virus,”
Transplantation, 89
(3), 299
–306
(2010). http://dx.doi.org/10.1097/TP.0b013e3181c9b51c TRPLAU 0041-1337 Google Scholar
A. Sonatoet al.,
“Enhanced sensitivity azimuthally controlled grating-coupled surface plasmon resonance applied to the calibration of thiol-poly(ethylene oxide) grafting,”
Sens. Actuator B-Chem., 181 559
–566
(2013). http://dx.doi.org/10.1016/j.snb.2013.02.022 SABCEB 0925-4005 Google Scholar
S. JiangZ. Cao,
“Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications,”
Adv. Mater., 22
(9), 920
–932
(2010). http://dx.doi.org/10.1002/adma.200901407 ADVMEW 0935-9648 Google Scholar
T. M. Blättleret al.,
“High salt stability and protein resistance of poly(l-lysine)-g-poly(ethylene glycol) copolymers covalently immobilized via aldehyde plasma polymer interlayers on inorganic and polymeric substrates,”
Langmuir, 22
(13), 5760
–5769
(2006). http://dx.doi.org/10.1021/la0602766 LANGD5 0743-7463 Google Scholar
A. LarssonB. Liedberg,
“Poly(ethylene glycol) gradient for biochip development,”
Langmuir, 23
(22), 11319
–11325
(2007). http://dx.doi.org/10.1021/la700729q LANGD5 0743-7463 Google Scholar
F. J. Xuet al.,
“Spatially well-defined binary brushes of poly(ethylene glycol)s for micropatterning of active proteins on anti-fouling surfaces,”
Biosens. Bioelectron., 24
(4), 773
–780
(2008). http://dx.doi.org/10.1016/j.bios.2008.06.055 BBIOE4 0956-5663 Google Scholar
H. Wanget al.,
“Fabrication and anti-fouling properties of photochemically and thermally immobilized poly(ethylene oxide) and low molecular weight poly(ethylene glycol) thin films,”
J. Colloid Interface Sci., 354
(1), 160
–167
(2011). http://dx.doi.org/10.1016/j.jcis.2010.10.018 JCISA5 0021-9797 Google Scholar
C.-H. Chaoet al.,
“Detection of urine cofilin-1 from patients hospitalized in the intensive care unit using the metal-enhanced fluorescence technique,”
Sens. Actuator B-Chem., 173 184
–190
(2012). http://dx.doi.org/10.1016/j.snb.2012.06.076 SABCEB 0925-4005 Google Scholar
|