|
|
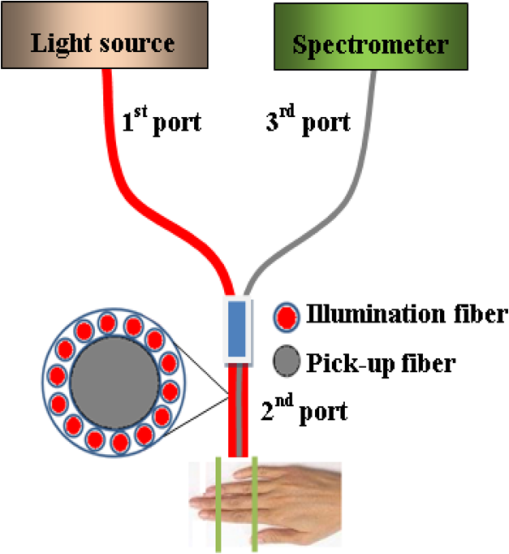
1.IntroductionMotivated by the growing maturity of laser treatment and optical imaging diagnosis, optical techniques, such as optical coherence tomography (OCT), confocal microscopy, nonlinear microscopy, and laser spectroscopic methods, are widely used in many fields. However, the complicated morphological nature of human tissue and variations of the refractive indices with internal different components make biotissues become a high scattering medium for visible and near-infrared wavelengths, i.e., the therapeutic and diagnostic optical window.1–5 Multiple scattering and absorption attenuate the effective light intensity of reaching internal tissue and diminish the detecting depth. Therefore, they limit the clinical application of optical imaging techniques. Currently, osmotic chemical agents used for optical clearing of biotissue have become a considerable interest. Optical clearing technique has been successfully developed to reduce the scattering properties and improve the light penetration depth by application of optical clearing agents (OCAs) with hyperosmolarity and biocompatibility.6 It has the significant potential to improve the application of spectroscopic and optical imaging techniques in clinic. OCAs, such as polyethylene glycol,7 glucose,8,9 glycerol,10–22 propylene glycol,16 and dextran,23 can reduce the scattering and enhance light penetration in biotissue. Generally, they are almost hydrophilic agents. However, it is a relatively slow process for these hydrophilic agents to penetrate the stratum corneum, when they are applied on the surface of skin. The agents with high concentration can achieve good osmosis effect, but they have their limitations in clinical applications considering the safety. A noninvasive way of incorporating a permeation enhancer can improve the osmosis effect in the stratum corneum. Some lipophilic agents has been added in OCAs to improve the delivery of agents in skin so as to achieve a better optical clearing effect, such as polypropylene glycol-based polymers,17 dimethyl sulfoxide (DMSO),19,22 oleic acid,19 azone,20 thiazone,24,25 and liquid paraffin.26–28 Optical wave bands used by different optical technologies are usually different. Many studies have been reported that biotissue in vivo and in vitro has different absorption coefficient (), scattering coefficient (), and refractive index () for different light wavelengths.4 Therefore, it is necessary to evaluate the optical clearing effect of an OCA thoroughly over the therapeutic and diagnostic optical window. Because of the excellent comprehensive characteristics of liquid paraffin, its synergistic effect as the penetration enhancer of glycerol is further studied by spectroscopy in this paper, whose purpose is to provide its anticipative result when it will be used in different optical technologies. A commercial spectrometer was used to measure the surface reflection of human skin before and after applying liquid paraffin glycerol mixtures, and then the reduction of diffuse reflectance was calculated. It was shown that anhydrous glycerol and different concentrations of liquid paraffin glycerol mixtures had different optical clearing effects on in vivo fingers for the spectra ranging from 600 to 1400 nm. It was also found that the mixtures had different effects for light with different wavelengths from the fluctuation of spectra. 2.Materials and Methods2.1.Samples and Chemical AgentsIn our experiment, 12 volunteers’ fingers were measured, including 10 males and 2 females, whose ages are from 22 to 43. All of them are healthy without any aberrance, such as scar, fibroma, pigmented nevus, or other diseases on their fingers. According to Ref. 26, when liquid paraffin is mixed with anhydrous glycerol by volume ratio to , the mixed solution displays the best optical clearing effect for in vivo samples. In addition, it can keep the structure of the tissue from deforming, which means it can balance between dehydration and moisture retention over a long time. Therefore, the same concentration range was used in our experiment. That is, four kinds of solution were used in this study, including anhydrous glycerol and three different concentrations of liquid paraffin whose volume fractions in mixed solutions are 30, 40, and 50%, respectively. Glycerol (WEICHEN Brand) and liquid paraffin (WENDA Brand), made by Tianjin Weichen Chemical Reagents Company Limited and Tianjin Yingda Rare Chemical Reagents Factory, Tianjin, China, respectively, were used in our study. 2.2.Experimental SystemA fiber-based spectrum analyzer system was set up in our experiment, whose sketch map is shown in Fig. 1. A specially designed Y-type fiber bundle was used in the system. Its first port is a fiber bundle composed of 14 same fibers whose core diameter is 100 μm, which is connected with a halogen tungsten lamp through a fiber optic connector of SMA 905. Its second port is also a fiber bundle composed of an inner fiber with a core diameter of 600 μm and the 14 fibers evenly distributed all around, as shown in Fig. 1. The broadband light from the halogen tungsten lamp uniformly illuminates the sample by the outer fibers and the reflection light from the sample is collected by the inner fiber. Its third port is only the inner fiber, which is connected with the spectrum analyzer (AQ-6315E, ANDO). Human fingers are fixed on a platform. In order to maintain the identical sampling in the whole measuring process, the distance between the second end and the sample is held at about 1 mm. In addition, the sample is nearly perpendicularly irradiated and the returned light is also collected perpendicularly as Ref. 26. 2.3.Evaluation of the Reduction of Diffuse ReflectionReflection spectrum collected by the spectrum analyzer system can be directly used to evaluate the diffuse reflectance. Some parameters have been used to evaluate it quantitatively.22,29 The optical clearing effect of OCAs can be revealed by the reduction of diffuse reflectance from the finger skin. Intensity ratio of reflectance () similar to Ref. 29 is used in our paper to calculate quantitatively the reduction of diffuse reflectance before and after treatment with agents. It is defined as follows: where is the measured diffuse reflectance at 1054.4 nm before treatment with agents and is the minimal measured diffuse reflectance in the whole measurement process at 1054.4 nm. The reason for choosing 1054.4 nm for the assessment of reflectance is because the spectrum analyzer detects the maximal power of the reflection at this wavelength.2.4.Measurement MethodThe experiment was carried out at 22°C room temperature. During the measuring process, the finger was fixed on the platform. In order to calculate the reduction of diffuse reflectance, the reflection spectra without the agent were first collected three times and the average of values was regarded as the baseline for each sample. Then, the agent was topically applied onto the surface of the finger, and spectra were collected at time intervals of 5 min. In order to evaluate the reflectance exactly, the distance between the second end of the Y-type fiber bundle and the sample remained unchanged while collecting all reflection spectra for a certain agent. Every volunteer was measured three times for each concentration. 3.Experimental Results and DiscussionWe can evaluate the change of reflection directly based on their reflection spectra before and after application of the agents. Figure 2 illustrates an example of the diffuse reflection spectra over a range from 600 to 1400 nm when the finger of a person was applied with different solutions. Figures 2(a), 2(b), 2(c), and 2(d) correspond to the results of anhydrous glycerol and 30, 40, and 50% liquid paraffin glycerol mixtures, respectively. The curves in each figure were obtained at different time intervals. It can be seen that the results from all four agents have similar trends qualitatively; that is, the diffuse reflection decreases gradually with time elapsing over the whole wavelength range investigated. In addition, we can see the reduced extent is different among these agents. From Fig. 2, we also find there is better effect at short wavelengths than that at long wavelengths, which is similar to the results of many agents.16,22,24 Fig. 2Changes of diffuse reflectance for human finger skin before and after application of different agents over the range of 600 nm - 1400 nm. (a), (b), (c) and (d) are anhydrous glycerol, 30%, 40% and 50% liquid paraffin glycerol mixtures, respectively. The different curves in each figure are corresponding to different time intervals from top to bottom, (a) no agent, 5, 15, 30, and 45 min, (b) no agent, 5, 15, 30, and 45 min, (c) no agent, 5, 15, 25, and 35 min, and (d) no agent, 5, 10, 20, and 30 min. 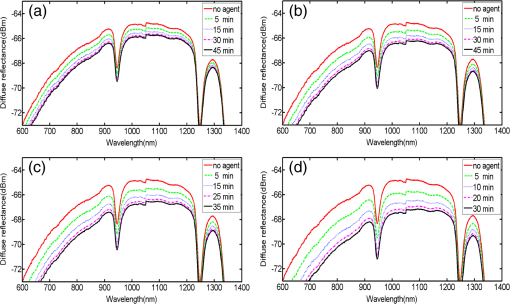 In order to compare the diffuse reflectance decrease, the data of samples without and with the agents treatment were extracted where the spectrum analyzer detected the minimal diffuse reflectance at the wavelength 1054.4 nm (), respectively. The statistical results of all 12 volunteers are shown in Fig. 3, where the negative percentage represents the decrease in reflectance compared to the control. The average diffuse reflectance decreased , 28.8, 33.8, and 43.5% for anhydrous glycerol, 30, 40, and 50% liquid paraffin glycerol mixtures, respectively. Three mixed solutions deliver more effective capability of decrease of diffuse reflectance than glycerol alone does. It indicates the trend that the more liquid paraffin is added, the larger decrease of diffuse reflectance is caused, which is similar to the effect of DMSO as shown in Ref. 22. Fig. 3Average reduction of diffuse reflectance () at 1054.4 nm for anhydrous glycerol and 30%, 40%, and 50% liquid paraffin glycerol mixtures, respectively. 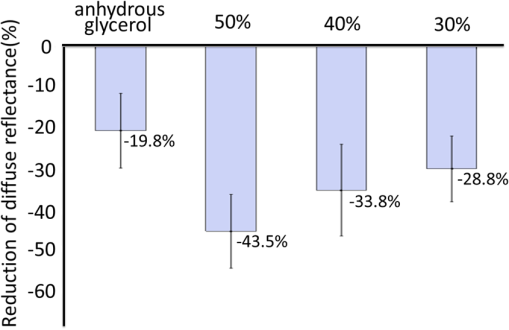 Two examples of the reduction of diffuse reflectance at 1054.4 nm with time elapsing of anhydrous glycerol, 30, 40, and 50% liquid paraffin glycerol mixtures are given in Fig. 4. They are shown with red, blue, black, and green curves, respectively. It can be seen from Fig. 4 that all the agents make the diffuse reflectance decrease. Thirty to fifty percentage liquid paraffin glycerol mixtures have much better effect than that of anhydrous glycerol. Furthermore, the mixtures improve the speed of the reduction of diffuse reflectance, which is accompanied by the increase of liquid paraffin. It further proves that OCAs combining hydrophilic agents with lipophilic agents improve the speed of percutaneous penetration over the whole spectrum investigated. Fig. 4Two examples of the reduction of diffuse reflectance at 1054.4 nm with time elapsing of anhydrous glycerol and 30%, 40%, and 50% liquid paraffin glycerol mixtures. 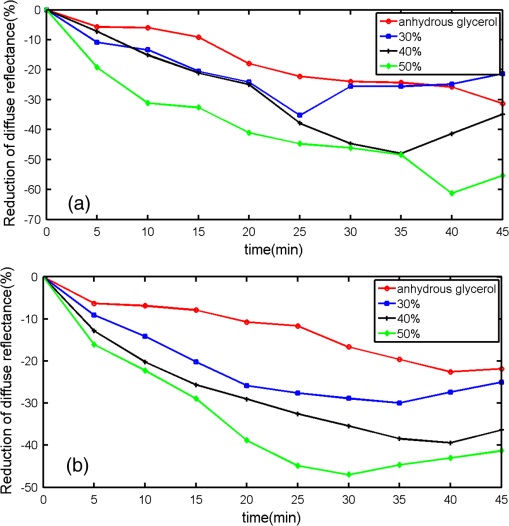 It can be seen from Fig. 4 that the curves of diffuse reflection within 45 min after applying 30 to 50% liquid paraffin glycerol mixtures have the common trend of first decreasing and then increasing, and have a maximum reduction of diffuse reflection, although they have slight difference for different persons [Figs. 4(a) and 4(b)]. This phenomenon further proves the scattering characteristics of biotissues are reversible after applying liquid paraffin glycerol mixture, which is an essential characteristic of OCAs. For all these solutions, 50% liquid paraffin glycerol mixture has the largest decreasing speed. However, the optimal reduction of diffuse reflection of anhydrous glycerol is not reached within 45 min. In addition, we can see from Fig. 4 that the reduction of diffuse reflectance is larger than 20% after 15 min mixture treatment, and this can be maintained for more than 25 min. We find 50% concentration has the largest reduced effect in Figs. 3 and 4. According to Ref. 26, 30% concentration has the best optical clearing effect under the surface of 700 μm. It has been known that blood flows in blood containing layers (living epidermis, dermis) will wash away a part of agent periodically for in vivo samples after the penetration of the osmotic agents. Most researchers think the mechanism of the internal biotissue enhancement includes the match of refractive indices and tissue dehydration due to the osmotic properties of OCAs. The gross volume of the mixture is the same for 30 to 50% mixture in this study and Ref. 26. After excluding other possibilities, as a possible explanation, we think that under the synergistic effect of the liquid paraffin more glycerol for 30% mixture can penetrate into the tissue and reach the deeper tissue. Although there are numerous studies about optical clearing techniques, the mechanism of optical clearing is still not completely clear; so evaluating an OCA thoroughly from different aspects is necessary before it can be used in clinic. Based on the results of this manuscript and Ref. 26, we think the optical clearing effect should be evaluated by combining the reduction of diffuse reflection from the surface (such as the reflectance spectrum) with the improvement of the returned light from the deep biotissue (such as OCT images). In addition, influence of interaction of osmotic agents and in vivo skin surface on light diffuse reflection and penetration should be further studied in the future. 4.ConclusionThe synergistic effect of reduction of diffuse reflection on human finger induced by different concentrations of liquid paraffin combined with glycerol was investigated by spectroscopy. From the experimental results, 30 to 50% mixed solutions are more effective and efficient than anhydrous glycerol over visible and near-infrared (600 to 1400 nm) wave band. The speed of the reduction of diffuse reflectance is accelerated by adding liquid paraffin over the whole spectrum investigated. In addition, there is better effect at short wavelengths than at long wavelengths. AcknowledgmentsThe authors acknowledge the support from the National Natural Science Foundation of China (Grant No. 11374167) and the Tianjin Foundation of Natural Science (No. 09JCZDJC18300). The authors thank Dr. Yan Li for helpful discussions. She is from the Department of Dermatology, Tianjin Medical University General Hospital. ReferencesR. K. WangV. V. Tuchin,
“Optical tissue clearing to enhance imaging performance for OCT,”
Optical Coherence Tomography: Technology and Applications, 855
–886 Springer, Berlin Heidelberg
(2008). Google Scholar
V. V. Tuchin,
“Optical clearing of tissues and blood using the immersion method,”
J. Phys. D: Appl. Phys., 38
(15), 2497
–2518
(2005). http://dx.doi.org/10.1088/0022-3727/38/15/001 JPAPBE 0022-3727 Google Scholar
L. V. WangH. Wu, Biomedical Optics: Principles and Imaging, John Wiley & Sons Inc., Hoboken, New Jersey
(2007). Google Scholar
V. V. Tuchin, Tissue Optics: Light Scattering Methods and Instruments for Medical Diagnosis, 2nd ed.SPIE, Bellingham, Washington
(2007). Google Scholar
E. A. GeninaA. N. BashkatovV. V. Tuchin,
“Tissue optical immersion clearing,”
Expert Rev. Med. Devices, 7
(6), 825
–842
(2010). http://dx.doi.org/10.1586/erd.10.50 1743-4440 Google Scholar
V. V. TuchinX. XuR. K. Wang,
“Dynamic optical coherence tomography in studies of optical clearing, sedimentation and aggregation of immersed blood,”
Appl. Opt., 41
(1), 258
–271
(2002). http://dx.doi.org/10.1364/AO.41.000258 APOPAI 0003-6935 Google Scholar
V. V. Tuchinet al.,
“Light propagation in tissues with controlled optical properties,”
J. Biomed. Opt., 2
(4), 401
–417
(1997). http://dx.doi.org/10.1117/12.281502 JBOPFO 1083-3668 Google Scholar
A. N. Bashkatovet al.,
“Optical clearing of skin tissue produced by application of glucose solution: in vivo study,”
Proc. SPIE, 6163 616313
(2006). http://dx.doi.org/10.1117/12.697313 PSISDG 0277-786X Google Scholar
E. A. Geninaet al.,
“Optical clearing of the eye sclera in vivo caused by glucose,”
Quantum Electron., 36
(12), 1119
–1124
(2006). http://dx.doi.org/10.1070/QE2006v036n12ABEH013337 QUELEZ 1063-7818 Google Scholar
G. Vargaset al.,
“Use of an agent to reduce scattering in skin,”
Lasers Surg. Med., 24
(2), 133
–141
(1999). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
H. Chenget al.,
“Hyperosmotic chemical agent’s effect on in vivo cerebral blood flow revealed by laser speckle,”
Appl. Opt., 43
(31), 5772
–5777
(2004). http://dx.doi.org/10.1364/AO.43.005772 APOPAI 0003-6935 Google Scholar
J. W. FluhrR. DarlenskiC. Surber,
“Glycerol and the skin: holistic approach to its origin and functions,”
Br. J. Dermatol., 159
(1), 23
–34
(2008). http://dx.doi.org/10.1111/j.1365-2133.2008.08643.x BJDEAZ 1365-2133 Google Scholar
C. G. Rylanderet al.,
“Dehydration mechanism of optical clearing in tissue,”
J. Biomed. Opt., 11
(4), 041117
(2006). http://dx.doi.org/10.1117/1.2343208 JBOPFO 1083-3668 Google Scholar
A. T. Yehet al.,
“Reversible dissociation of collagen in tissues,”
J. Invest. Dermatol., 121
(6), 1332
–1335
(2003). http://dx.doi.org/10.1046/j.1523-1747.2003.12634.x JIDEAE 0022-202X Google Scholar
J. Hirshburget al.,
“Collagen solubility correlates with skin optical clearing,”
J. Biomed. Opt., 11
(4), 040501
(2006). http://dx.doi.org/10.1117/1.2220527 JBOPFO 1083-3668 Google Scholar
X. XuQ. Zhu,
“Feasibility of sonophoretic delivery for effective skin optical clearing,”
IEEE Trans. Biomed. Eng., 55
(4), 1432
–1437
(2008). http://dx.doi.org/10.1109/TBME.2007.912416 IEBEAX 0018-9294 Google Scholar
M. H. Khanet al.,
“Optical clearing of in vivo human skin: implications for light-based diagnostic imaging and therapeutics,”
Lasers Surg. Med., 34
(2), 83
–85
(2004). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
X. Wenet al.,
“In vivo skin optical clearing by glycerol solutions: mechanism,”
J. Biophoton., 3
(1–2), 44
–52
(2010). http://dx.doi.org/10.1002/jbio.200910080 JBOIBX 1864-063X Google Scholar
J. JiangR. K. Wang,
“Comparing the synergistic effects of oleic acid and dimethyl sulfoxide as vehicles for optical clearing of skin tissue in vitro,”
Phys. Med. Biol., 49
(23), 5283
–5294
(2004). http://dx.doi.org/10.1088/0031-9155/49/23/006 PHMBA7 0031-9155 Google Scholar
X. XuQ. Zhu,
“Evaluation of skin optical clearing enhancement with azone as a penetration enhancer,”
Opt. Commun., 279
(1), 223
–228
(2007). http://dx.doi.org/10.1016/j.optcom.2007.06.055 OPCOB8 0030-4018 Google Scholar
X. XuR. K. Wang,
“The role of water desorption on optical clearing of biotissue: studied with near infrared reflectance spectroscopy,”
Med. Phys., 30
(6), 1246
–1253
(2003). http://dx.doi.org/10.1118/1.1576228 MPHYA6 0094-2405 Google Scholar
X. XuR. K. Wang,
“Synergistic effect of hyperosmotic agents of dimethyl sulfoxide and glycerol on optical clearing of gastric tissue studied with near infrared spectroscopy,”
Phys. Med. Biol., 49
(3), 457
–468
(2004). http://dx.doi.org/10.1088/0031-9155/49/3/008 PHMBA7 0031-9155 Google Scholar
M. Brezinskiet al.,
“Index matching to improve optical coherence tomography imaging through blood,”
Circulation, 103
(15), 1999
–2003
(2001). http://dx.doi.org/10.1161/01.CIR.103.15.1999 CIRCAZ 0009-7322 Google Scholar
D. Zhuet al.,
“Imaging dermal blood flow through the intact rat skin with an optical clearing method,”
J. Biomed. Opt., 15
(2), 026008
(2010). http://dx.doi.org/10.1117/1.3369739 JBOPFO 1083-3668 Google Scholar
X. Wenet al.,
“Enhanced optical clearing of skin in vivo and optical coherence tomography in-depth imaging,”
J. Biomed. Opt., 17
(6), 066022
(2012). http://dx.doi.org/10.1117/1.JBO.17.6.066022 JBOPFO 1083-3668 Google Scholar
J. Wanget al.,
“Evaluation of optical clearing with the combined liquid paraffin and glycerol mixture,”
Biomed. Opt. Express, 2
(8), 2329
–2338
(2011). http://dx.doi.org/10.1364/BOE.2.002329 BOEICL 2156-7085 Google Scholar
J. W. Wilsonet al.,
“Optical clearing of archive-compatible paraffin embedded tissue for multiphoton microscopy,”
Biomed. Opt. Express, 3
(11), 2752
–2760
(2012). http://dx.doi.org/10.1364/BOE.3.002752 BOEICL 2156-7085 Google Scholar
H. Shanet al.,
“Study on application of optical clearing technique in skin diseases,”
J. Biomed. Opt., 17
(11), 115003
(2012). http://dx.doi.org/10.1117/1.JBO.17.11.115003 JBOPFO 1083-3668 Google Scholar
H. Zhonget al.,
“Synergistic effect of ultrasound and thiazone-PEG 400 on human skin optical clearing in vivo,”
Photochem. Photobiol., 86
(3), 732
–737
(2010). http://dx.doi.org/10.1111/php.2010.86.issue-3 PHCBAP 0031-8655 Google Scholar
|