|
|
1.IntroductionStem cell therapy has been proposed as a treatment for brain damage, spinal cord injuries, cancer, cardiovascular disease, and other conditions.1–4 In fact, intravenous (i.v.) stem cell delivery for treatment has been increasingly used in animal models and humans.5,6 One challenge associated with the therapeutic effects of stem cells is the lack of reliable in vivo imaging methods to evaluate the biology of transplanted stem cells.7 An appropriate in vivo live imaging method is important to understand basic stem cell biology, including cell survival, migration, and differentiation after transplantation.1 Recently, nanotechnologies have contributed to advances in high resolution in vivo imaging for stem cell tracking.8–10 Magnetic nanoparticle- or radionuclide-labeled stem cells can be visualized by magnetic resonance imaging (MRI) or by positron emission tomography (PET). MRI imaging provides high spatial resolution and anatomical information but has limited sensitivity.11,12 PET imaging has high sensitivity but low spatial resolution, does not provide anatomical data, and the radioisotopes have a short half-life. 8,12 Fluorescence imaging (FI) with nanoparticles is another noninvasive imaging method for in vivo tracking. FI has advantages in high sensitivity and resolution at the subcellular level with microscopy, but has a limited penetration depth through tissues.12 Near-infrared fluorescence imaging (NIRFI) has improved penetration depth and provides more specific signals, but has the limitation of photobleaching.12,13 To overcome the limitations of each single method, multimodal imaging methods have been developed.14–16 In the present study, we monitored the human adipose-derived stem cells (hASCs) in Tg2576, an Alzheimer’s disease (AD) mouse model, using a multimodal MRI nanoparticle with enhanced NIR fluorescence.12 hASCs were labeled with fluorescent magnetic nanoparticles (LEO-LIVE™-Magnoxide 675 or 797) and administered by tail–vein injection. Since blood–brain barrier (BBB) leakage in AD brains has been reported,17,18 it was expected that intravenously injected hASCs could pass through the BBB. We checked the cellular internalization of nanoparticles into hASCs in vitro and performed sequential in vivo imaging at 0, 1, 3, and 10 days after injection using Maestro Imaging System. As a result, we could follow fluorescence signals up to 10 days after injection in live animals and found strong signals in the brains extracted from hASC transplanted Tg2576 mice up to 12 days after injection. 2.Methodology2.1.Cell PreparationhASCs were prepared under GMP conditions in the Stem Cell Research Center of RNL BIO (Seoul, Korea), with approval from the Institutional Review Board of ASAN Medical Center. All hASCs were isolated from human adipose tissues obtained from disposed lower abdomen by agreement and primarily cultured as previously described.19 hASCs were stained with fluorescent magnetic NEO-LIVE™-Magnoxide 675 or 797 (BITERIALS, Korea) nanoparticles at for in vitro assays or i.v. injection. All procedures were performed according to the manufacturer’s instructions. hASCs were incubated in growth medium containing Magnoxide for 24 h and washed with phosphate-buffered saline (PBS). 2.2.AnimalsAll animal procedures were performed according to the National Institutes of Health Guidelines for the Humane Treatment of Animals, with approval from the Institutional Animal Care and Use Committee of Seoul National University (IACUC No. SNU-091208-1). Tg2576 mice, which express mutant human APP, Swedish (K670N/M671L) mutation, were obtained from Taconic Farms (Germantown, New York) and were bred by mating male mice with C57B16/SJL F1 females as described previously.20 Mouse genotyping was performed as recommended by Taconic Farms. Eleven-month-old male Tg2576 mice or age-matched WT mice were injected with 150 μl of hASCs suspension through the tail vein. 2.3.ImmunocytochemistryhASCs labeled with Magnoxide 675 were fixed with 4% paraformaldehyde in PBS for 10 min at room temperature (RT), permeabilized with 0.2% Triton X-100 in PBS for 5 min, and blocked with 5.0% normal goat serum (Vector Laboratories, California) in PBS for 1 h at RT. They were then incubated with anti-human nuclei antibody (, Millipore, Massachusetts) for 1 h at RT, washed with PBS three times and incubated with secondary antibody, Alexa Fluor® 488 Anti-Mouse IgG (, Invitrogen, California) and 4’,6-diamidino-2-phenylindole (DAPI) for 1 h at RT. After washing with PBS three times, the coverslips were mounted and observed under a Zeiss LSM 510 confocal imaging system (Zeiss, Heidelberg, Germany). 2.4.In vivo fluorescence imagingFluorescence images were obtained using a Maestro Imaging System (CRI Inc., Woburn, Massachusetts) for data acquisition and analysis. For effective detection, fur on the dorsal and ventral sides of the mice was removed 1 day prior to imaging. The mice were anesthetized and hASCs labeled with NEO-LIVE™-Magnoxide 675 or 797 were injected through the tail–vein. The first in vivo images were captured at 5 min after the injections. With the mice under anesthesia, sequential in vivo fluorescence measurements were taken at 1, 3, and 10 days postinjection using the Maestro equipment. To examine the distribution of the transplanted cells, all organs were extracted at 5 or 12 days after injection and images were captured. 3.Results and DiscussionThe effective cellular internalization of nanoparticles into hASCs was observed by confocal microscopy as shown in Fig. 1. Cells were incubated with Magnoxide 675 in culture medium for 24 h and stained with anti-human nuclei antibody to detect nuclei of human cells. Magnoxide 675 was internalized into the cells and mainly resided in the cytoplasm. In the previous report, Park et al.21 showed that internalized nanoparticles are stable in the cells and are cytocompatible. We also confirmed that there were no cytotoxicity and no change on the overall growth rate or morphology of hASCs (Fig. 1). Fig. 1(a) Confocal microscope images of human adipose-derived stem cells (hASCs) labeled with NEO-LIVE™-Magnoxide 675 (purple). Cells were stained with anti-human nuclei (green) and DAPI (blue). Scale bar, 50 μm (b) Magnified images of the red squares in the left panels. Scale bar, 20 μm. 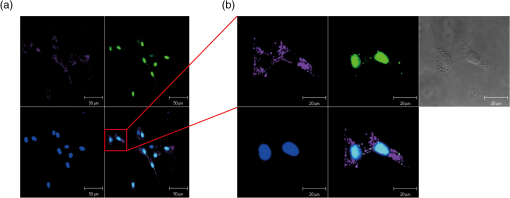 For in vivo cell tracking, cells were labeled with Magnoxide 797 1 day before injection. Labeling of cells was confirmed using Maestro Image System before the tail–vein injection [Fig. 2(a)]. In vivo fluorescence images were captured according to the experimental scheme in Fig. 2(b). The first images, which were obtained 5 min after injection, showed almost no fluorescence signal [Fig. 2(c), left panel]. One day after injection, hASCs were detected in the tail, body, and brain especially in the Tg2576 mouse (Tg-hASC). Since the AD brain shows leakage of the BBB, it is likely that hASCs could pass through the BBB and could be detected in the brain. Otherwise, no signal was observed in WT-hASC mice brain [Fig. 2(c), second panel]. At 3 days after injection, the fluorescence signals from hASCs in the brains were higher than those on any other days. To exclude autofluorescence signals, we took images of Tg-hASC mice together with WT-Sham (injected with saline) mice in the same field. Although the fluorescence signals decayed as time passed, we found weak signals at 10 days after injection. No signal was observed at 17 and 30 days after injection (data not shown). Fig. 2(a) Labeled cells with NEO-LIVE™-Magnoxide 797 in EP tube were visualized using Maestro Imaging System. (b) Experimental scheme of image measurements. (c) Sequential in vivo tracking was performed in mice injected with NEO-LIVE™-Magnoxide 797-labeled hASCs. Fluorescent images were taken at the indicated times. WT/Tg-hASC: injected with nanolabeled hASCs, WT-Sham: injected with saline. 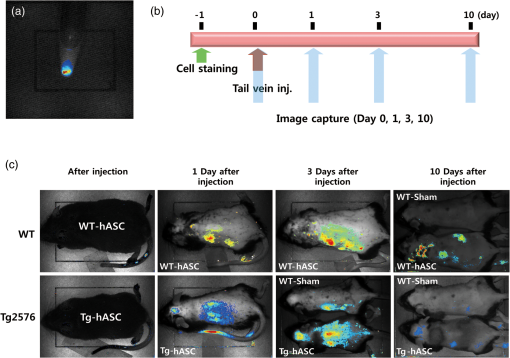 To examine the organ distribution of the transplanted cells, organs were extracted at 5 or 12 days after injection and then visualized using Maestro Image System. These mice were injected with saline or stem cells labeled with NEO-LIVE™-Magnoxide 675. Compared with WT-Sham or WT-hASC mice, Tg2576 mice showed high fluorescence signals in all organs including brain, gastrointestinal (GI) tract, kidney, liver, and bladder. Because the GI tract showed too strong signal to analyze other organs, we excluded it from the result [Fig. 3(a)]. Tg-hASC-2, which was sacrificed at 5 days after injection, showed much higher signals than Tg-hASC-1, sacrificed at 12 days after injection [Fig. 3(a)]. Additionally, we compared all signals from the brains in the same field and found strong signals in the Tg-hASC mice brains [Fig. 3(b)]. Fig. 3(a) Mice were injected with saline, NEO-LIVE™-Magnoxide 675-labeled hASCs or nanoparticle only. Each organ was extracted at 5 or 12 days after injection (only Tg-hASC-2 was sacrificed at 5 days after injection) and images were captured using Maestro Imaging System. (b) Brain images were captured separately in the same field. WT-Sham: injected with saline, Tg-Nano: injected with nanoparticles, and WT/Tg-hASC: injected with nanolabeled hASCs. 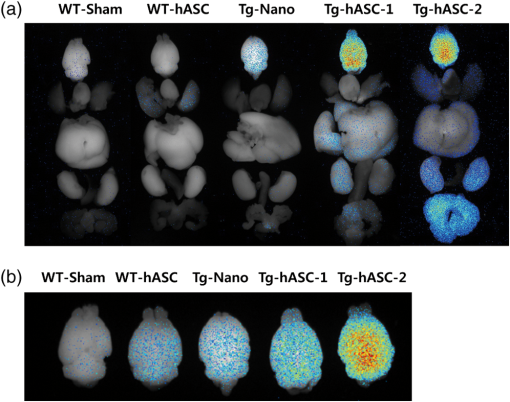 There were weak signals in the brains in the WT-ASC or Tg-Nano mice brain. According to recent report which showed transmigration of the mesenchymal stem cells across the BBB,22 it is likely that some cells are able to enter the WT mice brains. However, labeled hASCs were shown much more in the brains of Tg mice than in those of WT mice (Fig. 3). Stability of internalized nanoparticles has been previously confirmed, so it might be inferred that fluorescent signals in the brains came from hASC.21 Since NEO-LIVE™-Magnoxide 675 is a multimodal nanoparticle which is suitable for optical imaging and MRI, MRI was performed to examine further anatomical information of transplanted cells in the brain. The result indicated that some signals from hASCs labeled with nanoparticles were detected at 2 and 7 days after transplantation in the cortex area (data not shown). For more defined information, additional confirmation is required. In summary, for in vivo imaging of stem cells in AD mouse model, we intravenously administered stem cells labeled with fluorescent magnetic nanoparticles through the tail–vein. We successfully follow the fluorescent signals from hASCs in AD mouse model until 10 days after injection. Up to 12 days after injection, strong signals were found in the brains extracted from hASC-transplanted Tg2576 mice. These results suggest that in vivo imaging with this multimodal nanoparticle may provide a useful tool for stem cell tracking and understanding stem cell biology in other neurodegenerative diseases. AcknowledgmentsThis work was supported by the Gachon University research fund of 2012 (GCU-2012-M084). ReferencesS. C. Liet al.,
“A biological global positioning system: considerations for tracking stem cell behaviors in the whole body,”
Stem Cell Rev., 6
(2), 317
–333
(2010). http://dx.doi.org/10.1007/s12015-010-9130-9 SCRTCI 1550-8943 Google Scholar
M. I. PhillipsY. L. TangK. Pinkernell,
“Stem cell therapy for heart failure: the science and current progress,”
Future Cardiol., 4
(3), 285
–298
(2008). http://dx.doi.org/10.2217/14796678.4.3.285 FCUAAZ 1479-6678 Google Scholar
N. SanduB. Schaller,
“Stem cell transplantation in brain tumors: a new field for molecular imaging?,”
Mol. Med., 16
(9–10), 433
–437
(2010). http://dx.doi.org/10.2119/molmed.2010-00035 MOMEE2 0268-2680 Google Scholar
A. C. LeporeN. J. Maragakis,
“Stem cell transplantation for spinal cord neurodegeneration,”
Methods Mol. Biol., 793 479
–493
(2011). http://dx.doi.org/10.1007/978-1-61779-328-8 MMBIED 0097-0816 Google Scholar
U. M. Fischeret al.,
“Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect,”
Stem Cells Dev., 18
(5), 683
–691
(2009). http://dx.doi.org/10.1089/scd.2008.0253 SCDTAE 1547-3287 Google Scholar
U. Krauseet al.,
“Intravenous delivery of autologous mesenchymal stem cells limits infarct size and improves left ventricular function in the infarcted porcine heart,”
Stem Cells Dev., 16
(1), 31
–37
(2007). http://dx.doi.org/10.1089/scd.2006.0089 SCDTAE 1547-3287 Google Scholar
J. ChungP. C. Yang,
“Molecular imaging of stem cell transplantation in myocardial disease,”
Curr. Cardiovasc. Imaging Rep., 3
(2), 106
–112
(2010). http://dx.doi.org/10.1007/s12410-009-9001-4 1941-9066 Google Scholar
C. Villaet al.,
“Stem cell tracking by nanotechnologies,”
Int. J. Mol. Sci., 11
(3), 1070
–1081
(2010). http://dx.doi.org/10.3390/ijms11031070 IJMCFK 1422-0067 Google Scholar
L. Ferreiraet al.,
“New opportunities: the use of nanotechnologies to manipulate and track stem cells,”
Cell Stem Cell, 3
(2), 136
–146
(2008). http://dx.doi.org/10.1016/j.stem.2008.07.020 CSCEC4 1875-9777 Google Scholar
R. SinghH.S. Nalwa,
“Medical applications of nanoparticles in biological imaging, cell labeling, antimicrobial agents, and anticancer nanodrugs,”
J. Biomed. Nanotechnol., 7
(4), 489
–503
(2011). http://dx.doi.org/10.1166/jbn.2011.1324 JBNOAB 1550-7033 Google Scholar
V. S. Lelyveldet al.,
“Challenges for molecular neuroimaging with MRI,”
Int. J. Imaging Syst. Technol., 20
(1), 71
–79
(2010). http://dx.doi.org/10.1002/ima.v20:1 IJITEG 0899-9457 Google Scholar
J. S. Kimet al.,
“Development and in vivo imaging of a PET/MRI nanoprobe with enhanced NIR fluorescence by dye encapsulation,”
Nanomedicine, 7
(2), 219
–229
(2012). http://dx.doi.org/10.2217/nnm.11.94 1743-5889 Google Scholar
S. NagarajanY. Zhang,
“Upconversion fluorescent nanoparticles as a potential tool for in-depth imaging,”
Nanotechnology, 22
(39), 395101
(2011). http://dx.doi.org/10.1088/0957-4484/22/39/395101 NNOTER 0957-4484 Google Scholar
C. K. Sunget al.,
“Dual-modal nanoprobes for imaging of mesenchymal stem cell transplant by MRI and fluorescence imaging,”
Korean J. Radiol., 10
(6), 613
–622
(2009). http://dx.doi.org/10.3348/kjr.2009.10.6.613 1229-6929 Google Scholar
A. Tennstaedtet al.,
“Noninvasive multimodal imaging of stem cell transplants in the brain using bioluminescence imaging and magnetic resonance imaging,”
Methods Mol. Biol., 1052 1
–14
(2013). http://dx.doi.org/10.1007/978-1-62703-559-0 MMBIED 0097-0816 Google Scholar
L. Stelteret al.,
“Modification of aminosilanized superparamagnetic nanoparticles: feasibility of multimodal detection using 3T MRI, small animal PET, and fluorescence imaging,”
Mol. Imaging Biol., 12
(1), 25
–34
(2010). http://dx.doi.org/10.1007/s11307-009-0237-9 1536-1632 Google Scholar
G. L. Bowmanet al.,
“Blood-brain barrier impairment in Alzheimer disease: stability and functional significance,”
Neurology, 68
(21), 1809
–1814
(2007). http://dx.doi.org/10.1212/01.wnl.0000262031.18018.1a NEURAI 0028-3878 Google Scholar
B. D. Zipseret al.,
“Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease,”
Neurobiol. Aging, 28
(7), 977
–986
(2007). http://dx.doi.org/10.1016/j.neurobiolaging.2006.05.016 NEAGDO 0197-4580 Google Scholar
J. C. Raet al.,
“Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans,”
Stem Cells Dev., 20
(8), 1297
–1308
(2011). http://dx.doi.org/10.1089/scd.2010.0466 SCDTAE 1547-3287 Google Scholar
K. Hsiaoet al.,
“Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice,”
Science, 274
(5284), 99
–102
(1996). http://dx.doi.org/10.1126/science.274.5284.99 SCIEAS 0036-8075 Google Scholar
K. S. Parket al.,
“Characterization, in vitro cytotoxicity assessment, and in vivo visualization of multimodal, RITC-labeled, silica-coated magnetic nanoparticles for labeling human cord blood-derived mesenchymal stem cells,”
Nanomed. Nanotechnol. Biol. Med., 6
(2), 263
–276
(2010). http://dx.doi.org/10.1016/j.nano.2009.07.005 1549-9634 Google Scholar
M. N. Linet al.,
“Involvement of PI3K and ROCK signaling pathways in migration of bone marrow-derived mesenchymal stem cells through human brain microvascular endothelial cell monolayers,”
Brain Res., 1513 1
–8
(2013). http://dx.doi.org/10.1016/j.brainres.2013.03.035 BRREAP 1385-299X Google Scholar
|

