|
|
1.IntroductionDecrease of cerebral blood flow (CBF) reduces oxygen and substrate for brain metabolism and gives rise to brain ischemia, which is involved with pathological synaptic dysfunction,1 circulatory deficiencies, neuronal loss, and memory deficits.2,3 The progression of cerebral amyloid angiopathy affects vasodilation and promotes neurovascular units to release vasoconstrictive substances to suppress CBF and amplify cellular stress, which ultimately contribute to cognitive defects.4 In addition, epidemiological, pharmacotherapy, and clinical imaging studies indicate that vascular changes play an important role in the reduction of CBF present in the early stages of Alzheimer’s disease (AD) pathogenesis.5,6 Astrocytes, electrically nonexcitable cells, are clearly involved in the response and progression of neurodegeneration; they have a protective role in the initial response to neurodegeneration, but later may exert a negative effect through inflammation.7 Astrocytes are the key component coupling the neurovascular unit and supply energy and oxygen for neuronal metabolism by converting glucose to lactate.3,8–10 In addition, astrocytes have long been hypothesized to be involved in blood–brain material exchange11 and cerebrovascular regulation through direct interaction between the astrocyte endfoot and arterioles that play a key role in arteriole vasodilation and increase of local blood flow in the capillary.12–14 Though certain optical imaging approaches, such as two-photon intravital microscopy, dynamic light scattering, and spectrally enhanced microscopy, have been used to image the vascular network and measure blood microcirculation,15,16 their focus has been on observation and diagnosis, but not intervention, of CBF or brain ischemia. The in vivo photomodulation technique reported in this study can not only serve an observational and diagnostic function, but also provide a real-time, interactive strategy to increase local CBF of the live animal under observation and thus, serve as a powerful tool to delineate and understand the circuitry and dysfunction of neurovasculature. Recently, astrocyte activation has been proposed as a potential means to increase local CBF13 and as a novel therapeutic target for neurodegeneration, such as AD.7 It is well known that astrocyte activation plays a key role in protection from neurodegeneration and causes arteriole vasodilation to provide more energy for brain metabolism.17 Specific activation of the astrocyte endfoot may be the most direct method to control this interaction. Calcium uncaging, optogenetic activation, electromechanical stimulation, and pharmacological application have been deployed to activate astrocytes.13,18,19 Due to the limitation of light scattering and diffusion, single-photon calcium uncaging with ultraviolet (UV) light cannot provide the high spatial resolution to specifically target the astrocyte endfoot around the arterioles, particularly those more than 30 μm below the cortical surface. To this end, the optogenetic approach can be applied to specific targets and to activate astrocytes for chronic treatments with high spatiotemporal resolution.18 However, the introduction of external opsin proteins into the astrocyte membrane through viral expression limits its application in human patients. Mechanical and electrical stimulation would also need the invasive insertion of electrodes into the brain to activate astrocytes with uncertain side effects. Here we report a new strategy of photomodulation that applies infrared (IR) two-photon laser irradiation to specifically target the endfeet of astrocytes around arterioles and performs calcium uncaging in the deep brain with high spatial resolution to increase local CBF in downstream capillaries in vivo, with the goal to prevent brain starvation and ischemic neuronal damage. 2.Materials and Methods2.1.Animal PreparationThree-month-old male C57BL/6J mice were anesthetized by the inhalation of isoflurane (4% for induction; 1.5 to 2% for surgery, and 1 to 1.5% for imaging). After anesthesia, dexamethasone and buprenorphine were subcutaneously administered, and 20 to 30 μl 1% lidocaine solution was injected into the scalp to reduce pain. Fifteen minutes after the removal of the scalp, a high-speed microdrill was used to make a 3-mm-diameter craniotomy over the primary somatosensory cortex (centered 1 to 2 mm posterior to the bregma and 2 to 3 mm from the midline) under the dissecting microscope and a custom-made metal plate was glued on the skull with dental acrylic cement. After injecting the mixture of dyes into the cortex, 1% agarose gel and a 5 mm cover slip were added onto the exposed cortex to protect the exposed cortex and to reduce movement artifacts caused by respiration. Body temperature was monitored by a rectal probe and maintained at 37.1°C by a heating blanket (Homeothermic blanket systems, Harvard Apparatus, Holliston, Massachusetts). Experiments were performed only if the physiological variables remained within normal limits. All experiments were performed under the Institutional Animal Care and Use Committees approval of Houston Methodist Research Institute. 2.2.Dye InjectionPluronic F-127 (20% dissolved in dimethyl sulfoxide) was used to dissolve (acetyloxy)methyl ester (AM) ester dyes, and these dyes were diluted to the specific concentration [Oregon Green® 488 BAPTA-1 AM (OGB-1 AM), 1 mM; o-nitrophenyl 6,9-dioxa-3,12-diazatetradecanedioic acid, 3,12-bis(carboxymethyl)-4-(2-nitrophenyl) (NP-EGTA), 200 mM] using saline solution. Under the two-photon microscope, the mixture of OGB-1, Sulforhodamine 101 (SR 101), and NP-EGTA-AM was administered via an IM-300 microinjector (Narishige, Japan) into the somatosensory cortex (200 μm below the surface); 10 psi air pressure was used to perform dye injection for 90 s.20,21 After washing and removing the dyes or blood left on the surface, we poured saline containing 1% agarose onto the exposed cortex and mounted a cover slip (5 mm diameter). Thirty to forty-five minutes after the recovery of the mouse from surgery, imaging was performed under intravital two-photon microscopy. Five minutes before imaging, fluorescein isothiocyanate-dextran (FITC-dextran, 70,000 kDa, ) was systemically administered into the tail vein to visualize cerebral vasculature and blood flow. 2.3.In Vivo Two-Photon Imaging and Calcium UncagingThe upright laser scanning microscope (BX61WI, Olympus) was attached to a Ti:sapphire femtosecond pulsed laser system (80 MHz repetition rate, pulse width, Spectra Physics, Santa Clara, California) and the software (Fluoview 1000) was used for two-photon fluorescence imaging. air, water-immersion [NA, 1.05; working distance (WD), 2 mm, Olympus], and water-immersion objectives (NA 0.80, WD; 3.3 mm, Olympus) were selectively chosen for fluorescence imaging in vivo. To excite OGB-1 and SR 101 simultaneously, 800-nm irradiation was used, and emission light was detected with 515/50 and 605/55 filters, respectively. In addition, we visualized pial arteries and veins under widefield fluorescence and identified penetrating arteries or collecting veins (10 to 35 mm diameter) by following the direction of flow from the pial surface.22 Capillaries were identified by their diameter (). The average laser power for imaging was . Arterioles, capillaries, and veins were discriminated by vessel diameter and blood flow direction. Series stacks of images (step-size: 1 μm) were acquired from the cortical surface to depths below by vertically translating the objective of the two-photon intravital microscopy system. For calcium uncaging, the IR optical system (Ti:sapphire femtoseond pulse laser) was applied for photolysis; 800-nm IR laser (the output of average power 40 to 60 mW, pulse width, 80 MHz repetition, physics spectra) was chosen to pinpoint target astrocyte endfeet surrounding arterioles and astrocyte soma to cause calcium uncaging. We started with low laser power and steadily increased the power until calcium uncaging could be visualized. The output power for the laser stimulation was controlled from 15 to 60 mW and stimulation duration was 0.5 to 1 ms. 2.4.Data AnalysisIn all the experiments, we analyzed and processed image data using software Image J (NIH) and MATLAB®. The intensity of fluorescence signals was defined as , where and were fluorescence intensity in the astrocytes and background signal at the same time point; fluorescence intensity of the images before the stimulation was averaged as baseline intensity and relative calcium change was calculated based on , where and were fluorescence intensity in the astrocytes at any given time point and baseline intensity, respectively. The diameter of arteriole lumen was determined based on the distance between paired points across the arteriole directly adjacent to an identified endfoot and arteriole diameter of the images before stimulation averaged as baseline diameter. So the relative change of arterioles was defined as , where was arteriole lumen diameter at any given time point and was baseline diameter, respectively. 3.ResultsBrain ischemia results from the deficiency of blood flow.1 To characterize the role of astrocytes in the regulation of cerebral blood flow, a tunable, finely controllable Ti:sapphire femtosecond laser was employed to perform the visualization of astrocyte–vasculature interactions over the exposed somatosensory cortex. To determine the imaging area of interest, a small vessel area was chosen to avoid the interference of large vessel shadows according to the maximum intensity projection of our imaging stack. In order to enhance image contrast, we introduced two specific dyes (FITC-dextran, SR 101) to visualize cerebral vasculature [Fig. 1(a): green] and astrocytes [Fig. 1(a): red], respectively. In addition, astrocyte endfeet, the processes ensheathed around the arteriole [Fig. 1(a), right], can be visualized while vessels, including arteriole, capillary, and vein, can be discriminated based on blood flow direction and diameter size of vessel lumen. Fig. 1Imaging of cerebral vasculature, calcium signal, and astrocytes. (a) Image of the interaction of cerebral vasculature and astrocytes; to the right, the magnification of the white square; red, SR101 labeled astrocytes, green, FITC-dextran labeled cerebral vasculature. (b) Images of calcium signal and astrocytes; left, calcium signal imaging; middle, SR101 labeled astrocytes; and right, overlap left and middle. (c) Calcium transient for cells 1 and 2 in (b). 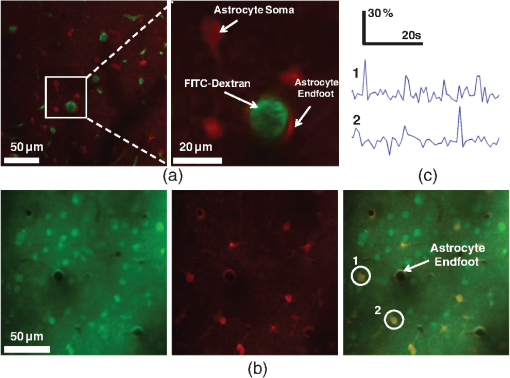 Astrocytes release glial transmitters in response to calcium influx specifically in the endfoot. In order to monitor physiological dynamics and signal transmission in astrocytes, the calcium signal indicator (OGB-1 AM) was administered into the somatosensory cortex 150 to 200 mm below the surface to monitor the change of thev calcium signal [Fig. 1(b), left]. Due to nonspecific labeling of OGB-1, the astrocyte-specific dye SR 101 was introduced to ensure the accuracy of astrocyte visualization [Fig. 1(b), middle and right]. Also, spontaneous calcium transients were regarded as the index to ensure the targeted astrocyte’s viability [Fig. 1(c)]. UV light calcium uncaging of astrocyte endfeet around the arteriole gives rise to an increase of lumen diameter of the arteriole in the healthy animal model.12,13 However, this method has the limitations of lower spatial resolution and lower stimulation depth due to light scattering. In order to finely control astrocyte activity and arteriole lumen diameter, we introduced the stimulation of IR two-photon irradiation to perform calcium uncaging in astrocytes and to measure the effect on arteriole vasodilation [Fig. 2(a)]. The mixture of NP-EGTA AM, OGB-1 AM, and SR 101 was administered into the area of interest in the exposed somatosensory cortex, and we used the same optical path as the two-photon imaging system to perform laser stimulation to perform calcium uncaging in astrocytes [Fig. 2(a)]. IR two-photon laser was used to specifically stimulate astrocyte somas, disregarding the vessel; the laser power was adjusted from low to high until the calcium signal increase was detected. We found that the calcium signal increases after laser stimulation and reaches its maximum 10 s later [Figs. 2(b) and 2(c)]. Fig. 2A schematic illustration of calcium imaging and calcium uncaging system. (a) Schematic illustration of calcium uncaging system using IR two-photon laser irradiation. (b) Time-series images of calcium uncaging exposed to the stimulation of IR two-photon laser irradiation. (c) Time-course tracings show that photostimulation causes a rapid increase of calcium signal and arterial vasodilation in the experiment shown in (b). 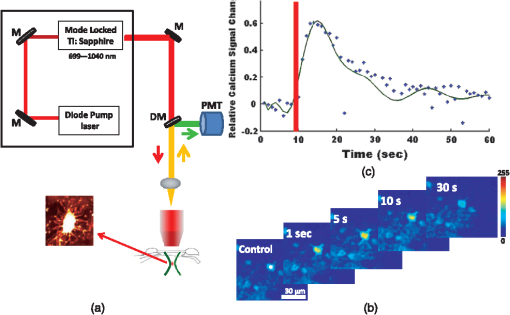 We investigated whether or not two-photon astrocyte activation gives rise to vasodilation in the arteriole in vivo. In our study, calcium signal in the endfoot increased rapidly and reached its maximum 10 s later; in addition, it lasted 60 to 80 s after laser stimulation on the endfoot. The increase of arteriole lumen followed the increase of the calcium signal and reached its maximum 20 s later with a duration of . As shown in Figs. 3(a) and 3(b), the diameter of arteriole lumen increased [Figs. 3(a) and 3(b)]. Zhao et al.23 suggested that a femtosecond pulse laser could target astrocyte membranes and increase calcium in cultured astrocytes; this raised the question of whether the vasodilation was not directly caused by calcium uncaging, but by laser stimulation on the astrocyte membrane. Thus, we injected the mixture of OGB-1 and SR 101 into the cortex, excluding NP-EGTA AM, and repeated the same experiment, but the same effect did not occur [Fig. 3(c)]; this result indicates that arteriole vasodilation was caused by calcium uncaging in the astrocyte endfoot. Fig. 3Calcium uncaging in astrocyte endfoot causes vasodilation in the arteriole. (a) Time-series images of arterioles diameter and calcium uncaging exposed to the stimulation of IR two-photon laser irradiation. Calcium uncaging triggered a rapid increase of in astrocyte endfoot and arterial vasodilation. White spot indicates the position of photostimulation. (b) Time-course tracings show that photostimulation causes a rapid increase of calcium signal and arterial vasodilation in the experiment shown in (a). (c) Photostimulation has no effect on both increase in astrocyte endfoot and arteriole vasodilation without NP-EGTA AM injection in the somatosensory cortex. White circle represents arteriole diameter, red dash curve represents the border of arterioles after photoactivation, blue curve indicates relative calcium signal change, and green curve indicates relative arterioles diameter change. 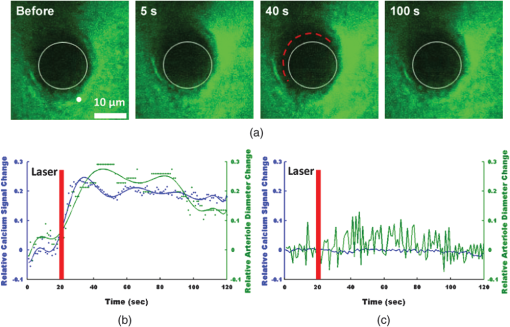 Due to the large size of the astrocyte soma, specific targeting of the soma was more readily done than on the astrocyte processes. Therefore, we addressed another question: could we specifically stimulate the soma, instead of the endfoot, to increase the lumen diameter of arteriole? When calcium uncaging in the astrocyte soma occurred under the stimulation of IR two-photon laser, the calcium signal increased and reached the maximum 10 s after laser stimulation, but vasodilation in the neighboring arteriole did not occur [Figs. 4(a) and 4(b)]. In addition, astrocyte endfeet ensheathed around the vessel, including the arteriole, the capillary, and the vein, so under microscopy, blood flow direction is a good objective criteria to discriminate the arteriole and vein, but this introduced some difficulties in finding a stimulation site to perform calcium uncaging. To investigate whether the endfoot around the arteriole is the only potential stimulation site for vasodilation, we tested whether calcium uncaging on the astrocyte endfoot around the vein increased the lumen diameter of the vein; the results, however, showed that calcium increases in the endfoot around the vein had no effect on the vasodilation [Figs. 4(c) and 4(d)]. Hence, we concluded that IR two-photon laser irradiation could be employed to target astrocyte endfeet in order to perform calcium uncaging studies. Moreover, only calcium uncaging in astrocyte endfeet triggered by femtosecond laser caused the increase of arteriole lumen and subsequently increased the local blood flow in downstream capillaries. Fig. 4Calcium uncaging in astrocyte soma and endfoot does not cause vasodilation in arteriole and vein, respectively. (a) The relative change of arteriole diameter and calcium signal in astrocytes soma before and 10 s after calcium uncaging in an astrocyte soma. (b) Time-course tracings of change in astrocyte soma and arteriole diameter change. (c) The relative change of vein diameter and calcium signal in astrocytes endfoot around the vein before and 10 s after calcium uncaging in an astrocyte endfoot. (d) Time-course tracings of change in astrocyte endfoot around the vein and the diameter change of vein’s lumen. Note: color intensity in (a) and (c) represents calcium signal intensity in astrocytes. 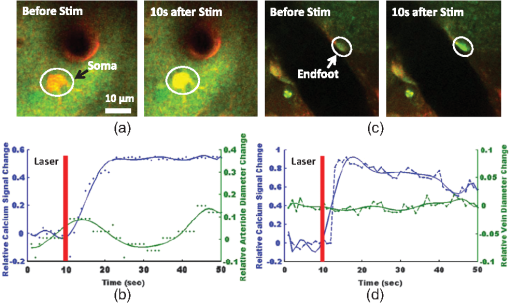 4.Conclusions and DiscussionsRecent studies24,25 demonstrate that decreased cerebral microcirculation, resulting in a deficiency of energy supply, causes neuronal dysfunction and chronic brain ischemia and has a crucial role in the progression of a variety of neurological diseases, including AD. Our experimental results show that activation of individual astrocyte endfeet around the arteriole increases lumen diameter of the arteriole by 25%, which should subsequently increase local CBF to supply energy for brain metabolism. Furthermore, because gap junctions transmit signals between adjoined astrocytes,26–28 the effect of vasodilation may spread to relatively distant areas and contribute to the same effect. These findings suggest that improving CBFflow for brain metabolism would be an effective and potentially therapeutic strategy to prevent or treat neurovascular or neurodegenerative diseases. Though UV light has been used to perform calcium uncaging in astrocyte endfeet and increase local blood flow in downstream capillaries,12,13 the stimulation site cannot be narrowed down to a subcellular structure due to light scattering and diffraction. Because of the small focal volume and reduced light scattering for IR two-photon excitation, our imaging technique can resolve this problem by targeting stimulation volumes down to femtoliters and can be used to address specific targets in astrocytes, as well as study and modulate physiology dynamics of the astrocytes in deep brain regions. In addition, some calcium-caging reagents have been developed for use under IR femtosecond laser stimulation:17 DM-nitrophen and azid-1 can not only be excited by UV irradiation, but also can have a maximum excitation at the specific long wavelength stimulation between 700 and 800 nm. Under 700-nm femtosecond laser irradiation and high-numerical-aperture objective, azid-1 can be photolyzed with a 10-μs pulse train of 7 mW average power.17 However, during the two-photon imaging process, the lower energy output of IR femtosecond pulse laser will make the occurrence of chemically irreversible calcium uncaging possible, which will interfere with calcium imaging. Compared with these calcium caging reagents, NP-EGTA AM is not as sensitive to femtosecond IR laser uncaging and needs relatively high energy to cause photolysis, which can reduce the possibility of unnecessary calcium uncaging during two-photon imaging. Label-free stimulation by IR two-photon lasers has been introduced to nondisruptively and reproducibly activate astrocytes. Using a high-numerical-aperture objective, the femtosecond, pulsed laser can be focused on the cell membrane and lead to photoporation with uncertain mechanism, but accurately targeting the plasma membrane is not easily achievable and this method lacks a specific molecular target.23,29 However, our technique not only confines calcium uncaging to less than femtoliter volumes,30 but also enables astrocyte activation more operationally without a specific focus on plasma membrane for photostimulation. In conclusion, calcium uncaging caused by femtosecond laser stimulation using intravital two-photon microscopy imaging offers a promising strategy to target specific regions, especially subcellular structures, in astrocytes and trigger calcium uncaging with finely controlled, high spatiotemporal resolution, and high stimulation accuracy in vivo. Coupling with the nonlinear excitation of a long-wavelength IR pulse laser and high-numerical-aperture objective, this strategy can reduce or even avoid out-of-focus photobleaching and photodamage while improving depth penetration for photostimulation in vivo. Owing to its high peak intensity and low pulse power, the femtosecond laser seldom damages the cells while offering high efficiency and precision.31,32 Therefore, the reported optical technique of astrocyte activation has the potential to facilitate physiological dynamics of astrogenesis-related vasodilation in deep brain regions in vivo and improve CBF in order to prevent brain ischemia, subsequently leading to the restoration of neurovascular function in neurodegeneration.33 AcknowledgmentsThis research is supported by TT and WF Chao Foundation and John S Dunn Research Foundation to S.T.C.W. Y.C. is partially supported by Nantz National Alzheimer Center at Houston Methodist Hospital. ReferencesY. Wenet al.,
“Transient cerebral ischemia induces site-specific hyperphosphorylation of tau protein,”
Brain Res., 1022
(1–2), 30
–38
(2004). http://dx.doi.org/10.1016/j.brainres.2004.05.106 BRREAP 1385-299X Google Scholar
E. FarkasP. G. Luiten,
“Cerebral microvascular pathology in aging and Alzheimer’s disease,”
Prog. Neurobiol., 64
(6), 575
–611
(2001). http://dx.doi.org/10.1016/S0301-0082(00)00068-X PGNBA5 0301-0082 Google Scholar
C. Iadecola,
“Neurovascular regulation in the normal brain and in Alzheimer’s disease,”
Nat. Rev. Neurosci., 5
(5), 347
–360
(2004). http://dx.doi.org/10.1038/nrn1387 NRNAAN 1471-0048 Google Scholar
R. Deaneet al.,
“RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain,”
Nat. Med., 9
(7), 907
–913
(2003). http://dx.doi.org/10.1038/nm890 1078-8956 Google Scholar
J. C. de la Torre,
“Alzheimer disease as a vascular disorder—nosological evidence,”
Stroke, 33
(4), 1152
–1162
(2002). http://dx.doi.org/10.1161/01.STR.0000014421.15948.67 SJCCA7 0039-2499 Google Scholar
Y. Heet al.,
“Regional coherence changes in the early stages of Alzheimer’s disease: a combined structural and resting-state functional MRI study,”
Neuroimage, 35
(2), 488
–500
(2007). http://dx.doi.org/10.1016/j.neuroimage.2006.11.042 NEIMEF 1053-8119 Google Scholar
A. W. Kraftet al.,
“Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice,”
FASEB J., 27
(1), 187
–198
(2013). http://dx.doi.org/10.1096/fj.12-208660 FAJOEC 0892-6638 Google Scholar
J. L. StobartC. M. Anderson,
“Multifunctional role of astrocytes as gatekeepers of neuronal energy supply,”
Front Cell Neurosci., 7 38
(2013). http://dx.doi.org/10.3389/fncel.2013.00038 Google Scholar
B. V. Zlokovic,
“Neurovascular mechanisms of Alzheimer’s neurodegeneration,”
Trends Neurosci., 28
(4), 202
–208
(2005). http://dx.doi.org/10.1016/j.tins.2005.02.001 TNSCDR 0166-2236 Google Scholar
R. Deaneet al.,
“Clearance of amyloid-beta peptide across the blood-brain barrier: implication for therapies in Alzheimer’s disease,”
CNS Neurol. Disord. Drug Targets, 8
(1), 16
–30
(2009). http://dx.doi.org/10.2174/187152709787601867 1871-5273 Google Scholar
B. RansomT. BeharM. Nedergaard,
“New roles for astrocytes (stars at last),”
Trends Neurosci., 26
(10), 520
–522
(2003). http://dx.doi.org/10.1016/j.tins.2003.08.006 TNSCDR 0166-2236 Google Scholar
M. Zontaet al.,
“Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation,”
Nat. Neurosci., 6
(1), 43
–50
(2003). http://dx.doi.org/10.1038/nn980 NANEFN 1097-6256 Google Scholar
T. Takanoet al.,
“Astrocyte-mediated control of cerebral blood flow,”
Nat. Neurosci., 9
(2), 260
–267
(2006). http://dx.doi.org/10.1038/nn1623 NANEFN 1097-6256 Google Scholar
X. Wanget al.,
“Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo,”
Nat. Neurosci., 9
(6), 816
–823
(2006). http://dx.doi.org/10.1038/nn1703 NANEFN 1097-6256 Google Scholar
V. Kalchenkoet al.,
“In vivo characterization of tumor and tumor vascular network using multi-modal imaging approach,”
J. Biophotonics, 4
(9), 645
–649
(2011). http://dx.doi.org/10.1002/jbio.201100033 JBOIBX 1864-063X Google Scholar
J. D. Driscollet al.,
“Two-photon imaging of blood flow in the rat cortex,”
Cold Spring Harb. Protoc., 2013
(8), 759
–767
(2013). http://dx.doi.org/10.1101/pdb.prot076513 1940-3402 Google Scholar
E. B. Brownet al.,
“Photolysis of caged calcium in femtoliter volumes using two-photon excitation,”
Biophys. J., 76
(1), 489
–499
(1999). http://dx.doi.org/10.1016/S0006-3495(99)77217-6 BIOJAU 0006-3495 Google Scholar
T. Sasakiet al.,
“Application of an optogenetic byway for perturbing neuronal activity via glial photostimulation,”
Proc. Natl. Acad. Sci. U. S. A., 109
(50), 20720
–20725
(2012). http://dx.doi.org/10.1073/pnas.1213458109 PNASA6 0027-8424 Google Scholar
V. Vedam-Maiet al.,
“Deep brain stimulation and the role of astrocytes,”
Mol. Psychiatry, 17
(2), 124
–131
(2012). http://dx.doi.org/10.1038/mp.2011.61 MOPSFQ 1359-4184 Google Scholar
C. Stosieket al.,
“In vivo two-photon calcium imaging of neuronal networks,”
Proc. Natl. Acad. Sci. U. S. A., 100
(12), 7319
–7324
(2003). http://dx.doi.org/10.1073/pnas.1232232100 PNASA6 0027-8424 Google Scholar
J. SchummersH. B. YuM. Sur,
“Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex,”
Science, 320
(5883), 1638
–1643
(2008). http://dx.doi.org/10.1126/science.1156120 SCIEAS 0036-8075 Google Scholar
A. F. McCaslinet al.,
“In vivo 3D morphology of astrocyte-vasculature interactions in the somatosensory cortex: implications for neurovascular coupling,”
J. Cereb. Blood Flow Metab., 31
(3), 795
–806
(2011). http://dx.doi.org/10.1038/jcbfm.2010.204 JCBMDN 0271-678X Google Scholar
Y. Zhaoet al.,
“Photostimulation of astrocytes with femtosecond laser pulses,”
Opt. Express, 17
(3), 1291
–1298
(2009). http://dx.doi.org/10.1364/OE.17.001291 OPEXFF 1094-4087 Google Scholar
M. Cortes-Canteliet al.,
“Fibrinogen and beta-amyloid association alters thrombosis and fibrinolysis: a possible contributing factor to Alzheimer’s disease,”
Neuron, 66
(5), 695
–709
(2010). http://dx.doi.org/10.1016/j.neuron.2010.05.014 NERNET 0896-6273 Google Scholar
T. O’Connoret al.,
“Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis,”
Neuron, 60
(6), 988
–1009
(2008). http://dx.doi.org/10.1016/j.neuron.2008.10.047 NERNET 0896-6273 Google Scholar
N. J. MaragakisJ. D. Rothstein,
“Mechanisms of disease: astrocytes in neurodegenerative disease,”
Nat. Clin. Pract. Neurol., 2
(12), 679
–689
(2006). http://dx.doi.org/10.1038/ncpneuro0355 NCPND4 1745-834X Google Scholar
R. Dermietzelet al.,
“Gap-junctions between cultured astrocytes—immunocytochemical, molecular, and electrophysiological analysis,”
J. Neurosci., 11
(5), 1421
–1432
(1991). JNRSDS 0270-6474 Google Scholar
M. V. L. Bennettet al.,
“New roles for astrocytes: gap junction hemichannels have something to communicate,”
Trends Neurosci., 26
(11), 610
–617
(2003). http://dx.doi.org/10.1016/j.tins.2003.09.008 TNSCDR 0166-2236 Google Scholar
M. Choiet al.,
“Label-free optical activation of astrocyte in vivo,”
J. Biomed. Opt., 16
(7), 075003
(2011). http://dx.doi.org/10.1117/1.3600774 JBOPFO 1083-3668 Google Scholar
W. Denk,
“Two-photon scanning photochemical microscopy: mapping ligand-gated ion channel distributions,”
Proc. Natl. Acad. Sci. U. S. A., 91
(14), 6629
–6633
(1994). http://dx.doi.org/10.1073/pnas.91.14.6629 PNASA6 0027-8424 Google Scholar
W. Watanabeet al.,
“In vivo manipulation of fluorescently labeled organelles in living cells by multiphoton excitation,”
J. Biomed. Opt., 13
(3), 031213
(2008). http://dx.doi.org/10.1117/1.2939401 JBOPFO 1083-3668 Google Scholar
U. K. TirlapurK. Konig,
“Targeted transfection by femtosecond laser,”
Nature, 418
(6895), 290
–291
(2002). http://dx.doi.org/10.1038/418290a NATUAS 0028-0836 Google Scholar
Y. Chenet al.,
“In vivo optical activation of astrocytes as a potential therapeutic strategy for neurodegenerative diseases,”
Proc. SPIE, 8565 85655K
(2013). http://dx.doi.org/10.1117/12.2004712 PSISDG 0277-786X Google Scholar
|

