|
|
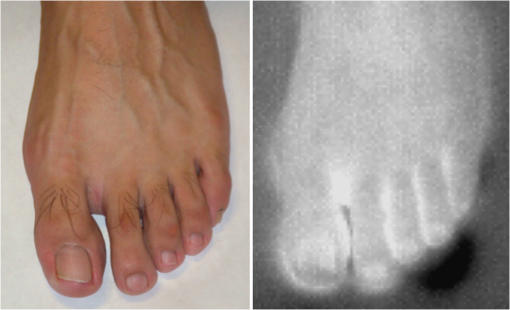
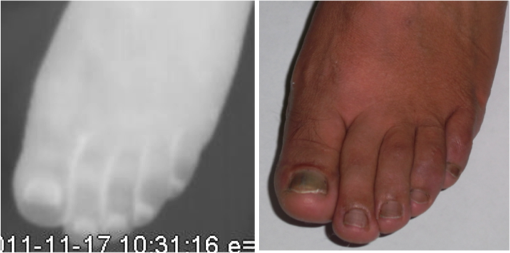
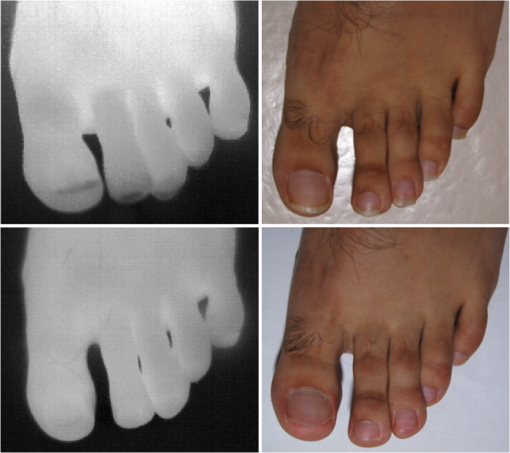
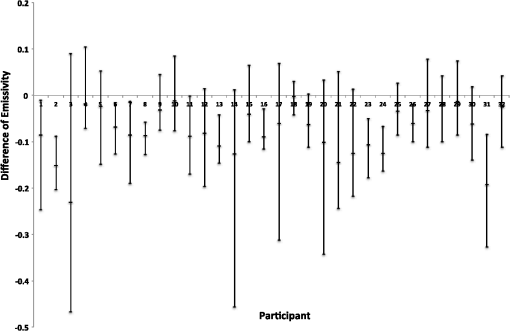
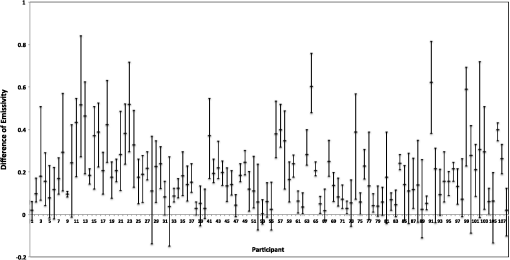
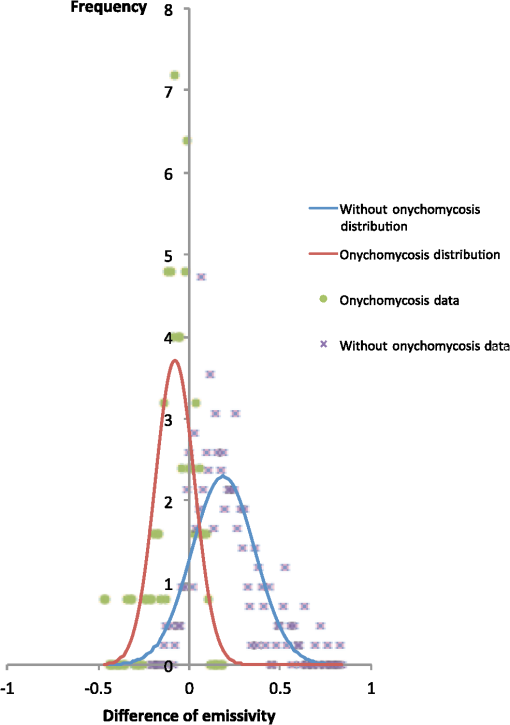
1.IntroductionA nail can be unhealthy due to different reasons, but maybe the best known is fungal infection. It can be present in one or more nails, and it may begin as a white or yellow spot under the tip of a fingernail or toenail.1–4 As the nail fungus spreads deeper into the nail, it may cause nail discoloration and thickening, and development of crumbling edges, an unsightly and potentially painful problem.4–6 Such a condition is commonly called onychomycosis. Onychomycosis is a chronic infection of the nail plate and nail bed caused by dermatophytes, yeasts, and nondermatophyte molds.1,2,7 Onychomycosis accounts for approximately 50% of all nail problems and 30% of all cases of dermatophytosis. However, it can be said that not all patients with dystrophic nails have onychomycosis.4,8–11 Commonly considered as “just a cosmetic problem,” onychomycosis can lead to serious, long-term and permanent damage to the digits.1,2,4,5 The disease is approximately 20 times more common in toenails than in fingernails.1,2,12 Additionally, it is known that patients with psoriasis, diabetes, and immunosuppression are more prone to onychomycosis.12–16 Sports activity and age also increase the risk of onychomycosis.4,9–11 Finally, genetics may be another factor governing the epidemiology of onychomycosis.9,17 Recurrence, either by re-infection or relapse of a previous infection, is seen in up to 50% of cases.18 Smoking and peripheral arterial disease are independent predictors of onychomycosis infection. Gupta et al.10 found a significant relationship between patients with peripheral arterial disease and onychomycosis. Diabetes is another factor that promotes prevalence of onychomycosis. Different studies have shown that diabetic patients with extremity complications are at least twice as likely to suffer from onychomycosis as normal individuals.12,19,20 Additionally, in patients suffering from HIV, it was observed that most of the infections in extremities are associated with onychomycosis.20 There are four major clinical presentations of onychomycosis: distal subungual (on the distal cutting edge of the nail), proximal subungual (just next to the cuticle), superficial (above the nail), and total dystrophic onychomycosis. Distal subungual infection is the most common and is subdivided into distal lateral subungual and subungual onychomycosis. Both of these conditions begin at the distal section of the nail and then spread under the nail, infecting the nail bed.1,2,7,9 Total dystrophic types of onychomycosis infection involve the whole nail and can be considered as a combination of all the types of diseases.9,21 Therefore, a diagnosis of onychomycosis needs to be established to select appropriate therapy1,7,22,23 and to avoid the common problem to prescribe medications for onychomycosis when it is not present in the nail. Normally, diagnosis can be achieved by means of direct microscopy, a fungal culture, and the histological analysis of the nail plate,1,7,8,9,24 and more recently, through the extraction of DNA and the analysis of its sequence.25 Of these, fungal cultures and DNA analysis are the only methods that will identify the genus and species of the causative pathogen, which is necessary for the selection of an antifungal agent.1,7 The clinician should be aware of the possibility of false-negative results, which occur at a rate of approximately 5% to 15%.26 Superficial fungal infections can be easily managed once a proper diagnosis is made.1,27 Care should be taken to correctly identify signs and symptoms of other diseases that clinically mimic onychomycosis. These include psoriasis (the most common such disorder), lichen planus, bacterial infections, contact dermatitis, traumatic onychodystrophies, pachyonychia congenita, nail bed tumors, yellow-nail syndrome (rare), and idiopathic onycholysis.1,28,29 Here, a new diagnostic method based on infrared (IR) images analysis is proposed, with which it is possible to determine when a dystrophic nail suffers from onychomycosis, and which offers advantages such as being a noninvasive, completely innocuous, fast, and being a low cost procedure. This methodology is very quick to implement and it only includes the uncovering of the extremity under study, so that at that moment, a reaffirmation or dissertation can be made about the presence of onychomycosis in a dystrophic nail. The procedure allows for making a visual evaluation on the spot to acquire the image, and then a detailed evaluation will allow diagnostic verification through digital image analysis. However, at the moment, the procedure does not allow one to determine between different dermatophytes, and its use is only recommended to determine if a nail has onychomycosis or not. The first approach to diagnosis can be done visually, since nails showing low energy indicate high probability of the presence of onychomycosis. Later image analysis consists of computing the differences of emissivity between the nail and the surrounding skin on the toe, since correlation has been found between negative differences and the presence of onychomycosis in nails and its grade of affectation. The main advantage of the proposed procedure is the short time that is required for diagnosis. It only takes minutes to confirm whether a dystrophic nail is due to onychomycosis, and even to validate the degree of affectation of the nail. It is known that in many hospitals prevention guidelines are in place to examine patients in search of fungi due to health reasons, because an infected patient may contaminate many others. In order to prevent such problems, a diagnosis of the patient is carried out upon his or her arrival to the hospital, but this kind of diagnosis is time consuming and thus reduces the validity of the prevention culture. The procedure laid out here requires only a few minutes to find out whether a patient has onychomycosis or not, returning to the prevention culture the importance that it must have. 2.TheoryAll bodies emit energy () through radiation.30,31 The type of radiation depends additionally on wavelength, on temperature, and emissivity of the body surface. To compute the emissivity, which is not directly measurable from the body, it is necessary to calculate or to measure the total energy emitted by a blackbody ()30 at the same temperature of the body under study, and then compute the emissivity () using In this case, the body under study is the nail, and to understand how the energy or heat is produced and then radiated, it is necessary to know that nutrients, oxygen, and heat are delivered to the nail by blood in arteries.32 These arteries pass through the lateral nail fold and then run deep into the dermis. The living tissue is fixed to the bone, which contains capillaries and glands that feed and regulate the temperature of the tissue, so the heat is conducted to the superficial epidermis, the layer just beneath the nail bed, which moves forward with the nail plate. Tiny arterial branches, called capillaries, carry blood to the nail bed dermis tissue, resulting in its healthy pink appearance, but these capillaries do not reach into the nail plate. Therefore, the nail plate receives no blood and its radiated energy corresponds to transmitted heat from the nail bed by means of conduction (remember that the nail plate is formed by dead cells).33–35 As radiated energy from the nail depends on temperature and emissivity, problems such as vascular disease can be the most important variable affecting this emission, since, this disease affects directly the temperature of the nail, and the temperature of all the extremity and of course, its energies. This causes the energy received in the detector to be low, as compared to energy emitted by a nail with normal vasculature. A dystrophic nail may present altered temperature due to different reasons, such as a separation of the nail bed from the nail plate, which produces bad heat conduction from one to the other. In consequence, the nail is only heated through conducted heat from the cuticle, the eponychum, and the lateral fold. This condition produces lower radiated energy than that of normal nails. The same thing happens in the free margin or cutting edge of the nail, since capillaries and tissues are not directly in contact with this part of the nail. Therefore, distal edge temperature is not directly controlled by and is lower than that of the nail plate. A thick nail is another example of low temperature because the heat is poorly conducted through it and then it presents low energy. In addition to temperature alterations, emissivity changes may appear in the nail. It occurs when a dystrophic nail suffers from the presence of some conditions such as fungi, which alter its radiant emission. Skin and nails also have different emissivity values, and they can be computed to determine some types of dystrophies. To compute the emissivities of the nail and surrounding skin, it is possible to take advantage of Eq. (1) once the corresponding energies have been computed. Here, we propose to use an IR camera in the middle range as a detector. This provides a wide spectral range of detection (commonly from 7 to 14 μm), and allows computing both energies, nail and blackbody, using digital image techniques. Those images need to be acquired using the same parameters in the camera, which are emissivity adjustments, temperature ranges, and all the parameters involved in the IR camera used, such as humidity, room temperature, distance from the camera to the body, temperature correction, and others. Additionally, the blackbody temperature needs to be set at the same temperature as the nail to ensure that the emissivity has been correctly computed in the end. To compute the energy of the nail, skin, and blackbody through digital IR images, Michel’s equation, that comes from the general equation to compute energy in a continuum range, may be used.36,37 To do this, it is necessary to use a program that can read the images, allow the selection of a specific area, and show the corresponding pixel gray levels in that area. With the gray levels that directly correspond to the intensity level received for the detector at the moment of its acquisition, it is possible to compute the energy of that area () using Here, and correspond to the axes on the image, and are the limits of these axes’ area, and is the gray level in the position on the selected area. Once both energies have been computed, Eq. (1) can be applied and emissivity obtained.For the procedure proposed above, it was necessary to compute differences of emissivity between nail and skin of the toe. To quantify the damage of the nail, it was carried out through the operation emissivity of the nail minus emissivity of the skin of the toe using 3.MethodTo conduct this study, 141 people between 30 and 75 years old were recruited, of which 65 were women and 76 were men. 58 were diabetic type II. All of them were randomly invited to participate in the study, and once the institutional ethical committee approval was achieved, the participants signed the informed consent form. The inclusion criteria for the participant was that, on the day the images were acquired, the participant had clean feet without having used any talc powder, body lotion, petrolatum, or any other cleaning or cosmetic product (such as nail polish), and that they had not used antifungal products in the four weeks prior to their appointment. 3.1.Acquisition of the IR ImagesThe patient was met at the laboratory and asked to keep his or her feet uncovered for 20 min to avoid the influence of kind of shoes and conditions of arrival, so that they adapted to the environmental conditions of the place. An effort was made to keep room temperature at . Once the participant had adapted, he or she was asked to sit straight on the spot where the cameras had been placed for image acquisition. Images were taken of both naked feet placed on a plastic template (acrylic piece) to avoid direct contact with the floor in order to prevent excessive heat loss to the floor. To document the procedure, a digital picture of both feet was taken using a Samsung ST65 digital camera (Samsung Electronics Co., Seoul, South Korea) For the acquisition of the IR images, two medium-range IR cameras were used: a FLIR E45 camera (FLIR Systems, Inc., Wilsonville, OR), and an SDS Infrared Hotfind LX camera (SDS Infrared Ltd., Guangzhou, China). The two cameras were used to comparatively validate the results of the study. For the FLIR camera, the temperature range was 18 to 38°C, with a reflected room temperature of 20°C. For the Hotfind camera, the temperature range was 18 to 38°C, sphere temperature from to 250°C, level 28°C and spam 20°C, and a temperature correction of 0°C. Room temperature for both cameras was 20°C, with a humidity of 60% (it was the average in the laboratory).Emissivity was 0.95 for both (since that was the emissivity of the blackbody used as a reference).Both cameras were set as eight-bit grayscale where the dark levels represent the lower energies. Regarding the focus for the cameras, it was adjusted manually to achieve a sharp and well-focused image at a distance of 60 cm from the participant’s foot. This distance was determined in such a way that the acquired imaged allowed the visualization of all toes and toenails, and they occupied most of the image. Once all the images had been taken (IR and conventional), temperature of the nails, fingertips, and phalanges (on the upper side, next to the nail, and the instep) was taken using a calibrated Fluke 54II digital thermometer (Fluke Co., Everett, WA), thus setting the basis for calculation of the skin and nail emissivities. In order to calculate the emissivities, the blackbody had to be set at the same temperature. Therefore, it was necessary to consider that the thermocouple has a stabilization time of around 1 min, and it was necessary that all nails and skin of toes wait this time. For the calculation of emissivities, images of an OMEGA BB701 (OMEGA Engineering Inc., Stamford, Connecticut) blackbody were acquired using the same two IR cameras, which was at the same temperature of the body part under study (nail or skin), at the same distance, and using the same adjustments with which the participant images had been taken. 3.2.Procedure of Microbiological Analysis LaboratoryAfter having acquired the IR images, digital pictures, and temperatures, a nail biopsy was taken following the conventional procedure of any microbiological analysis lab. The nail that showed clinical signs of onychomycosis was chosen, lightly disinfected with alcohol on gauze and then scraped from center to edge, crossing the lesion margin, using a sterile scalpel blade. The sample taken was then deposited into a Petri dish to be later used for a culture and analyzed by direct microscopy. It is important to first take the images, then immediately measure the temperature with the contact thermometer, and finally do the nail scraping, so that the emission conditions of the nail are not altered by the procedure. To validate the methodology proposed here, the sample was inspected under a microscope to search for the presence of hyphae, which are characteristic shapes of dermatophytes at advanced stages that can be seen under a microscope. Once it was documented whether the sample had hyphae or not, a traditional fungal culture of the sample was made, which on average takes 4 weeks. This culture was carried out by two clinical analysis laboratories in order to reduce the percentage of false negative results. Once the study protocol was finished for all participants, images were processed and analyzed, the participant and blackbody image energies had been calculated, and emissivities and their differences were obtained. 4.ResultsIt was found that the use of IR procedures to diagnose onychomycosis allows evaluating the condition of a nail in two ways. First, it has been found that nails that suffer from onychomycosis emit less energy than nails that are free of it, and as the ailment progresses, this energy emission decreases even more. Consequently, if the detector is used to measure the energy in an IR camera, such a decrease in energy will be seen in an image.The computed emissivity differences also allow a quantitative evaluation of the damage caused by onychomycosis. For the configuration of the IR cameras used in this study, it was chosen that the lowest energy levels be represented by lower gray levels and the darker areas in the image correspond to zones having less energy (the used scale was 0 to 255 gray levels, thus, level 255 corresponds to maximum energy). An example of this is shown in Fig. 1. In such an image, feet of six different participants having nails with Trichophyton rubrum are presented as nails with dark zones. It was observed that such darkness on the nail is independent of foot vascularity. Since it is assumed that darker feet have less blood flow than those showing higher brightness, those dark feet correspond to diabetic people with peripheral artery disease (two upper right images). Fig. 1Infrared (IR) images of nails with onychomycosis (from top to bottom and left to right): the first five correspond to diabetic participants and the last is not a diabetic participant. 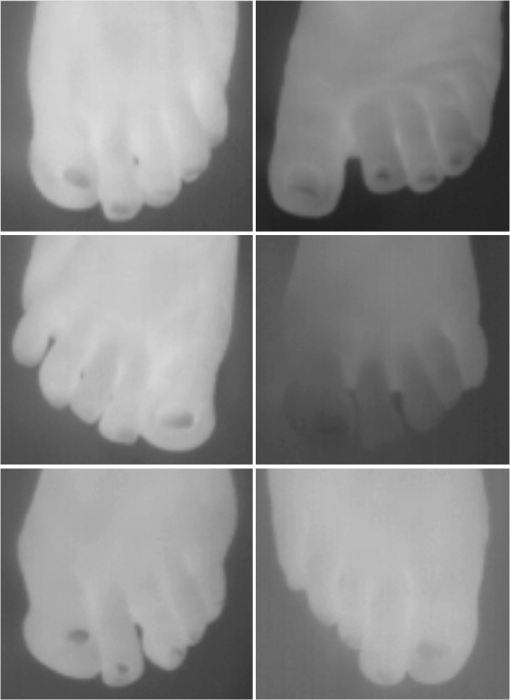 A quick qualitative evaluation of the nail condition can be attained with the naked eye. A nail or a portion of it that appears to be darker than the corresponding toe or than other areas of the nail itself, evidences a high probability of suffering from onychomycosis. An example of this is shown in Fig. 2, in which the photographs show the evidence of a dystrophic nail in the hallux (big toe) of both feet, and low energy is observed in the IR images. This is indicative of the presence of onychomycosis, which was corroborated by means of positive fungal culture developed in both toes for T. rubrum. Fig. 2Onychomycosis is in the hallux. Upper images are the IR images and the bottom ones are corresponding photographs for a diabetic participant. 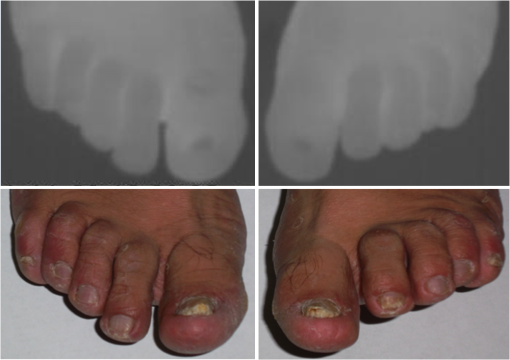 An example of feet of some participants that had nails without onychomycosis is shown in Fig. 3. In these IR images, it is possible to see nails with the same or more radiant energy than the skin of toes, which were found to be an indication of the absence of onychomycosis. This higher energy emitted for the nail, in spite of its lower temperature than skin, is due to the higher emissivity of the nail. Again, the vascularity of the feet (directly responsible for the energy of the foot) does not affect the evaluation because the presence of onychomycosis can be evaluated comparatively with other sections of the same limb of the participant and darkening of the nail is compared to the same toe of the participant, thus some toes may be less energetic than others, but this does not affect the evaluation, as is the case in the left central image of a diabetic participant that is shown in Fig. 3. Fig. 3IR images of nails without onychomycosis (from top to bottom and left to right), images one and five correspond to nondiabetic participants, three is a diabetic participant with peripheral artery disease, and the right column corresponds to diabetic participants without onychomycosis. 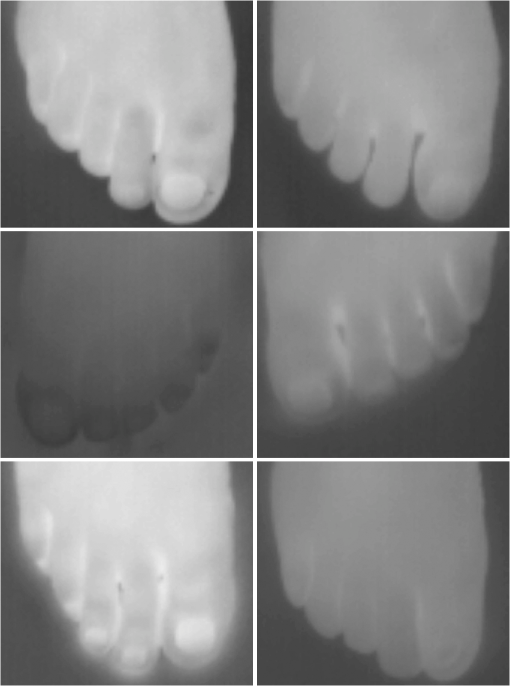 Healthy nails of diabetic and nondiabetic participants without any visible dystrophies, were always found to be more energetic than the surrounding skin. An example of this is shown in Fig. 4, in which the nails emit more energy than the toes. Some cases may present a nail that looks dystrophic to the naked eye, but when it is analyzed using IR images, it appears as a nail without onychomycosis.An example of this is shown in Fig. 5. All the cases considered in this study were corroborated through fungal cultures and direct microscopy. It was found that long nails may generate confusion when making the diagnosis, since the end of the nail’s edge presents a lower temperature than the nail plate, and in consequence, it emits lower energy. It is important that the nail be short to make a correct diagnosis. An example of this case is shown in Fig. 6, in which is presented a case where a participant arrived with long nails, but without any dystrophy. He was invited to cut his nails and after having waited around 20 min, he was re-examined. Visual assessment of onychomycosis through IR images may be affected by the subjectivity and ability of the evaluator at the time of assessing the case. To avoid this, it is recommended that additional to the first visual assessment through IR images, the energies and emissivities of the nail and the adjacent skin are also evaluated. Since emissivity is dependent on temperature which normally varies between individuals and between fingers or toes, the emissivity of the nail is directly affected. High variation was found to exist in toe and toenail temperature from person to person. The average toes’ temperature of the participants was found to be with a range from 22.0 to 36.3°C. For participants without diabetes, it was , and for diabetic participants, it was . Toenail temperature average was with a range from 21.7 to 34.1°C in nondiabetic participants and in diabetic people it was . Due to this, high variation on temperature was not possible to find a correlation between onychomycosis and nail emissivity. Thus, in order to make a good diagnosis, it was decided to calculate the differences in emissivity between the nail and the skin right next to it in the same patient, and that this difference in emissivity would be used as an indicator of the presence or absence of onychomycosis. This threw good correlation. Considering the difference in emissivity between nail and toe in the same individual (nail emissivity minus toe emissivity), a good correlation was found between these differences and the presence of onychomycosis. The graphs shown in Figs. 7 and 8 present these computed differences in emissivity for each foot. The first graph (Fig. 7) corresponds to the presence of onychomycosis, and then to the absence of it (Fig. 8) among the group of patients who took part in the study. Having analyzed the experimental data, an adjustment was carried out to have two distribution curves, and it was found that it is possible to statistically distinguish the two groups of participants. That is, two data distributions that include (separately) participants with onychomycosis and those who do not suffer from it were obtained, and it was corroborated that the participants with onychomycosis were correctly grouped with those diagnosed with direct microscopy and positive culture. That is, those participants with onychomycosis were classified in the relevant group. These two distribution curves are shown in Fig. 9. An analysis of temperature showed that there is sufficient statistical evidence to support that means of nails’ temperatures of participants with onychomycosis and without it are different. A statistical test was developed to determine if the mean of nail temperature in participants with onychomycosis was lower than the mean of nail temperature in participants without onychomycosis. The statistical test produced sufficient evidence to asseverate that temperature in a nail with onychomycosis is lower than the temperature of a nail without onychomycosis (with a value of 0.015). To develop this test, it was considered that the mean temperature of nails with onychomycosis was 25.59°C with a standard deviation of 2.12, while the mean temperature of nails without onychomycosis was 27.64°C with a standard deviation of 2.42. Additionally, it was verified that there was a difference between the mean temperature of toenails and of toes in the participants, because the emissivity differences are computed from these two parts. It was found that in participants with onychomycosis did not have statistical difference between means of nail temperature and of toe temperature (mean temperature of toe was 25.95°C with standard deviation of 2.26), and in participants without onychomycosis, enough evidence was found to support that a nail without onychomycosis has a lower temperature than toe temperature (mean temperature of toe was 29.09°C with standard deviation of 3.16). This also supported the asseveration that a nail with onychomycosis has a lower temperature than one without it. Additionally, it was found that nail temperature is lower than the temperature at distal part of the toes, but statistically equal to that of the tips of the toes (), and that in the case of healthy nails, the increment in the energy of the nail with respect to the toe observed in the IR images is due to a higher emissivity. The energy of healthy nail was found higher than the energy of toe skin, even when skin temperature is higher than nail temperature. This is indicative that the emissivity of the nail is higher than the emissivity of the skin. A nail without onychomycosis has a lower temperature than toe skin, but has a higher emission of energy. This high emission is due to higher emissivity of the nail. However, when the nail is affected by onychomycosis, two processes are presented; a diminishing in the emissivity of the nail, and also a diminishing in the temperature of the nail, both of which contribute to a decrease the energy of the nail. 5.DiscussionApplying the suggested diagnostic method, some considerations have to be taken into account to reduce the probability of false positive cases. For example, in the cases where there are clinically damaged nails (dystrophic nail) which, due to blood circulation problems, also look darker (less energized) to the naked eye, it is advisable to obtain the difference in emissivities between the nail and the tip of the toes, since if there is peripheral arterial disease, the energy of the toes is diminished by the decrease in temperature, and the same happens to the temperature of the nail. This causes a confusion to the naked eye, but not to the difference of emissivities. Another situation to consider is detachment of the nails not caused by onychomycosis. A first visual examination has to be done to discard this situation. False positives in our test will be those cases where the calculated difference in emissivities turns out to be negative, indicating the presence of onychomycosis when there is actually no such thing. This may be due to a detachment of the nail plate because of some other reason, or to a different case of nail dystrophy. The mistake can be fully made if a nail anomaly is detected visually, which might contribute to a false positive. However, if the nail looks fine to the naked eye, the presence of onychomycosis can be then discarded. The false negative cases will be those in which there is onychomycosis, and that neither in the IR images nor in the calculation of differences in emissivity can one detect the presence of onychomycosis. However, in the present work, it was not possible to make such cases evident. A good diagnosis of onychomycosis through the method suggested in this work must include the following considerations: the nail is to be free of any kind of ointment, body lotion, make-up, or any other substance that might modify the nail emissivity in a superficial manner, so that if a decrease in emissivity is detected, it can be linked to onychomycosis. Also, the nail must be well trimmed, and there should be no detachment of any kind from the skin. If there were energy reduction in a nail that is too long or somehow detached, the decrease might be attributed to a decrease in nail temperature, and not to a change in emissivity caused by onychomycosis. Like in other diagnoses, it is suggested to carry out the two tests for onychomycosis, direct microscopy and culture of a sample of the nail, to make sure whether it is present or not. Here, it is suggested to carry out, in addition to IR analysis, a complementary test to determine the type of dermatophyte present in the nail. All positive cases of onychomycosis considered here were corroborated by positive fungal culture, but unfortunately all presented T. rubrum as dermatophyte in the nail. Results shows that IR images’ evaluation is a good tool to diagnose onychomycosis. However, more research is needed to evaluate the possibility of using the same technology for discrimination of different kind of fungus affecting a nail. 6.ConclusionsDystrophic nails of the participants, which were corroborated with positive fungal culture and/or hyphae by direct microscopy to suffer onychomycosis showed low energy emission. This energy decrease is mostly due to a diminution on the nail emissivity. It was found that nails with lower energy than adjacent skin have a very high possibility of suffering from onychomycosis, and nails with higher energy than adjacent skin have a very low possibility of suffer onychomycosis in people with diabetes and without it. Darker areas on the nail are indicative of onychomycosis. It can be seen with a naked eye on the IR image and can be corroborated through calculating the differences of emissivity through the IR image. The procedure described herein offers an advantage that is hard to match by the methods currently in use for the diagnosis of the ailment, since it is a completely noninvasive method, leading to a higher prevention of contagion, to the avoidance of the susceptibility of a culture and of its contamination, and to a much faster response time and a relatively low cost. In this work, it was correlated to the use of IR techniques in the diagnosis of onychomycosis, which is most frequently caused by dermophyte fungi. However, there are other factors whose behavior is still to be studied, and which also damage nails and correspond to the other percentages that were left out of this work, such as yeasts, which are known to have different epidemiologies and etiologies. ReferencesB. E. Elewski,
“Onychomycosis: pathogenesis, diagnosis, and management,”
Clin. Microbiol. Rev., 11
(3), 415
–429
(1998). CMIREX 0893-8512 Google Scholar
B. E. Elewski,
“Clinical pearl: diagnosis of onychomycosis,”
J. Am. Acad. Dermatol., 32
(3), 500
–501
(1995). http://dx.doi.org/10.1016/0190-9622(95)90075-6 JAADDB 0190-9622 Google Scholar
R. Derbyet al.,
“Novel treatment of onychomycosis using over-the-counter mentholated ointment: a clinical case series,”
J. Am. Board Fam. Med., 24
(1), 69
–74
(2011). http://dx.doi.org/10.3122/jabfm.2011.01.100124 1557-2625 Google Scholar
A. K. Guptaet al.,
“Prevalence and epidemiology of onychomycosis in patients visiting physicians offices: a multicenter Canadian survey of 15,000 patients,”
J. Am. Acad. Dermatol., 43
(2), 244
–248
(2000). http://dx.doi.org/10.1067/mjd.2000.104794 JAADDB 0190-9622 Google Scholar
B. E. Elewski,
“The effect of toenail onychomycosis on patient quality of life,”
Int. J. Dermatol., 36
(10), 754
–756
(1997). http://dx.doi.org/10.1046/j.1365-4362.1997.00163.x IJDEBB 0011-9059 Google Scholar
B. SigurgeirssonO. Steingrímsson,
“Risk factors associated with onychomycosis,”
J. Eur. Acad. Dermatol. Venereol., 18
(1), 48
–51
(2004). http://dx.doi.org/10.1111/jdv.2004.18.issue-1 JEAVEQ 0926-9959 Google Scholar
B. E. ElewskiR. J. Hay,
“Update on the management of onychomycosis: highlights of the third annual international summit on cutaneous antifungal therapy,”
Clin. Infect. Dis., 23
(2), 305
–313
(1996). http://dx.doi.org/10.1093/clinids/23.2.305 CIDIEL 1058-4838 Google Scholar
R. C. SummerbellJ. KaneS. Krajden,
“Onychomycosis, tinea pedis and tinea manuum caused by non-dermatophytic filamentous fungi,”
Mycoses, 32
(12), 609
–619
(1989). http://dx.doi.org/10.1111/j.1439-0507.1989.tb02192.x MYCSEU 1439-0507 Google Scholar
J. FaergemannR. Baran,
“Epidemiology, clinical presentation and diagnosis of onychomycosis,”
Br. J. Dermatol., 149
(s65), 1
–4
(2003). http://dx.doi.org/10.1046/j.1365-2133.149.s65.4.x BJDEAZ 1365-2133 Google Scholar
A. K. Guptaet al.,
“The epidemiology of onychomycosis: possible role of smoking and peripheral arterial disease,”
J. Eur. Acad. Dermatol. Venereol., 14
(6), 466
–449
(2000). http://dx.doi.org/10.1046/j.1468-3083.2000.00124.x JEAVEQ 0926-9959 Google Scholar
A. Iglesiaset al.,
“Prevalence and nature of nail alterations in pediatric patients,”
Pediatr. Dermatol., 18
(2), 107
–109
(2001). http://dx.doi.org/10.1046/j.1525-1470.2001.018002107.x 0736-8046 Google Scholar
A. K. Guptaet al.,
“Prevalence and epidemiology of toenail onychomycosis in diabetic subjects: a multicentre survey,”
Br. J. Dermatol., 139
(4), 665
–671
(1998). http://dx.doi.org/10.1046/j.1365-2133.1998.02464.x BJDEAZ 1365-2133 Google Scholar
A. K. Guptaet al.,
“A higher prevalence of onychomycosis in psoriatics compared with non-psoriatics: a multicentre study,”
Br. J. Dermatol., 136
(5), 786
–789
(1997). http://dx.doi.org/10.1111/j.1365-2133.1997.tb03673.x BJDEAZ 1365-2133 Google Scholar
N. Gregory,
“Special patient populations: onychomycosis in the HIV-positive patient,”
J. Am. Acad. Dermatol., 35
(3:2), S13
–S16
(1996). http://dx.doi.org/10.1016/S0190-9622(96)90064-X JAADDB 0190-9622 Google Scholar
G. Pierard,
“Onychomycosis and other superficial fungal infections of the foot in the elderly: a pan-European survey,”
Dermatology, 202
(3), 220
–224
(2001). http://dx.doi.org/10.1159/000051640 DERMEI 0742-3217 Google Scholar
R. Caputoet al.,
“Prevalence of superficial fungal infections among sports-active individuals: results from the Achilles survey, a review of the literature,”
J. Eur. Acad. Dermatol. Venereol., 15
(4), 312
–316
(2001). JEAVEQ 0926-9959 Google Scholar
N. Zaiaset al.,
“Autosomal dominant pattern of distal subungual onychomycosis caused by Trichophyton rubrum,”
J. Am. Acad. Dermat., 34
(2:1), 302
–304
(1996). http://dx.doi.org/10.1016/S0190-9622(96)80142-3 JAADDB 0190-9622 Google Scholar
B. Sigurgeirssonet al.,
“Long-term effectiveness of treatment with terbinafine vs itraconazole in onychomycosis: a 5-year blinded prospective follow-up study,”
Arch. Dermatol., 138
(3), 353
–357
(2002). http://dx.doi.org/10.1001/archderm.138.3.353 ARDEAC 0003-987X Google Scholar
D. M. Saunteet al.,
“Prevalence of toe nail onychomycosis in diabetic patients,”
Acta Derm.-Venereol., 86
(5), 425
–428
(2006). http://dx.doi.org/10.2340/00015555-0113 ADVEA4 0001-5555 Google Scholar
D. Dompmartinet al.,
“Onychomycosis and AIDS. Clinical and laboratory findings in 62 patients,”
Int. J. Dermatol., 29
(5), 337
–339
(1990). http://dx.doi.org/10.1111/ijd.1990.29.issue-5 IJDEBB 0011-9059 Google Scholar
I. WeitzmanR. C. Summerbell,
“The dermatophytes,”
Clin. Microbiol. Rev., 8
(2), 240
–259
(1995). CMIREX 0893-8512 Google Scholar
T. K. Harrellet al.,
“Onychomycosis: improved cure rates with itraconazole and terbinafine,”
J. Am. Board Fam. Pract., 13
(4), 268
–273
(2000). http://dx.doi.org/10.3122/15572625-13-4-268 0893-8652 Google Scholar
R. K. Scheret al.,
“Onychomycosis: diagnosis and definition of cure,”
J. Am. Acad. Dermatol., 56
(6), 939
–944
(2007). http://dx.doi.org/10.1016/j.jaad.2006.12.019 JAADDB 0190-9622 Google Scholar
J. Leibner-Ciszaket al.,
“Evaluation of a PCR melting profile method for intraspecies differentiation of Trichophyton rubrum and Trichophyton interdigitale,”
J. Med. Microbiol., 59
(2), 185
–192
(2010). http://dx.doi.org/10.1099/jmm.0.013458-0 JMMIAV 0022-2615 Google Scholar
A. DobrowolskaJ. DębskaP. Stączek,
“Molecular identification of T. rubrum and T. mentagrophytes by PCR-RFLP targeting of the DNA chitin synthase 1 gene,”
Mikol. Lek., 15
(4), 193
–196
(2008). Google Scholar
B. E. Elewski,
“Large-scale epidemiological study of the causal agents of onychomycosis: mycological findings from the multicenter onychomycosis study of terbinafine,”
Arch. Dermatol., 133
(10), 1317
–1318
(1997). http://dx.doi.org/10.1001/archderm.1997.03890460143029 ARDEAC 0003-987X Google Scholar
R. T. BrodellB. E. Elewski,
“Superficial fungal infections: errors to avoid in diagnosis and treatment,”
Postgrad. Med., 101
(4), 279
–287
(1997). http://dx.doi.org/10.3810/pgm.1997.04.209 POMDAS 0032-5481 Google Scholar
P. RichR. K. Scher, An Atlas of Diseases of the Nail, 9
–45 The Parthenon Publishing Group Inc., New York
(2005). Google Scholar
J. C. SzepietowskiA. Reich,
“Onychomycosis and quality of life,”
Int. Acad. Cosmet. Dermatol., 4
(1), 85
–87
(2009). Google Scholar
W. L. Wolfe,
“Radiation Theory,”
The Infrared Handbook, 132
–176 ERIM, Ann Arbor, MI
(1978). Google Scholar
R. W. Boyd, Radiometry and the Detection of Optical Radiation, John Wiley & Sons, New York
(1983). Google Scholar
D. D. Schoon, Nail Structure and Product Chemistry, 61
–77 2nd ed.Thomson Delmar Learning(2005). Google Scholar
J. E. Crouch, Functional Human Anatomy, 4th ed.Lea & Febiger(1985). Google Scholar
B. Parasharet al.,
“Natural therapy of fungal nail disease: review,”
Pharma Innovation, 1
(4), 46
–60
(2012). PIHNBQ 2277-7695 Google Scholar
H. Feneis, Pocket Atlas of Human Anatomy, 392
–393 5th ed.Thieme(2007). Google Scholar
R. C. GonzalezR. E. Woods, Digital Image Processing, Addison Wesley, Reading, MA
(1992). Google Scholar
J. TangE. PeliS. Acton,
“Image enhancement using a contrast measure in the compressed domain,”
IEEE Signal Process. Lett., 10
(10), 289
–292
(2003). http://dx.doi.org/10.1109/LSP.2003.817178 IESPEJ 1070-9908 Google Scholar
|