|
|
1.IntroductionOsteoarthritis (OA) is a progressive degenerative disease characterized by loss of articular cartilage, subchondral bone remodeling, joint space narrowing, and osteophyte formation.1,2 According to the World Health Organization (WHO) Scientific Group on Rheumatic Diseases, 10% of the world’s population older than 60 years has significant clinical problems that can be attributed to OA.3–5 Obesity, trauma, age, genetic factors, and mechanical forces constitute risk factors and can initiate the process of cartilage degeneration.6,7 Furthermore, clinical symptoms involve joint pain, stiffness, local inflammation, and loss of mobility, which lead to a significant reduction of the quality of life.8 The OA treatment options include painkillers and anti-inflammatory drugs, physical exercises and, in the most serious cases, surgical interventions.6,9 In this context, there is a critical need to develop innovative technologies capable of treating the degenerative process related to OA and enhancing cartilage repair.10 Low level laser therapy (LLLT) has been indicated for several therapeutic purposes, including cartilage repair.11,12 It is shown that the LLLT has anti-inflammatory effects, increases tissue metabolism and neoangiogenesis, and stimulates collagen production by fibroblasts and tissue regeneration.13,14 In cartilage tissue, in vitro and in vivo studies demonstrated positive effects of laser therapy.1,12,15,16 Kushibiki et al.15 showed an increased chondrocyte differentiation and higher chondrogenic messenger RNA expression in prechondrogenic cells after laser irradiation. Furthermore, the LLLT is able of reducing swelling,12 modulating inflammation,17 decreasing cartilage destruction,18,19 stimulating angiogenesis, and reducing fibrosis formation1 in OA animal models. Lin et al.19 demonstrated that 810-nm LLLT can improve cartilage structure, prevent articular cartilage degradation, and significantly decrease the expression of caspase-3 in the knees of the rats submitted to an anterior cruciate ligament transection (ACLT). Despite the positive effects of the LLLT on tissue regeneration, there are limited evidences demonstrating the effects of this therapeutic approach on cartilage repair. Moreover, the mechanism by which the LLLT acts on cartilage is not fully understood and for many, the use of LLLT as a treatment modality is still controversial.20 Also, different authors use a wide range of doses which make difficult the comparison of published results and the choice for an ideal protocol. In this context, it was hypothesized that laser therapy could biomodulate the inflammation and prevent the degenerative process on an OA, providing a treatment with additional advantages for clinical use. Consequently, the present study was carried out in order to analyze the effects of lasertherapy at two different fluencies, on cartilage damage in an OA experimental model in knees of rats. Histology analysis was used to evaluate the dose responses of laser application in cartilage repair, after 5- and 8-weeks postsurgery. Also, immunohistochemistry evaluation of inflammatory markers involved in the process of cartilage degeneration [tumor necrosis factor (TNF-) and interleukin-1 (IL-), proteolitic enzymes related to the matrix degradation metalloprotein 13 (MMP 13) and Collagen type 1 (Col-1)]. 2.Methods2.1.Experimental GroupsThis study was approved and conducted in accordance with the Animal Care Committee guidelines of the Federal University of São Carlos (CEP 040/2010). Animals were maintained at 19°C to 23°C on a 12:12 h light-dark cycle in the Animal Experimentation Laboratory of the Federal University of São Carlos. Rats were housed in plastic cages and had free access to water and standard food. Eighty male Wistar rats (weighing , 12 to 13 weeks) were randomly distributed into four groups (): intact control group (IG), injured control group (CG), injured laser treated group at (L10), and injured laser treated group at (L50). The animals were distributed into two subgroups, with different periods of sacrifice (5- and 8-weeks postsurgery). 2.2.Anterior Cruciate Ligament Transection (ACLT)The animals were submitted to general anesthesia induced by intraperitoneal injection of xilazin (Syntec®, R. Soluções Lar, Cotia, São Paulo, Brasil, , IP) and ketamin (Agener®, Av. do Café, São Paulo, Brasil at , IP) and subjected to the ACLT of the left hind paw. The left knee was shaved, then sterilized and draped in sterile fashion. A medial arthrotomy was performed. Then, the patella was dislocated and the anterior cruciate ligament was isolated and transected. The ACLT was confirmed with Lachman testing by the surgeon and an observer.21 After being irrigated with sterile saline solution, the wounds were closed in layers and antiseptically treated. Rats were given appropriate postoperative care and allowed free activities in individual cages. 2.3.TreatmentsLaser treatment started 2 weeks after the surgery and it was performed for 15 and 30 sessions for each subgroup, using the following protocol: five consecutive days of irradiation with an interval of 2 days, for 3 and 6 weeks, respectively. The LLLT was applied at two points (on the medial and lateral sides of the joint), using the punctual contact technique. A low-energy Ga-Al-As laser (Theralaser, DMC® São Carlos, São Paulo, Brazil) was used at 830 nm, continuous wave diode, with a beam diameter, a power output of 30 mW, fluence at (irradiation time of 10 s, energy per point 0.3 J), and fluence at (irradiation time of 47 s, energy per point 1.4 J). On the respective days, animals were euthanized individually by carbon dioxide asphyxia. The knee joints were removed for analysis. 2.4.Histological AnalysisAfter harvesting, the specimens were fixated in 4% formaldehyde for 2 days, followed by decalcified in 4% ethylenediaminetetraacetic acid . The specimens were divided into two pieces, using a blade, at the mean point between both condyles, perpendicular to the articular surface. Samples were embedded in paraffin blocks and histological sections were done. Therefore, thin sections (6 μm) were prepared in the sagittal plane, starting from the medial margin of the joint using a micrometer (Leica RM—2145, Germany). Laminas were stained with hematoxylin and eosin (HE, Merck, Germany), Safranin-O (Merck, Germany), and Picrossirius-Red (Merck, Germany). Moreover, three sections were obtained for the imunohistoquemical analysis. 2.5.Histological Descriptive AnalysisHistopathological alterations in the articular cartilage were evaluated by two blinded observers. For descriptive analysis, the samples were stained with HE to evaluate cartilage structure, amount of cells and cellular organization. The specimens were examined using a light microscopy () (Leica Microsystems AG, Wetzlar, Germany). 2.6.Semi-Quantitative AnalysisThe severity of the OA lesion was graded on a scale using the Mankin scoring system (Table 1).22,23 Samples were stained with Safranin- O and histological evaluation system was performed along all of the extension. At least three sections of each specimen were examined using light microscopy () (Leica Microsystems AG, Wetzlar, Germany). The mean Mankin score of the two observers was calculated. Table 1Mankin score for the histological grading of cartilage degeneration.
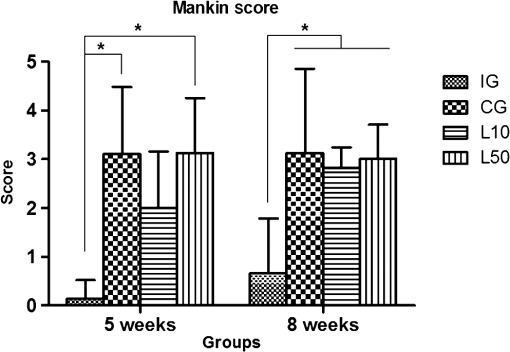
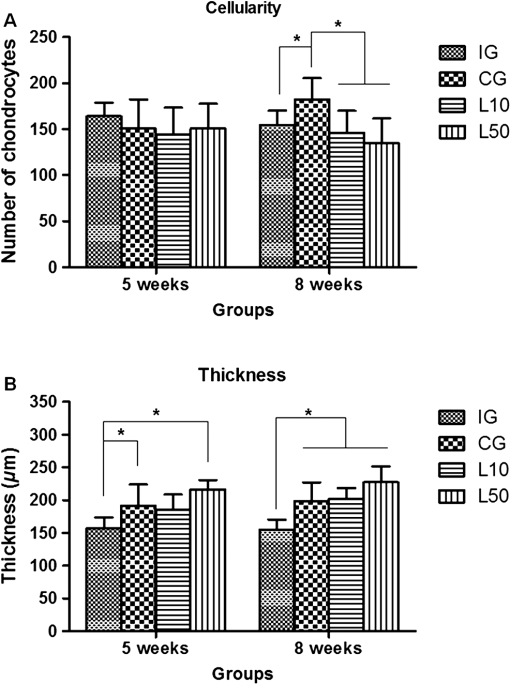
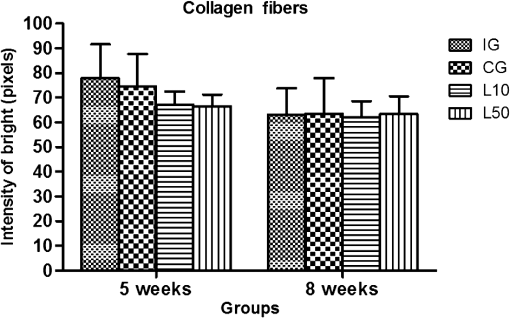
2.7.Morphometric AnalysisThe morphometric study was carried out using one randomized slide stained with HE. The cartilage thickness and number of chondrocytes in each area were quantitatively scored using the computer-based image analysis Axiovision 3.1 Image Analysis (Carl Zeiss, Oberkochen, Germany). To count the number of condrocytes, three areas of , at the anterior, central, and posterior region of each slide were chosen. Within each area, cells were marked and the chondrocytes average was calculated. Thickness was also measured in three regions, one central and two laterals (300-mm left and right from the first region), from subcondral bone to articular surface.24 2.8.Picrossirius Red AnalysisHistological sections stained by the Picrosirius-polarization method were viewed under polarized light (Carl Zeiss, Oberkochen, Germany) to assess the collagen organization in the cartilage tissue.25–28 Similarly to the morphometric analysis, three areas of 80,000 μm2 (anterior, central, and posterior region) of each slide were chosen to quantify the amount of collagen using the software ImageJ (Version 1.45, National Institutes of Health, Bethesda, Maryland). In each field, an indirect evaluation of the total collagen fibers organization based on the birefringence of the collagen fiber bundles after staining with Picrosirius was performed. Two experienced observers (PO and AR) performed the scoring in a blinded manner.29 2.9.ImmunohistochemistryThe TNF-, IL-, MMP 13, and Col-1 immunoexpression were determined using the streptavidin-biotin-peroxidase method. Sections at 3 μm were deparaffinized in three changes of xylene and rehydrated in a graded series of ethanol to distilled water. For antigen retrieval, slides were placed in 0.01 M citrate-buffer pH 6.0 and heated in a microwave for three cycles of 5 min each at 850 W. Endogenous peroxidases were quenched by incubation in 3% for 20 min at room temperature. Sections were incubated overnight at 4°C with primary antibody: TNF- (polyclonal rabitt anti-rat, ab6671, Abcam, Cambrige, MA, UK), interleukin-1 (IL-) (polyclonal rabitt anti-rat, sc-7884, Sta Cruz biotechnology, California, USA), metalloprotein 13 (MMP 13) (polyclonal rabitt anti-rat, ab75606, abcam, Cambrige, MA, UK) and Collagen type 1 (Col-1) (anti-Col 1A monoclonal primary antibody, Sta Cruz Biotechnology, USA). Subsequently, sections were incubated. Which tissue was used as positive control for each antibody tested in immunohistochemistry technique with biotinylated secondary antibody (LSA B, Dak ocytomation) for 30 min, washed in phosphate-buffered saline, and incubated with streptavidin peroxidase conjugate (LSA B, Dakocytomation) for 30 min. Finally, the reaction was developed using 3,3′-Dianobenzidine tetrahydrocloride (Sigma, St. Louis, Missouri) for 5 min. Slides were briefly counterstained in hematoxylin and rehydrated, and cover slips added. Negative and positive controls were to run simultaneously. Positive controls were represented by mammary tissue. Negative controls were made by eliminating the primary antibody as established in previous studies conducted by our group.30 The TNF-, IL-, MMP 13, and Col-1 immunoexpressions were evaluated both qualitatively (presence of the immunomarkers) and quantitatively in predetermined fields using a light microscope ((Leica Microsystems AG, Wetzlar, Germany). 2.10.Immunohistochemical Semiquantitative AnalysisSections stained using immunohistochemistry were analyzed for the percentages of immunopositive cells in control and experimental animals. A total of 1000 cells were evaluated in 3 to 5 fields at magnification. These values were used as labeling indices. 2.11.Statistical AnalysisThe normality of all variables’ distribution was verified using the Shapiro–Wilk W test. For the variable that exhibited normal distribution, comparisons among the groups were made using one-way analysis of variance (ANOVA) with pos hoc Tukey’s test. For the variable that exhibited nonnormal distribution, Kruskal Wallis test was used. STATISTICA version 7.0 was used to carry out the statistics analysis. Values of were considered statistically significant. 3.Results3.1.General FindingsNeither postoperative complications nor behavioral changes were observed. The rats rapidly returned to their normal diet and showed no weight loss during the experimentation. None of the animals died during the experiment and no infection in the surgical site was observed. 3.2.Histological Analysis3.2.1.Descriptive analysisHistopathological analysis revealed that after 5 weeks, cartilage tissue of IG presented a normal structure, without signs of fibrillation. Moreover, in the superficial region, the chondrocytes were displayed in a parallel arrangement and in the intermediate region they were organized in columns [Fig. 1(a)]. The CG presented signs of fibrillation, irregularities in the articular surface and chondrocytes displayed in a disorganized disposition [Fig. 1(c)]. In the laser treated groups, at both fluencies, the articular surface showed initial signs of fibrillation and condrocytes organized in a normal orientation, resembling the intact animals [Figs. 1(e) and 1(g)]. Fig. 1Representative photomicrographs the experimental groups. Organization of chondrocytes (thin arrow →), fibrillation and irregularities (thick arrow 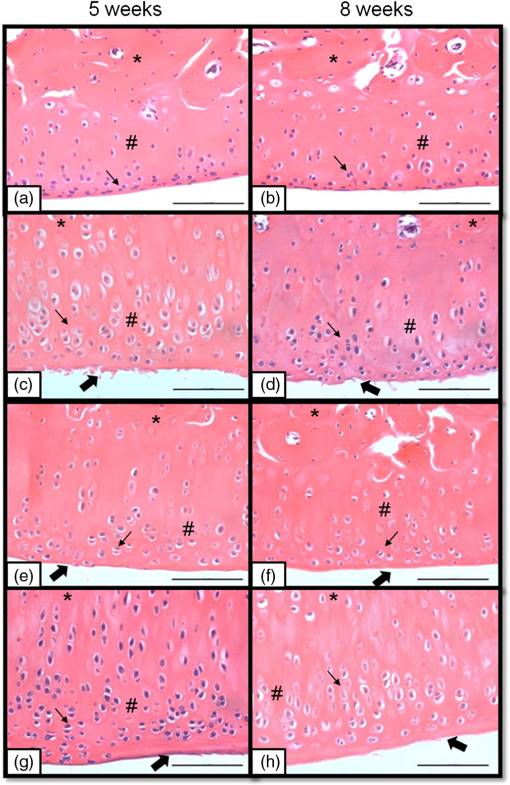 After 8 weeks, the histological findings revealed that IG presented normal cartilage structure [Fig. 1(b)]. In the control animals, the degenerative process progressed, presenting intense fibrillation, surface irregularities and hipercellularity, with chondrocytes displayed in a disorganized way [Fig. 1(d)]. The L10 presented a better tissue organization compared to CG, with a moderate amount of cells, with slight fibrillation, and irregularities [Fig. 1(f)]. Cartilage structure of the L50 was more disorganized compared to L10, with a moderate presence of chondrocytes and fibrillation in articular surface [Fig. 1(h)]. 3.2.2.Semi-quantitative analysisThe semi-quantitative analysis demonstrated that, after 5 weeks, IG presented significantly lower scores in comparison to CG and L50. After 8 weeks, Mankin scores observed in intact animals were significantly lower compared to CG, L10 and L50 (Fig. 2). No other difference was observed. 3.3.Morphometric Analysis3.3.1.CellularityFive weeks postsurgery, similar results in the cellularity analysis were found for all groups. In the second period evaluated, the number of condrocytes in the CG was higher compared to IG, L10 and L50 (, 0.00017, 0.00015, respectively) [Fig. 3(a)]. 3.3.2.ThicknessAfter 5 weeks, the IG presented lower cartilage thickness compared to CG () and L50 () [Fig. 3(a)]. After 8 weeks, IG had significantly lower cartilage thickness compared to the other groups (, 0.0021, 0.00019 to CG, L10, and L50, respectively) [Fig. 3(b)]. 3.3.3.Collagen fibersNo difference was observed in collagen fiber evaluation comparing the results found in the experimental groups at the two periods analyzed (Fig. 4). 3.4.ImmunohistochemistryFigures 5Fig. 6Fig. 7–8 showed the qualitative immunoexpression for IL-, TNF-, MMP-13, and Col-1, respectively. It is possible to observe that all the immunoreactivities were observed in the nucleus of chondrocytes. Fig. 5Representatives sections of IL- immunohistochemistry. (a) IG 5 weeks; (b) IG 8 weeks; (c) CG 5 weeks; (d) CG 8 weeks; (e) L10 5 weeks; (f) L10 8 weeks; (g) L50 5 weeks; (f) L50 8 weeks. Scale bar, 100 μm. 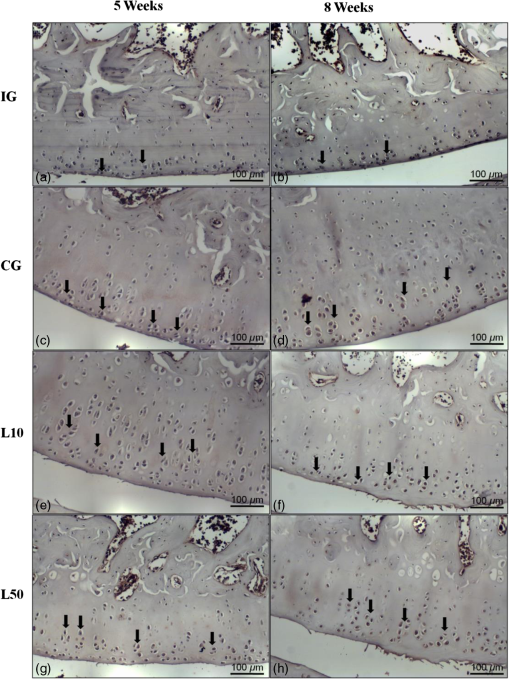 Fig. 6Representatives sections of tumor necrosis factor (TNF)- immunohistochemistry. (a) IG 5 weeks; (b) IG 8 weeks; (c) CG 5 weeks; (d) CG 8 weeks; (e) L10 5 weeks; (f) L10 8 weeks; (g) L50 5 weeks; (h) L50 8 weeks. Scale bar, 100 μm. 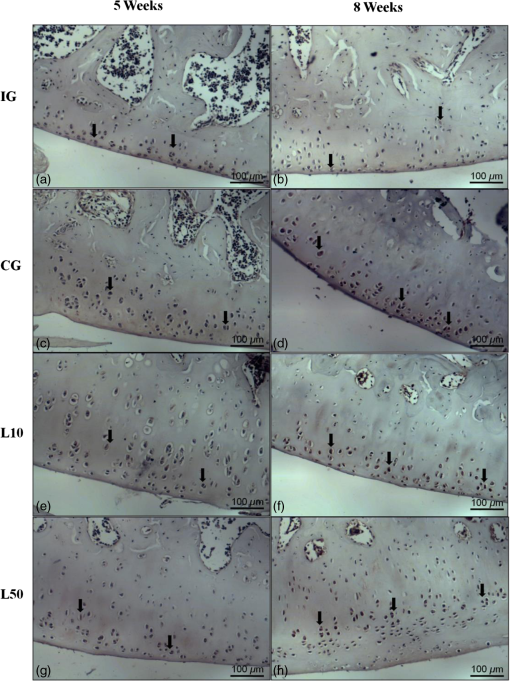 Fig. 7Representatives sections of metalloprotein (MMP)-13 immunohistochemistry. (a) IG 5 weeks; (b) IG 8 weeks; (c) CG 5 weeks; (d) CG 8 weeks; (e) L10 5 weeks; (f) L10 8 weeks; (g) L50 5 weeks; (h) L50 8 weeks. Scale bar, 100 μm. 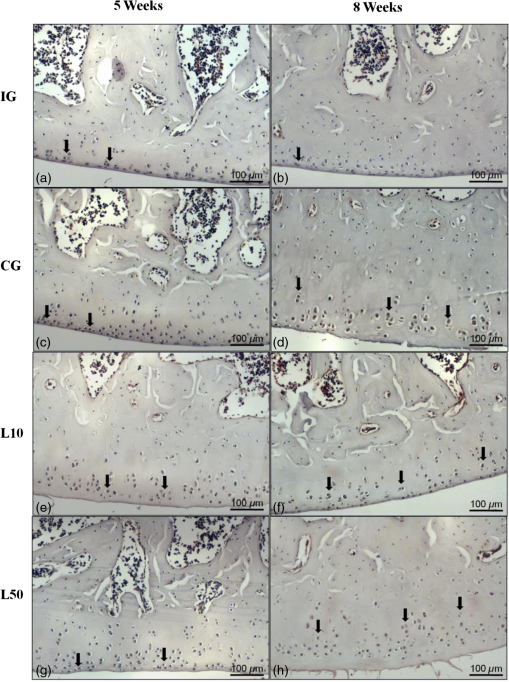 Fig. 8Representatives sections of collagen (Col)-1 immunohistochemistry. (a) IG 5 weeks; (b) IG 8 weeks; (c) CG 5 weeks; (d) CG 8 weeks; (e) L10 5 weeks; (f) L10 8 weeks; (g) L50 5 weeks; (h) L50 8 weeks. Scale bar, 100 μm. 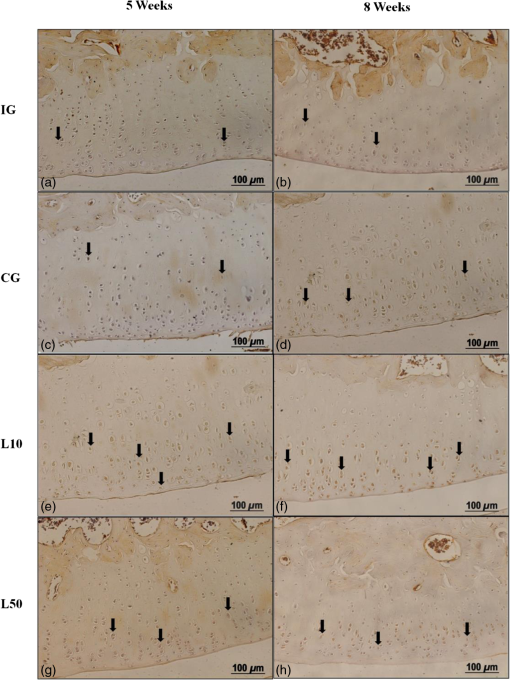 Figure 9 reveals the results quantitative of IL-, TNF-, MMP-13, and Col-1 immunoexpression. Results indicated that a lower IL- expression was observed in the IG compared to the other groups at both experimental periods analyzed (5- and 8-weeks postsurgery) [Fig. 9(a)]. Similar findings of IL- expression were demonstrated in the other experimental injured groups (treated and control groups). Fig. 9(a) Results of the TNF- expression. (b) Results of the IL- expression. (c) Results of the MMP-13 expression. IG: intact control group; CG: injured control group; L10: injured laser treated group at ; L50: injured laser treated group at . * . 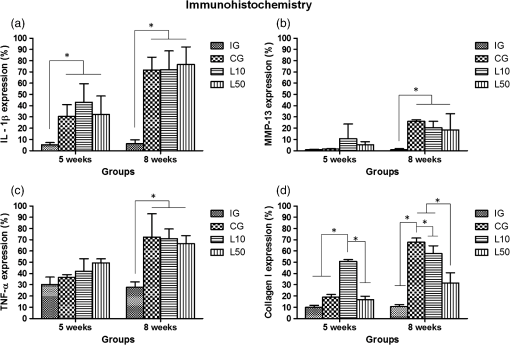 Quantitative analysis of TNF- showed that no difference was observed between the experimental groups after 5 weeks. Eight weeks postsurgery, the expression of TNF- was higher in the CG, L10, and L50 compared to IG. No other difference was detected [Fig. 9(b)]. Similar results were observed in the MMP-13 immunoexpression evaluation. At the first experimental period, no difference was observed. After 8 weeks, the expression of MMP-13 was higher in the treated and nontreated OA groups compared to IG [Fig. 9(c)]. The analysis of Col-1 showed that 5-weeks postsurgery, a significant increase of this protein was found in the L10 compared to the other groups. In the second experimental period, intact animals showed a lower expression of col-1 compared to the other groups. Moreover, CG and L10 showed a significantly higher expression compared to L50 [Fig. 9(d)]. 4.DiscussionIn this study, the effects of 830-nm laser on the degeneration of the articular cartilage of knees of rats submitted to an OA experimental model were investigated. The main histological findings revealed that lasertherapy, at , modulated the progression of the degenerative process, showing a better cartilage structure and lower number of condrocytes compared to the other OA animals. However, lasertherapy was not able to decrease the degenerative process measured by Mankin score and the increase of cartilage thickness related to the arthritic process. Moreover, similar findings in the expression of , TNF-, and MMP-13 were found in all experimental groups, and col-1 expression was decreased in irradiated animals, at . Many studies have recently demonstrated the positive effects of the LLLT on tissue metabolism.11,31,32 This therapy has been showed to accelerate cartilage cell proliferation in vitro studies15,33 and to stimulate cartilage repair in an OA experimental models.12,17,18 Also, it was demonstrated that the LLLT associated with exercises is effective in decreasing pain and improve articular function in patients with OA of the knees.16 Authors affirm that in the presence of a degenerative process, chondrocytes have their metabolic rate increased in an attempt to recover the damaged tissue.8 These modifications lead to an abnormal increase in the number of cells, which culminate in apoptosis.34–36 In this study, the positive effects of a laser, especially at on the qualitative histological findings and in the reduction in the number of cartilage cells, support the idea that lasertherapy had a positive influence in cell metabolism, preventing the hypercellularity. These findigs corroborate those of da Rosa et al.1 who observed that 808-nm laser stimulated angiogenesis and reduced the formation of fibrosis in an experimental model of the OA in rats. Structural modifications and increase of articular thickness are important indicators of cartilage degeneration. However, in this study, injured laser treated animals, at both fluencies, showed similar results in the Mankin evaluation and thickness measurement compared to CG. These results are in accordance with Bayat et al.37 who found an increase in the cartilage thickness after 632-nm laser irradiation, at in an OA experimental model. The authors considered this fact as a beneficial response in relation to tissue structure and attributed this to the laser having stimulated cellular metabolism. Picrossirius analysis showed that no difference in the amount of collagen was observed in the present study. However, immuhistochemistry showed that, 8-weeks postsurgery, a higher expression of Col-1 was observed in control animals and laser treated animals, at , suggesting a positive effect of the LLLT at . It is well known that the degenerative process culminates in a substitution of collagen type II for collagen type I.29,38 In this context, our immunhistochemistry findings after irradiation appeared to be a predictive factor for response to treatment. Furthermore, a series of anti-inflammatory markers are activated during the progression of OA, including IL-1, TNF, and MMP-13. The action of these inflammatory mediators lead to an increase in the catabolic pathways, inhibit matrix synthesis, and promote cellular apoptosis.39 The analysis of the immunoexpression of IL-, TNF-, and MMP-13 showed that laser irradiated animals presented similar findings compared to untreated animals, suggesting that the LLLT did not have any effect in the modulation of the inflammatory process related to OA. The anti-inflammatory effects of the LLLT on the degenerative process have been demonstrated by many authors, who observed decreased prostaglandin E2 and TNF expression after LLLT in OA experimental models.7,12 The disparate nature of our results may be due to a range of factors, including cellular physiology, fluences, and/or the LLLT wavelength employed. The disparity of the findings cited here supports the notion of probable cell/tissue and dose/wavelength specificities and the hypothesis that the parameters used in the present study were not capable of modulating the inflammatory process. Due to the lack of established parameters to treat the degenerative process in the cartilage tissue and based on the existence of a curve dose-response, the present study investigated the biological performance of two different fluences. Our results showed that the lower fluency () was more likely to produce morphological modifications in the tissue than the higher fluence (). It is important to stress that this parameter is extremely variable in laser therapy studies relating to cartilage regeneration, and a wide range of doses are used for different authors. Although the effects of the LLLT have been demonstrated by many authors, the regulatory mechanisms of laser on tissues are poorly understood.11 It may involve photochemical signaling, with laser light enhancing cell proliferation through changes in mitochondrial physiology, subsequently affecting RNA synthesis. Such effects then alter the expression of various cell regulatory proteins.39 It may be due also to the redox potential of the target cells, which is associated with stimulation of cell function if it shifts toward oxidation and with inhibition if towards reduction.40 However, the reasons for the stimulatory and inhibitory effects of laser on cartilage repair remain unclear and warrant further investigation. 5.ConclusionIn conclusion, the results found in present study indicate that 830-nm laser phototherapy modulates the proliferation of chondrocytes and prevented the increase of Col-1 in the experimental model of OA in rats, but had no effect on the modulation of the inflammatory process. Further investigations are required to investigate possible response mechanisms that may explain the contrasting outcomes obtained when examining laser irradiation, especially related to the modulation of the inflammatory process. Such future studies will undoubtedly contribute to a better understanding of the safety and efficacy of the LLLT to be used in clinical studies. AcknowledgmentsWe thank to Brazilian funding agencies Fapesp and CNPq for the financial support of this research. ReferencesA. S. da Rosaet al.,
“Effects of low-level laser therapy at wavelengths of 660 and 808 nm in experimental model of osteoarthritis,”
Photochem. Photobiol., 88
(1), 161
–166
(2012). http://dx.doi.org/10.1111/php.2011.88.issue-1 PHCBAP 0031-8655 Google Scholar
M. B. Goldring,
“The role of chondrocyte in osteoarthritis,”
Arthritis Rheum., 43
(9), 1916
–1926
(2000). http://dx.doi.org/10.1002/1529-0131(200009)43:9<1916::AID-ANR2>3.0.CO;2-I ARHEAW 0004-3591 Google Scholar
D. Pereiraet al.,
“The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review,”
Osteoarthritis Cartilage, 19
(11), 1270
–1285
(2011). http://dx.doi.org/10.1016/j.joca.2011.08.009 OSCAEO 1063-4584 Google Scholar
F. M. CornelisF. P. LuytenR. J. Lories,
“Functional effects of susceptibility genes in osteoarthritis,”
Discov. Med., 12
(63), 129
–139
(2011). 1539-6509 Google Scholar
K. Naitoet al.,
“Relationship between serum undercarboxylated osteocalcin and hyaluronan levels in patients with bilateral knee osteoarthritis,”
Int. J. Mol. Med., 29
(5), 756
–760
(2012). http://dx.doi.org/10.3892/ijmm.2012.897 IJMMFG 1107-3756 Google Scholar
D. T. Felsonet al.,
“The association of bone marrow lesions with pain in knee osteoarthritis,”
Ann. Intern. Med., 134
(7), 541
–549
(2001). http://dx.doi.org/10.7326/0003-4819-134-7-200104030-00007 AIMEAS 0003-4819 Google Scholar
H. Guoet al.,
“Comparing different physical factors on serum TNF- levels, chondrocyte apoptosis, caspase-3 and caspase-8 expression in osteoarthritis of the knee in rabbits,”
Jt., Bone, Spine, 78
(6), 604
–610
(2011). http://dx.doi.org/10.1016/j.jbspin.2011.01.009 1297-319X Google Scholar
K. R. Vincentet al.,
“The pathophysiology of osteoarthritis: a mechanical perspective on the knee joint,”
PM&R, 4
(5), S3
–S9
(2012). http://dx.doi.org/10.1016/j.pmrj.2012.01.020 0272-8710 Google Scholar
L. Xiaet al.,
“The effect of different treatment time of millimeter wave on chodrocytes apoptosis, caspase-3, caspase-8 an MMP-13 expression rabbit surgically induced model of knee osteoarthritis,”
Rheumatol. Int., 2
(9), 2847
–2856
(2001). http://dx.doi.org/10.1007/s00296-011-2080-y RHINDE 1437-160X Google Scholar
N. C. de Moraiset al.,
“Anti-inflammatory effect of low-level laser and light-emitting diode in zymosan-induced arthritis,”
Photomed. Laser Surg., 28
(2), 227
–232
(2010). http://dx.doi.org/10.1089/pho.2008.2422 PLDHA8 1549-5418 Google Scholar
A. C. Rennoet al.,
“The effects of laser irradiation on osteoblast and osteosarcoma cell proliferation and differentiation in vitro,”
Photomed. Laser Surg., 25
(4), 275
–280
(2007). http://dx.doi.org/10.1089/pho.2007.2055 PLDHA8 1549-5418 Google Scholar
A. P. Castanoet al.,
“Low-level laser therapy for zymosan-induced arthritis in rats: importance of illumination time,”
Lasers Surg. Med., 39
(6), 543
–550
(2007). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
F. Sorianoet al.,
“Photobiomodulation of pain and inflammation in microcrystalline arthropathies: experimental and clinical results,”
Photom. Laser Surg., 24
(2), 140
–150
(2006). http://dx.doi.org/10.1089/pho.2006.24.140 PLDHA8 1549-5418 Google Scholar
A. Guret al.,
“Efficacy of 904 nm gallium arsenide low level laser therapy in the management of chronic myofascial pain in the neck: a double-blind and randomize-controlled trial,”
Lasers Surg. Med., 35
(3), 229
–235
(2004). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
T. Kushibikiet al.,
“Chondrogenic mRNA expression in prechondrogenic cells after blue laser irradiation,”
J. Photochem. Photobiol. B, 98
(3), 211
–215
(2010). http://dx.doi.org/10.1016/j.jphotobiol.2010.01.008 JPPBEG 1011-1344 Google Scholar
P. P. Alfredoet al.,
“Efficacy of low level laser therapy associated with exercises in knee osteoarthritis: a randomized double-blind study,”
Clin. Rehabil., 26
(6), 523
–533
(2012). http://dx.doi.org/10.1177/0269215511425962 CEHAEN 0269-2155 Google Scholar
M. R. Guerinoet al.,
“Laser treatment of experimentally induced chronic arthritis,”
Appl. Surf. Sci., 154
(1), 561
–564
(2000). http://dx.doi.org/10.1016/S0169-4332(99)00460-2 ASUSEE 0169-4332 Google Scholar
H. J. Choet al.,
“Effect of low-level laser therapy on osteoarthropathy in rabbit,”
In Vivo, 18
(5), 585
–591
(2004). IVIVE4 0258-851X Google Scholar
Y. S. LinM. H. HuangC. Y. Chai,
“Effects of helium-neon laser on the mucopolysaccharide induction in experimental osteoarthritic cartilage,”
Osteoarthritis Cartilage, 14
(4), 377
–383
(2006). http://dx.doi.org/10.1016/j.joca.2005.10.010 OSCAEO 1063-4584 Google Scholar
L. Brosseauet al.,
“Low level laser therapy for osteoarthritis and rheumatoid arthritis: a metaanalysis,”
J. Rheumatol., 27
(8), 1961
–1969
(2000). JORHE3 Google Scholar
J. M. Williamset al.,
“Effects of surgically induced instability on rat Knee articular cartilage,”
J. Anat., 134
(1), 103
–109
(1982). JOANAY 0021-8782 Google Scholar
R. G. Pearsonet al.,
“Histopathology grading systems for characterisation of human knee osteoarthritis--reproducibility, variability, reliability, correlation, and validity,”
Osteoarthritis Cartilage, 19
(3), 324
–331
(2011). http://dx.doi.org/10.1016/j.joca.2010.12.005 OSCAEO 1063-4584 Google Scholar
H. J. Mankinet al.,
“Bochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hips II. Correlation of morphology with biochemical and metabolic data,”
J. Bone Joint Surg. Am., 53
(3), 523
–537
(1971). JBJSA3 1058-2436 Google Scholar
A. F. Renneret al.,
“The effect of a passive muscle stretching protocol on the articular cartilage,”
Osteoarthritis Cartilage, 14
(2), 196
–202
(2006). http://dx.doi.org/10.1016/j.joca.2005.08.011 OSCAEO 1063-4584 Google Scholar
L. C. JunqueiraG. S. MontesE. M. Sanchez,
“The influence of section thickness on the study of collagen by the Pricrosirius-polarization method,”
Histochemistry, 74
(1), 153
–156
(1982). http://dx.doi.org/10.1007/BF00495061 HCMYAL 0301-5564 Google Scholar
G. S. Montes,
“Structural biology of the fibres of the collagenous and elastic systems,”
Cell Biol. Int., 20
(1), 15
–27
(1996). http://dx.doi.org/10.1006/cbir.1996.0004 CBIIEV 1065-6995 Google Scholar
G. B. Andradeet al.,
“Use of the Picrosirius-polarization method to age fibrotic lesions in the hepatic granulomas produced in experimental murine schistosomiasis,”
Ann. Trop. Med. Parasitol., 93
(3), 265
–272
(1999). http://dx.doi.org/10.1080/00034989958528 ATMPA2 1364-8594 Google Scholar
I. Garavello-Freitaset al.,
“Low-power laser irradiation improves histomorphometrical parameters and bone matrix organization during tibia wound healing in rats,”
J. Photochem. Photobiol. B, 70
(2), 81
–89
(2003). http://dx.doi.org/10.1016/S1011-1344(03)00058-7 JPPBEG 1011-1344 Google Scholar
F. A. Vasilceacet al.,
“The remodeling of collagen fibers in rats ankles submitted to immobilization and muscle stretch protocol,”
Rheumatol. Int., 31
(6), 737
–742
(2011). http://dx.doi.org/10.1007/s00296-010-1371-z RHINDE 1437-160X Google Scholar
A. P. Paiottiet al.,
“Effect of COX-2 inhibitor lumiracoxib and the TNF- antagonist etanercept on TNBS-induced colitis in Wistar rats,”
J. Mol. Histol., 43
(3), 307
–317
(2012). http://dx.doi.org/10.1007/s10735-012-9400-8 JMHOAO 1567-2379 Google Scholar
F. Kamaliet al.,
“The therapeutic effect of low-level laser on repair of osteochondral defects in rabbit knee,”
J. Photochem. Photobiol. B, 88
(1), 11
–15
(2007). http://dx.doi.org/10.1016/j.jphotobiol.2007.04.010 JPPBEG 1011-1344 Google Scholar
J. M. Bjordalet al.,
“A systematic review of low level laser therapy with location-specific doses for pain from chronic joint disorders,”
Aust. J. Physiother., 49
(2), 107
–116
(2003). Google Scholar
B. J. Wonget al.,
“Identification of chondrocyte proliferation following laser irradiation, thermal injury, and mechanical trauma,”
Lasers Surg. Med., 37
(1), 89
–96
(2005). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
I. Fujitaet al.,
“Apoptosis of hypertrophic chondrocytes in rat cartilaginous growth plate,”
J. Orthop. Sci., 2
(5), 328
–333
(1997). http://dx.doi.org/10.1007/BF02488917 JOSCFS 0949-2658 Google Scholar
K. Kuhntet al.,
“Cell death in cartilage,”
Osteoarthritis Cartilage, 12
(1), 1
–16
(2004). http://dx.doi.org/10.1016/j.joca.2003.09.015 OSCAEO 1063-4584 Google Scholar
K. P. Pritzkeret al.,
“Osteoarthritis cartilage histopathology: grading and staging,”
Osteoarthritis Cartilage, 14
(1), 13
–29
(2006). http://dx.doi.org/10.1016/j.joca.2005.07.014 OSCAEO 1063-4584 Google Scholar
M. Bayatet al.,
“Effect of low-level helium-neon laser therapy on histological and ultrastructural features of immobilized rabbit articular cartilage,”
J. Photochem. Photobiol., 87
(2), 81
–87
(2007). http://dx.doi.org/10.1016/j.jphotobiol.2007.02.002 JPPBEG 1011-1344 Google Scholar
C. N. K. Diaset al.,
“Progression of articular cartilage degeneration after application of muscle stretch,”
Connect. Tissue Res., 53
(1), 39
–47
(2012). http://dx.doi.org/10.3109/03008207.2011.610476 CVTRBC 0300-8207 Google Scholar
T. I. KaruS. F. Kolyakov,
“Exact action spectra for cellular response relevant to phototherapy,”
Photomed. Laser Surg., 23
(4), 355
–361
(2005). http://dx.doi.org/10.1089/pho.2005.23.355 PLDHA8 1549-5418 Google Scholar
M. Mognatoet al.,
“Cell growth modulation of human cells irradiated in vitro with low-level laser therapy,”
Photomed. Laser Surg., 22
(6), 523
–526
(2004). http://dx.doi.org/10.1089/pho.2004.22.523 PLDHA8 1549-5418 Google Scholar
|
||||||||||||||||||||||||||||||||||||||||||||||