|
|
|
The first use of thermal preconditioning (PC) to confer tolerance to subsequent hyperthermia1 was later found to be a consequence of an adaptive heat shock response.2 In general, PC to environmental stimuli, and the adaptive responses to the PC, are well-established concepts rooted in the field of hormesis.3,4 Hyperthermia can lead to either thermal tolerance (limited exposure to 40.5–42.5°C) or cell death in a manner dependent upon both time and temperature (40–47°C).5 In fact, hyperthermia treatment of cells has been used to sensitize cancer cells to subsequent ionizing radiation or chemotherapeutic treatment regimes.5 To study thermal PC as a means of protecting cultured cells from photothermal damage we used an established artificially pigmented in vitro retinal model6,7 that uses the human-derived hTERT-RPE1 cell line. This experimental model takes advantage of the RPE cell’s ability to phagocytose melanosome particles (MPs) extracted from bovine eyes to become pigmented. The resulting pigmented cells display damage sensitivity to laser exposure relative to wavelength and exposure duration similar to that found using animal models.6 We successfully generated in the cultured system a mild hyperthermia thermal profile [see Fig. 1(a) for details] similar to a whole-body hyperthermia profile8 shown to produce protective responses against ischemia/reperfusion injury in the rat. Western analysis (data not shown) indicated the in vitro PC produced a typical heat shock response for heat shock protein-70 (maximum sevenfold induction at 18 and 24 h) and heat shock factor-1 (maximum twofold induction at 18 h). Fig. 1Thermal preconditioning (PC) schemes. (a) The thermal profile shown provides the temperature history for 48-well plates containing hTERT-RPE1 cells and/or MPs with . Plates were transferred from a 37°C incubator to one at 43.7°C for 40 min, and then returned to 37°C. Temperature of cells was estimated using a digital thermometer every 1 min in adjacent mock wells (0.3 mL medium alone). Plot is the of 13 independent mock pretreatments. (b) PC 1: cells were allowed to phagocytose MPs for 5 h prior to hyperthermia PC. PC 2: cells were preconditioned and then pigmented with nonpreconditioned MPs 1 h later. PC 3: cells and MPs were pretreated in separate wells of the same plate and added together 1 h later. All samples were exposed to laser the following day. 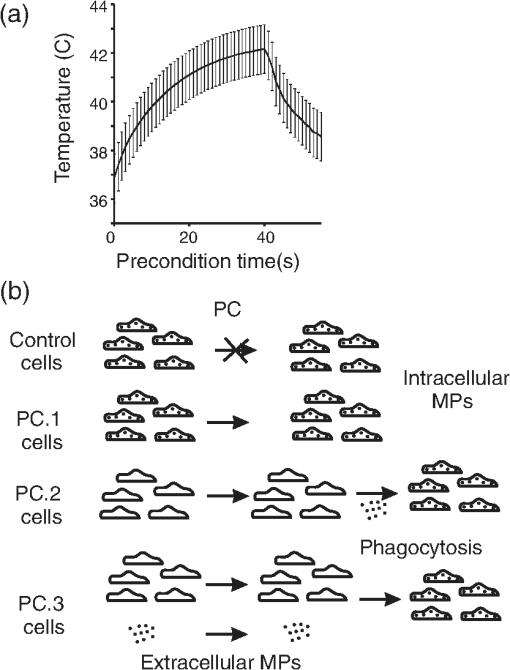 Contrary to protection from laser damage, we initially found the preconditioned artificially pigmented cells died at lower laser irradiances than did controls. Here, to study the role of the MPs in this observed sensitivity to laser damage, we repeated the pretreatments in three different ways, each varying the location of the pigment MPs during the hyperthermia PC [Fig. 1(b)]. All cells had intracellular MPs at the time of laser challenge, which was carried out 18–24 h post hyperthermia. The laser challenge phase of the experiment consisted of 16 independent replicates (center of eight separate wells of a 48-well plate on two different days) of 0.25-s exposures using a 0.93-mm diameter laser (flat top profile) at 514 nm with an irradiance of (, mean and standard deviation for all 64 exposures). This irradiance was chosen because it produced damage responses of 25–94% among all sample types. Figure 2(a) provides an example of a damage response in the in vitro retinal model. The representative fluorescent image identifies not only the laser-damaged area, as indicated by ethidium homodimer (red), but also the high degree of viability of the cells having undergone the hyperthermia PC (surrounding green fluorescence from Calcein outside the region of laser exposure). We compared the number of MPs remaining in the medium after overnight incubation and found no appreciable difference in phagocytosis due to hyperthermia. When provided equal amounts of extracellular MPs, only 4% and 7% were not phagocytosed by control cells and cells undergoing the hyperthermia, respectively. From the viability dye and phagocytosis analyses, we believe the cells were not injured by the hyperthermia PC treatment. Fig. 2Damage assessment. (a) Fluorescence image ( magnification). Staining of wells occurred 1 h after laser exposure (Calcein and ethidium homodimer indicator dyes). The entire well was treated as in PC 1 method. (b) Damage was assessed after 16 independent replicate exposures were delivered to each sample group. Laser exposures (0.25 s each) consisted of at 514 nm using a 0.93-mm diameter beam. (c) Raman microspectroscopy of individual MPs within control cells, or cells that were subjected to PC treatments 1 or 2. Raman spectra were collected using 532-nm excitation wavelength by focusing the incident radiation to about a 0.5-μm spot-size beam using a high-numerical objective. Raman signal was collected in backscattered geometry and averaged over 10 spots within the same sample. Details of the experimental setup and data analysis are presented in Ref. 9. 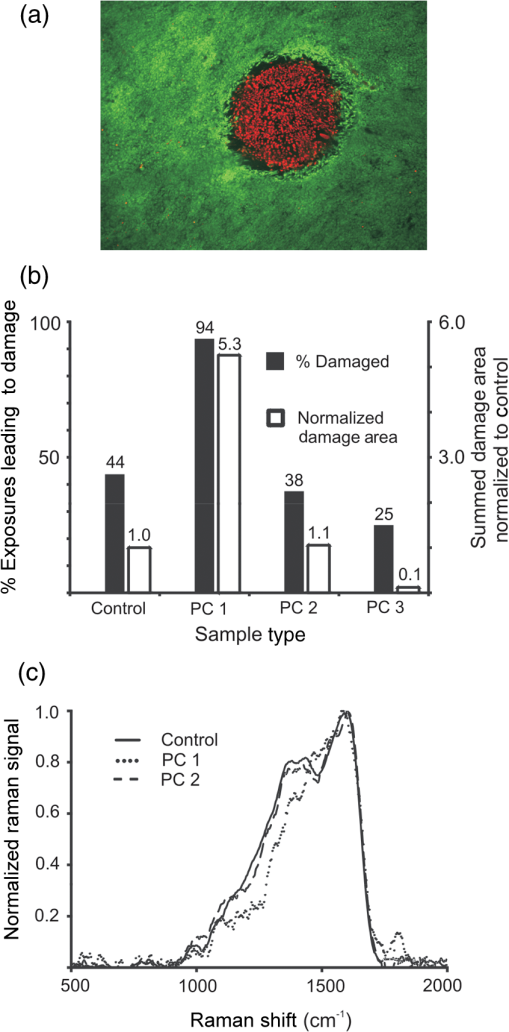 Figure 2(b) highlights our results in terms of damage frequency (percent damage out of 16 attempts) and the sum of all area damaged relative to control. Note that generated similar damage frequency in control (7 out of 16, or 44%) and PC 2 treatment (6 out of 16, or 38%). However, PC 1 and PC 3 treatments generated the opposite effects of sensitization and protection, respectively. The PC 3 cells had about one-half the damage occurrences (25%) relative to control cells and the damage areas were much smaller (10% of control). We believe this reduction in damage efficacy cannot be entirely the consequence of fewer MPs taken up by cells after PC (93% uptake compared to 96% in controls). Finally, and perhaps more importantly, PC of cells with intracellular melanosomes (PC 1) led to a doubling of the damage frequency (94%). If the average size of damage was the same as in control cells, this doubling would lead to a doubling of summed damage area as well. However, we found a fivefold increase in damaged area, which indicates damage spots that were at least twice the size as that in controls. At this point, it was unclear whether the hyperthermia-induced sensitivity was the consequence of a change in the optical properties of the MPs or the temperature at which the cells become damaged. To identify if the PC 1 and PC 3 treatments altered the cellular threshold temperature for death, we employed our microthermography method, as detailed in a previous article.10 Briefly, we collected spatially resolved, high-speed thermal images at the cellular level during each of the 64 laser exposures (all 16 replicates of the four sample types). We overlaid the respective thermal and fluorescence images to identify thermal data at the boundary of cell death (entirely around circumference of the damaged area), and thus a threshold temperature for each exposure leading to death. We found that cells from all four sample types (32 of the 64 total exposures) died at the same time-averaged temperature rise (), which implies the effects of hyperthermia treatment centered on the biology of the MPs rather than the cell’s “thermostat” for death. To understand the chemical changes in the MP that lead to PC-specific sensitivity, we assessed Raman microspectroscopy and bulk absorption. Figure 2(c) shows definitively that the chemistry of the MPs in PC 1 cells was grossly changed in the Raman fingerprint region (). Measurements (five wells, each with four flashes at four locations) of background-corrected bulk absorption at 560 nm were obtained from a Tecan GENios microplate reader for control () and PC 1 () cells 18-h post PC, showing a modest 20% increase due to PC treatment. The exact mechanisms by which sensitization occurs when pigmented RPE cells undergo mild hyperthermic treatment remain unknown. The chemistry of the MP is altered in a gross manner, but the bulk absorption (absorption plus scatter) of the cell monolayer is slightly changed overall. We would not expect to see a modification of the MP linear absorption by clumping alone, but this could lead to greater localization of the heat generated, which could prove deadly to cells at lower irradiances. Without further analysis of melanosome ultrastructure by high-magnification microscopy, it remains uncertain as to whether or not the PC treatment altered the MP’s size, shape, and intracellular distribution (including clumping). Due to the implications for enhanced laser sensitivity in humans undergoing mild hyperthermia (e.g. fever), we are currently studying the effects of PC on primary RPE cells having endogenously derived MPs. AcknowledgmentsThe opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Air Force. We thank Kurt Schuster for help with thermal data analysis. This work was supported by the Air Force Research Laboratory, Human Effectiveness Directorate, contract FA8650-08-D-6920 (M. L. D., G. D. N., M. S. F., L. E. E.) and the Air Force Office of Scientific Research, Grant No. 92HE04COR. This research was partially supported (V. V. Y.) by the NSF Grant Nos. DBI 1250361 and CBET 1250363. ReferencesE. W. GernerM. J. Schneider,
“Induced thermal resistance in HeLa cells,”
Nature, 256
(5517), 500
–502
(1975). http://dx.doi.org/10.1038/256500a0 NATUAS 0028-0836 Google Scholar
R. W. CurrieF. White,
“Trauma-induced protein in rat tissues: a physiological role for a ‘heat shock’ protein?,”
Science, 214
(4516), 72
–75
(1981). http://dx.doi.org/10.1126/science.7280681 SCIEAS 0036-8075 Google Scholar
E. J. Calabrese,
“Converging concepts: adaptive response, preconditioning, and the Yerkes–Dodson Law are manifestations of hormesis,”
Aging Res. Rev., 7
(1), 8
–20
(2008). http://dx.doi.org/10.1016/j.arr.2007.07.001 ARRGAK 1568-1637 Google Scholar
E. J. Calabrese,
“Hormesis: a revolution in toxicology, risk assessment and medicine,”
EMBO Rep., 5
(Suppl 1), S37
–S40
(2004). http://dx.doi.org/10.1038/sj.embor.7400222 ERMEAX 1469-221X Google Scholar
J. L. Roti Roti,
“Cellular responses to hyperthermia (40–46°C): cell killing and molecular events,”
Int. J. Hyperthermia, 24
(1), 3
–15
(2008). http://dx.doi.org/10.1080/02656730701769841 IJHYEQ 0265-6736 Google Scholar
M. L. Dentonet al.,
“In vitro model that approximates retinal damage threshold trends,”
J. Biomed. Opt., 13
(5), 054014
(2008). http://dx.doi.org/10.1117/1.2981831 JBOPFO 1083-3668 Google Scholar
M. L. Dentonet al.,
“In vitro model reveals a sharp transition between laser damage mechanisms,”
J. Biomed. Opt., 15
(3), 030512
(2010). http://dx.doi.org/10.1117/1.3449107 JBOPFO 1083-3668 Google Scholar
A. Gowdaet al.,
“Heat shock improves recovery and provides protection against global ischemia after hypothermic storage,”
Ann. Thorac. Surg., 66
(6), 1991
–1997
(1998). http://dx.doi.org/10.1016/S0003-4975(98)00905-9 0003-4975 Google Scholar
A. Sahaet al.,
“Raman microspectroscopy of melanosomes: the effect of long term light irradiation,”
J. Biophoton., 4
(11–12), 805
–813
(2011). http://dx.doi.org/10.1002/jbio.v4.11/12 JBOIBX 1864-063X Google Scholar
M. L. Dentonet al.,
“Spatially correlated microthermography maps threshold temperature in laser-induced damage,”
J. Biomed. Opt., 16
(3), 036003
(2011). http://dx.doi.org/10.1117/1.3548881 JBOPFO 1083-3668 Google Scholar
|

