|
|
1.IntroductionLaser phototherapy (LPT) has been clinically used to accelerate the wound healing in several diseases including those from the oral cavity.1 The LPT has proven to be efficient in treating slow healing skin ulcers,2 chronic diabetic leg and foot ulcers,3,4 and several oral diseases, such as chemotherapy- and radiotherapy-induced oral mucositis,5–8 herpes simplex infections,9 and bone osteonecrosis.10,11 Diverse cell types, like fibroblasts,12–14 osteoblasts,15 endothelial,16,17 and keratinocytes,18–20 when growing in culture in adverse conditions respond positively to LPT. This treatment improves cell proliferation, migration, and transcription of genes involved in wound healing.21 Epithelial cells play an important role in wound healing by restoring the epithelial barrier, followed by re-establishment of tissue homeostasis.22 Rapid migration and proliferation of epithelial cells located in the basal layer of the epithelium adjacent to the injured area are important events of wound healing. Several molecular pathways are activated during wound healing including the AKT/mTOR pathway.23–26 In fact, augmented epithelial migration and accelerated wound healing have been observed as a result of in vivo overexpression of the AKT/mTOR pathway in genetically defined animal models.27 Additionally, mammalian target of rapamycin (mTOR) signaling pathway was proven to be a key player in the accelerated migration phenotype of epithelial and muscle cells.23,27 Only three studies analyzed the effects of LPT on epithelial cells, looking for understanding the mechanisms underlying the biostimulation in these cells.18–20 It is known that the mechanisms involved in the LPT are those related to the improvement in the respiratory metabolism28,29 and changes in mitochondrial membrane potential,30 leading to an increase in the mitochondrial respiration and ATP synthesis.31 However, it remains unclear how LPT influences epithelial cell proliferation and migration. Thus, this study searched the molecular mechanisms underlying the effects of LPT on oral keratinocytes proliferation and migration, and further the molecular circuitry involved in this process was dissected. 2.Materials and MethodsThe study received approval from the Research Committee of School of Dentistry, Universidade Federal do Rio Grande do Sul under the process number 24114. 2.1.Cell Lineage and Cell CultureNormal oral keratinocyte spontaneously immortalized (NOK-SI) cell line isolated from the retromolar area of the oral cavity was previously established and kindly provided by Dr. Gutkind from the National Institute of Dental and Craniofacial Research (NIDCR/NIH).32 The cells were maintained in Dulbecco’s-modified Eagle’s medium (DMEM, Hyclone, Thermo Fisher Scientific, Waltham, Massachusetts) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Thermo Fisher Scientific), penicillin, streptomycin, and anfotericyn B (Hyclone, Thermo Fisher Scientific) at 37°C in a humidified atmosphere with 5% . Cells plated on 6- or 96-well tissue-culture plates were grown to 70% of confluence. Cell stress was induced by using culture medium with low concentration of FBS (DMEM with 2% FBS, nutritional deficit).19,33 All cell culture experiments were performed under laminar flow (biosafety class II), and cells were monitored daily using a phase contrast microscope (AO Biostar, American Optical, Reichert, Depew, New York). 2.2.LPTThe irradiations were performed using a continuous-wave indium-gallium-aluminum-phosphide (InGaAlP) diode laser (MM Optics Ltd., São Carlos, SP, Brazil) with a spot size of , operating at a wavelength of 660 nm, and an output power of 40 mW. The energy densities used were 4 and corresponding to 4 and 20 s of exposure times, respectively. Laser was applied perpendicularly and in contact with the tissue-culture plates. When using 96-well culture plates [MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy phenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay], the laser was applied in a single point; whereas, using 6-well culture plates (“scratch assay”), the irradiations were applied in five points. Three sessions of irradiations were applied with 6-h intervals. The output power of the equipment was checked using a power meter (Laser Check; MM Optics LTDA, Sao Paulo, Brazil). The total energy per point and the total energy used in each culture plate after all the irradiations are described in Table 1. Table 1Irradiation patterns of laser phototherapy (LPT).
Because the distance between the laser source and the surface of application is critical, the LPT was performed through the bottom of the optically clear plates. The irradiation was carried out in partial dark conditions without the influence of other light. Control groups were treated under identical conditions without activating the laser source (sham irradiation). 2.3.In Vitro ‘Scratch Assay’In vitro scratch-induced wound model was chosen to assess the migratory ability of the cells upon irradiation with diode laser. NOK-SI cells were seeded on 6-well culture dishes maintained at 37°C and grown to confluence in normal growth media. Three groups were established: control (), laser group, and laser group. Two hours before the scratch, culture medium was substituted with nutritional-deficit media in all groups (DMEM with 2% FBS). A wound was made in the monolayer of the cells by completely scratching the cells in a line with a 200-μl pipette tip. The time of scratching the wound was designated as time zero. Three irradiations were applied in five points with 6-h intervals between each application. The first irradiation was performed immediately after the scratching (T0). Cells were allowed to proliferate and migrate into the scratch wound for 48 h since the time zero. Migration of the cells into the wound was photographed at 0, 12, 24, 36, and 48 h under a phase contrast microscope. Relative wound closure (%) was measured by using a computerized image analyzer system (AxioVision 4.8.1, Carl Zeiss, Thornwood, New York). “Scratch assay” was performed in triplicate. 2.4.Cell Mitochondrial Activity Analysis (MTS Assay)To determine the cell proliferation rate, the Cell Titer 96TM Aqueous nonradioactive cell proliferation kit (Promega, Fitchburg, Wisconsin) was used. Cell proliferation was determined by the reduction of MTS (inner salt) following the manufacturer’s protocol. In brief, cells were seeded onto the 96-well plates 24 h prior to irradiation. Culture media from all the samples were replaced with the nutritional-deficient media (2% FBS) after cellular attachment (6 h after seeding). Three groups were established: control (), laser group, and laser group. Cells received either sham (control) or laser irradiation in eight replicates for each treatment. Twenty-four hours after the first irradiation, the cells were incubated with MTS for 4 h at 37°C. The absorbance at 595 nm was measured using a microplate reader (Promega). 2.5.Western BlottingNOK-SI cells were seeded into the 6-well culture dishes and maintained at 37°C in a humidified atmosphere with 5% in DMEM supplemented with 10% FBS. Three groups were established: control (), laser group, and laser group. Six hours before irradiation, the medium was substituted with nutritional-deficit medium in all groups (DMEM with 2% FBS). Epithelial growth factor (EGF) was used as a positive control. The laser groups received three irradiations applied in five points with 6-h intervals. Eighteen hours after the first irradiation, the cells were washed with ice-cold PBS and lysed with radio immunoprecipitation assay (RIPA) buffer. Protein lysates were cleared of cellular debris by centrifugation at 4°C for 10 min, resolved in 10% SDS-PAGE gels, and transferred to polyvinyl difluoride membrane (Millipore, Billerica, Massachusetts). After the transfer of proteins, the membranes were blocked at room temperature for 1 h with 5% milk solution, washed three times with Tris-buffered saline (TBS)-Tween, and incubated overnight with anti-phospho S6 (Cell Signaling, Danvers, Massachusetts), anti-phospho (Cell Signaling), and GAPDH (Calbiochem, Gibbstown, New Jersey), which was used as a loading control. The membranes were washed three times for 5 min each with TBS-Tween and then incubated for 1 h at room temperature with appropriate secondary antibodies conjugated with horseradish peroxidase. The reactions were visualized using the ECL Super Signal West Pico Substrate (Pierce Biotechnology, Rockford, Illinois). 2.6.F-Actin Polarization AssayCulture medium containing 10% FBS was substituted by DMEM supplemented with 2% FBS 6 h before the first irradiation. Two groups were established: control group receiving (sham irradiation) and LPT group receiving . The LPT group received two irradiations with 6-h interval. F-actin staining was performed in two different time points: 10 min and 12 h after the first irradiation. In brief, cells were washed with cold PBS, fixed with fresh 4% paraformaldehyde, and permeabilized using 0.1% Triton for 5 min. Cells were incubated with 100-nM rhodamine/phalloidin for 30 min (Cytoskeleton, Denver, Colorado), followed by DNA staining using Hoechst 33342. Slides were mounted using aqueous mounting media, and images were acquired using QImaging ExiAqua monochrome digital camera attached to a Nikon Eclipse 80i Microscope and QCapturePro software (Nikon, Melville, New York). 2.7.Statistical AnalysisStatistical analyses were carried out using GraphPad Prism 5 (GraphPad Software, San Diego, California). Cellular migration was assessed by two-way analysis of variance (ANOVA), followed by the Bonferroni multiple comparison test. Statistical analysis of cellular proliferation was performed by one-way ANOVA, followed by Tukey’s multiple comparison test. Asterisks denote statistical significance [*; **; ***; and not significant (NS) ]. 3.Results3.1.LPT Accelerates Oral Keratinocyte MigrationIn vitro scratch-induced wound model was chosen to assess the migratory ability of the cells upon irradiation with diode laser [Fig. 1(a)]. Oral keratinocytes responded to diode laser biostimulation by accelerating the epithelial cell migration. Compared with sham-irradiated control group, LPT-treated cells showed significant acceleration of migration in 12 h after scratch ( *** and **), followed by wound closure within the first 24 h (* for 4 and ) [Fig. 1(b)]. Epithelial cells did not show significant differences in migration when irradiated by either 4 or energy density (NS ). Fig. 1Oral keratinocyte migration upon laser irradiation. (a) Scratch wound assays in NOK-SI oral keratinocytes cell line monolayers from control and LPT groups (4 and ) during whole experimental time (T0 to T48). Wounds were generated after cell confluence (T0). LPT irradiations were applied three times with 6-h interval. In vitro cell migration was assessed every 12 h. Scale bars represent 50 μm. (b) Graph shows epithelial cell migration, represented as the percentage of open wound of all groups in each experimental time (h). Compared with control group, LPT-treated cells show significant accelerated migration by 12 h after scratch (***— and **—), followed by accelerated wound closure within the first 24 h (*). There were no significant differences in migration when comparing both irradiated groups (4 and ) (NS ). 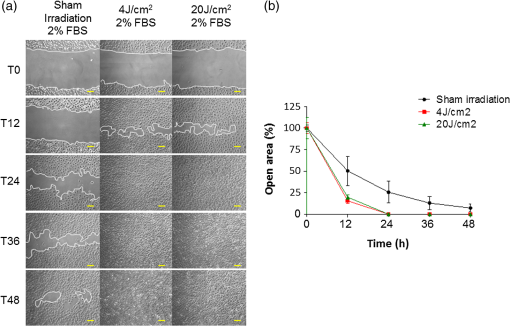 3.2.LPT Did Not Influence the Proliferation Rate of Oral KeratinocytesCell viabilities of control and irradiated groups 24 h after irradiation are represented in Fig. 2. There were no differences in the amount of viable cells among the groups (). Fig. 2Lack of proliferation of oral keratinocyte upon laser irradiation. Graphical representation of the number of viable cells 24 h after irradiation. Cell viability was determined by the reduction of MTS. Cells () were seeded onto the 96-well plates 24 h prior to irradiation. Culture media from all the samples were replaced with the nutritional-deficient media (2% FBS) after cellular attachment (6 h after seeding). There is no difference in the amount of viable cells among the established groups [control (sham group), laser group, and laser group]. Irradiation with LPT does not influence the proliferation rate of oral epithelial cells (NS ). 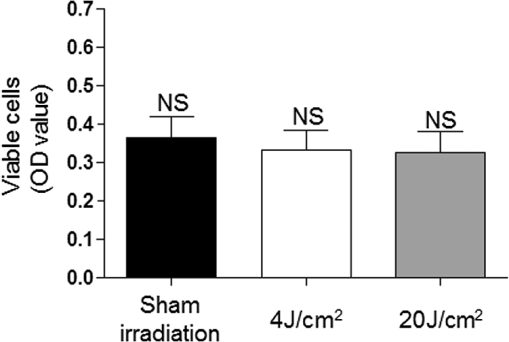 3.3.Diode Laser Irradiation Activates the AKT/mTOR Signaling Pathway in Oral KeratinocytesTo further explore the mechanistic details of accelerated epithelial cell migration, the involvement of the AKT/mTOR signaling pathway especially the phosphorylation levels of S6 protein, a downstream marker of mTOR signaling, was tested. LPT was able to induce the activation of the mTOR signaling in both irradiated groups (4 and ). Further, treatment of cells with rapamycin, a specific pharmacological inhibitor of mTOR, completely ablated the LPT-induced phosphorylation of S6 protein (Fig. 3). AKT phosphorylation at serine 473 () was not affected by LPT. As expected, an increase in AKT phosphorylation upon EGF treatment was noticed. GAPDH was used as a loading control. Fig. 3Upregulation of the AKT-mTOR pathway during laser-induced accelerated wound healing. Western blot analysis of phosho S6 and phospho AKT in NOK-SI oral keratinocytes cell line. Oral keratinocytes were seeded on 6-well culture dishes supplemented with 10% FBS. Six hours before irradiation, the medium was substituted by nutritional-deficit medium in all groups (DMEM with 2% FBS). Three groups were established: control (), laser group, and laser group. The laser groups received three irradiations with 6-h intervals. After 18 h of initial irradiation, Western blot analysis was performed for anti-phospho S6, anti-phospho , and GAPDH (loading control). EGF was used as a positive control. LPT induced the activation of the mammalian target of rapamycin (mTOR) signaling in both irradiated groups (4 and ). Treatment of cells with rapamycin, a specific pharmacological inhibitor of mTOR, completely ablated the LPT-induced phosphorylation of S6 protein. AKT phosphorylation at serine 473 () was not affected by LPT. An increase in AKT phosphorylation upon EGF treatment is noticed. 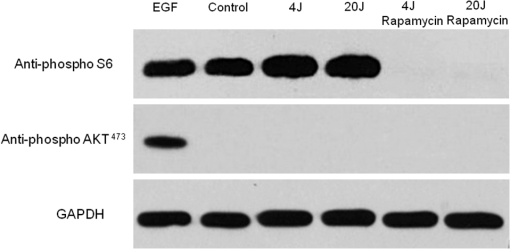 3.4.Low-Power Diode Laser Promotes F-Actin PolymerizationCell migration requires directional reorganization of the actin cytoskeleton and can be mediated by a variety of extracellular factors including growth factors and extracellular matrix. To test if LPT interferes with the cytoskeletal reorganization during epithelial cell migration, changes in F-actin organization were observed at two time points: 10 min and 12 h after the first irradiation using the optimal energy density of as previously established (Fig. 1). It was observed that LPT induced a robust accumulation of F-actin in NOK-SI cells compared with sham irradiation (Fig. 4). It was also observed that LPT induced the polarization of F-actin as early as 10 min after the initial irradiation, which was intensified after 12 h (Fig. 4, arrows). This was also accompanied by changes in cellular morphology (Fig. 4) to one that is typical of highly motile cells.34,35 Fig. 4Low-power diode laser promotes F-actin polymerization. Immunofluorescence images of F-actin staining in NOK-SI oral keratinocytes cell line. Oral keratinocytes cells were seeded on 6-well culture dishes supplemented with 10% FBS. Culture media were substituted by DMEM supplemented with 2% FBS 6 h before the initial irradiation. Two groups were established: control group () and laser group. The laser groups received three irradiations with 6-h intervals. F-actin staining was performed 24 h after administration of nutritional-deficit medium. Phalloidin (F-actin staining—red), DAPI (DNA staining—blue). LPT induced a robust accumulation of F-actin in treated cells (arrows), associated with the changes in the cellular morphology. Scale bars represent 50 μm. 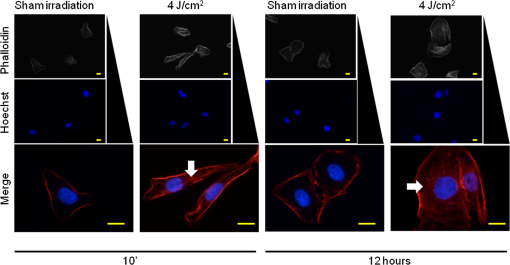 4.DiscussionWound healing is a key process essential for tissue repair and restoration of cellular homeostasis. It plays important roles in several physiological and pathological conditions involving ulcerated lesions that constitute significant clinical challenges such as burn ulcers, mucositis, diabetes with slow or nonhealing ulcers, pressure, and chronic venous ulcers.36–38 In an epitheliocentric view, wound healing is characterized by a multistep process comprising epithelial proliferation and migration. Notably, epithelial proliferation is often observed at the original wound edge, characterized by an increase in epithelial thickness. However, the initial stages in epithelial cell migration are mainly observed in the epithelial tongue, characterized by the layers of epithelial cells that migrate into the wound bed, thereby promoting healing of the wound. The epithelial tongue is primarily composed of migratory cells and a very few proliferating cells. It is interesting to note that the cells from the epithelial tongue do not undergo a terminal differentiation program compared with the cells localized at proliferating wound edge, suggesting that these two compartments are driven by distinct sets of active molecular processes.39 Clinically, LPT has been shown to be effective in wound healing by accelerating the closure of lesions in addition to reducing pain and discomfort.1–11,40 These observations suggest that the LPT may influence mucosal wound healing by accelerating epithelial cell migration over the wounded site. However, only three studies analyzed the effects of LPT on epithelial cells and the molecular mechanisms by which LPT can promote biostimulation in these cells.18–20 In the present study, we sought to explore the specific effects of diode laser irradiation on oral keratinocytes during wound healing using a keratinocyte cell line originally isolated from the retromolar area of the oral cavity.32 Our results suggest that the LPT accelerates the healing of oral mucosa by activation of oral keratinocytes migration and mTOR signaling pathway independent from cellular proliferative mechanisms. Recent studies including those from our laboratory have identified key molecules responsible for enhancing and impairing epithelial cell migration.27,32 We had shown that the PI3K/mTOR signaling pathway plays a crucial role in driving the re-epithelialization process. In fact, depletion of phosphatase and tensin homolog (PTEN), a negative modulator of the PI3K/mTOR signaling pathway, resulted in accelerated epithelial cell migration and overall wound healing.27 The identification of new therapeutic strategies to enhance the epithelial wound healing is aimed at the discovery of clinically viable solutions for slow healing ulcers. Recent advances in the field of photobiomodulation41,42 have shown a great deal of promise in this regard.1,12,18,21 However, molecular mechanisms have not been completely clear. The use of low-power diode laser or LPT has been shown to modulate several biological processes including wound healing; however, only a few reports have showed the effect of LPT on epithelial cells.18–20 In addition, the impact of LPT over keratinocytes from the oral mucosa and the associated molecular mechanisms involved in accelerated migration remain unknown. In the present study, we investigated the effect of LPT on wound-healing process of oral keratinocytes and explored the molecular circuitry involved in epithelial cell migration. To get in-depth analysis on cellular response to laser stimulation, we used two different LPT parameters: 660-nm diode , 4 and with same frequency of irradiation and irradiation time intervals. This choice was made based on the literature which showed that the small energy densities of irradiation lead to better effects compared with higher energy densities in wound healing.43–48 These studies were in accordance to the Arndt–Schultz law49 (e.g., a small stimulus can excite physiological activities, whereas a higher stimulus can inhibit them). Then, 4 and were used as lower and higher energy densities, respectively. By maintaining the same power in both groups, the cells were irradiated with energy per point of 0.16 J in the laser group and an energy per point five times higher (0.8 J) in the laser group. With these parameters, it was possible to observe the response of the cells to very different energies, and even though both 0.16 and 0.8 J per point could be considered low energies, they were different enough to induce diverse responses. Our results revealed that, independent of the energy density and energy per point, the laser irradiation had no effect on cell proliferation rate, but accelerated epithelial cell migration. In fact, the laser irradiation closed the open wound in half of the time compared with that of the control group. In addition to the accelerated cell migration, LPT also enhanced actin polymerization in epithelial cells, a hallmark of the highly motile cells. Using the same energy density in an in vivo animal model, our group observed that LPT was capable of accelerating the oral mucosa wound-healing process. Moreover, faster and more organized re-epithelialization and tissue healing of the oral mucosa were achieved with an energy density of in comparison with .40 Nonetheless, an increase in energy density has been shown to impair the wound healing in vivo.45 Other studies have predominantly attributed the inhibitory effect to higher power levels rather than energy density per se.50 We agree with this point of view, that the tissue response could not be only related to the energy density, but with the energy per point or total energy applied in tissue. In our study, the epithelial cells after LPT irradiation with 0.16 J energy per point ( laser group) and 0.8 J energy per point ( laser group) showed similar in vitro proliferative, migratory, and mTOR signaling pathway protein expression. The current mechanism by which LPT induces cellular response involves the activation of mitochondrial respiratory chain.1 LPT irradiation activates cellular cytochromes that promote a cascade of events leading to the generation of reactive oxygen species (ROS), changes in flux, no binding to cytochrome c oxidases that affect the levels of cyclic nucleotides, and DNA and RNA synthesis, thereby modulating a variety of cellular functions.51 We also found that LPT activates the mTOR signaling pathway in epithelial cells. Apart from its role in cell growth, shape, polarity, and size,52–58 mTOR also acts as a metabolic sensor and has been shown to regulate both the resting oxygen consumption and oxidative capacity. Therefore, mTOR activity correlates with the overall mitochondrial function, and pharmacological inhibition using rapamycin significantly reduces mitochondrial membrane potential.59 In all our experimental conditions, we noticed that LPT increased the phosphorylation of S6 protein and rapamycin treatment impaired this phosphorylation, indicating that LPT triggers mTOR signaling pathway. AKT phosphorylation was not influenced by LPT, suggesting that LPT activation of mTOR may be through mTORC1 and independent of mTORC2, which is known to phosphorylate AKT at serine 473. The physiological implications of this mechanism are yet to be elucidated in detail. Our findings have advanced the current understanding on the molecular circuitry involved in the use of a low-power diode laser in oral epithelial cells. Our data strongly indicate that LPT is a promising therapeutic strategy for diseases of the oral cavity such as oral mucositis, resulting from chemotherapy and radiotherapy, herpes simplex infections, and postsurgical management. Nonetheless, new studies are under way to better understand the effect of LPT over oral keratinocyte physiology and to further dissect the molecular mechanisms involved in photobiomodulation. AcknowledgmentsThis work was funded in part by the University of Michigan, School of Dentistry startup, by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and by the Universidade Federal do Rio Grande do Sul (UFRGS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There was no industrial funding for the study. ReferencesP. V. PeplowT. Y. ChungG. D. Baxter,
“Laser photobiomodulation of wound healing: a review of experimental studies in mouse and rat animal models,”
Photomed. Laser Surg., 28
(3), 291
–325
(2010). http://dx.doi.org/10.1089/pho.2008.2446 PLDHA8 1549- 5418 Google Scholar
J. Kubota,
“Defocused diode laser therapy (830 nm) in the treatment of unresponsive skin ulcers: a preliminary trial,”
J. Cosmet. Laser Ther., 6
(2), 96
–102
(2004). http://dx.doi.org/10.1080/14764170410014983 1476-4172 Google Scholar
D. G. Minatelet al.,
“Phototherapy promotes healing of chronic diabetic leg ulcers that failed to respond to other therapies,”
Lasers Surg. Med., 41
(6), 433
–441
(2009). http://dx.doi.org/10.1002/lsm.v41:6 LSMEDI 0196-8092 Google Scholar
A. Schindlet al.,
“Diabetic neuropathic foot ulcer: successful treatment by low-intensity laser therapy,”
Dermatology, 198
(3), 314
–316
(1999). http://dx.doi.org/10.1159/000018140 DERMEI 0742-3217 Google Scholar
R. G. CauwelsL. C. Martens,
“Low level laser therapy in oral mucositis: a pilot study,”
Eur. Arch. Paediatr. Dent., 12
(2), 118
–123
(2011). http://dx.doi.org/10.1007/BF03262791 1818-6300 Google Scholar
A. L. Rimuloet al.,
“Chemotherapy-induced oral mucositis in a patient with acute lymphoblastic leukaemia,”
Eur. Arch. Paediatr. Dent., 12
(2), 124
–127
(2011). http://dx.doi.org/10.1007/BF03262792 1818-6300 Google Scholar
A. F. Oton-Leiteet al.,
“Effect of low level laser therapy in the reduction of oral complications in patients with cancer of the head and neck submitted to radiotherapy,”
Spec. Care Dent., 33
(6), 294
–300
(2013). http://dx.doi.org/10.1111/scd.2013.33.issue-6 0275-1879 Google Scholar
L. Camposet al.,
“Improvement in quality of life of an oncological patient by laser phototherapy,”
Photomed. Laser Surg., 27
(2), 371
–374
(2009). http://dx.doi.org/10.1089/pho.2008.2300 PLDHA8 1549- 5418 Google Scholar
R. Navarroet al.,
“Low-level-laser therapy as an alternative treatment for primary herpes simplex infection: a case report,”
J. Clin. Pediatr. Dent., 31
(4), 225
–228
(2007). JCPDEX 1053-4628 Google Scholar
M. A. Martinset al.,
“Association of laser phototherapy with PRP improves healing of bisphosphonate-related osteonecrosis of the jaws in cancer patients: a preliminary study,”
Oral Oncol., 48
(1), 79
–84
(2012). http://dx.doi.org/10.1016/j.oraloncology.2011.08.010 EJCCER 1368-8375 Google Scholar
P. Vescoviet al.,
“Nd:YAG laser biostimulation in the treatment of bisphosphonate-associated osteonecrosis of the jaw: clinical experience in 28 cases,”
Photomed. Laser Surg., 26
(1), 37
–46
(2008). http://dx.doi.org/10.1089/pho.2007.2181 PLDHA8 1549- 5418 Google Scholar
F. G. Bassoet al.,
“In vitro wound healing improvement by low-level laser therapy application in cultured gingival fibroblasts,”
Int. J. Dent., 2012 719452
(2012). http://dx.doi.org/10.1155/2012/719452 IJDNB4 1687-8736 Google Scholar
C. A. Damanteet al.,
“Effect of laser phototherapy on the release of fibroblast growth factors by human gingival fibroblasts,”
Lasers Med. Sci., 24
(6), 885
–891
(2009). http://dx.doi.org/10.1007/s10103-008-0582-y LMSCEZ 1435-604X Google Scholar
M. M. Marqueset al.,
“Effect of low-power laser irradiation on protein synthesis and ultrastructure of human gingival fibroblasts,”
Lasers Surg. Med., 34
(3), 260
–265
(2004). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
N. A. FujiharaK. R. HirakiM. M. Marques,
“Irradiation at 780 nm increases proliferation rate of osteoblasts independently of dexamethasone presence,”
Lasers Surg. Med., 38
(4), 332
–336
(2006). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
A. Schindlet al.,
“Direct stimulatory effect of low-intensity 670 nm laser irradiation on human endothelial cell proliferation,”
Br. J. Dermatol., 148
(2), 334
–336
(2003). http://dx.doi.org/10.1046/j.1365-2133.2003.05070.x BJDEAZ 1365-2133 Google Scholar
N. Kipshidzeet al.,
“Low-power helium: neon laser irradiation enhances production of vascular endothelial growth factor and promotes growth of endothelial cells in vitro,”
Lasers Surg. Med., 28
(4), 355
–364
(2001). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
F. G. Bassoet al.,
“Biostimulatory effect of low-level laser therapy on keratinocytes in vitro,”
Lasers Med. Sci., 28
(2), 367
–374
(2013). http://dx.doi.org/10.1007/s10103-012-1057-8 LMSCEZ 1435-604X Google Scholar
F. P. Eduardoet al.,
“Cultured epithelial cells response to phototherapy with low intensity laser,”
Lasers Surg. Med., 39
(4), 365
–372
(2007). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
K. Ejiriet al.,
“High-frequency low-level diode laser irradiation promotes proliferation and migration of primary cultured human gingival epithelial cells,”
Lasers Med. Sci.,
(2013). http://dx.doi.org/10.1007/s10103-013-1292-7 LMSCEZ 1435-604X Google Scholar
W. Postenet al.,
“Low-level laser therapy for wound healing: mechanism and efficacy,”
Dermatol. Surg., 31
(3), 334
–340
(2005). http://dx.doi.org/10.1111/(ISSN)1524-4725 DESUFE 1076-0512 Google Scholar
R. M. CastilhoC. H. SquarizeJ. S. Gutkind,
“Exploiting PI3K/mTOR signaling to accelerate epithelial wound healing,”
Oral Dis., 19
(6), 551
–558
(2013). http://dx.doi.org/10.1111/odi.2013.19.issue-6 1354-523X Google Scholar
Y. W. Kimet al.,
“DA6034 promotes gastric epithelial cell migration and wound-healing through the mTOR pathway,”
J. Gastroenterol. Hepatol., 27
(2), 397
–405
(2012). http://dx.doi.org/10.1111/jgh.2012.27.issue-2 JGHEEO 1440-1746 Google Scholar
N. Ben-Dovet al.,
“Low-energy laser irradiation affects satellite cell proliferation and differentiation in vitro,”
Biochim. Biophys. Acta, 1448
(3), 372
–380
(1999). http://dx.doi.org/10.1016/S0167-4889(98)00147-5 BBACAQ 0006-3002 Google Scholar
G. Sheferet al.,
“Skeletal muscle cell activation by low-energy laser irradiation: a role for the MAPK/ERK pathway,”
J. Cell. Physiol., 187
(1), 73
–80
(2001). http://dx.doi.org/10.1002/(ISSN)1097-4652 JCLLAX 0021-9541 Google Scholar
M. J. Leeet al.,
“YAP and TAZ regulate skin wound healing,”
J. Invest. Dermatol., 134
(2), 518
–525
(2013). http://dx.doi.org/10.1038/jid.2013.339 JIDEAE 0022-202X Google Scholar
C. H. Squarizeet al.,
“Accelerated wound healing by mTOR activation in genetically defined mouse models,”
PloS One, 5
(5), e10643
(2010). http://dx.doi.org/10.1371/journal.pone.0010643 1932-6203 Google Scholar
B. Beauvoitet al.,
“Correlation between the light scattering and the mitochondrial content of normal tissues and transplantable rodent tumors,”
Anal. Biochem., 226
(1), 167
–174
(1995). http://dx.doi.org/10.1006/abio.1995.1205 ANBCA2 0003-2697 Google Scholar
C. E. CooperR. Springett,
“Measurement of cytochrome oxidase and mitochondrial energetics by near-infrared spectroscopy,”
Philos. Trans. R. Soc. London. B Biol. Sc., 352
(1354), 669
–676
(1997). http://dx.doi.org/10.1098/rstb.1997.0048 PTRBAE 0962-8436 Google Scholar
E. Alexandratouet al.,
“Human fibroblast alterations induced by low power laser irradiation at the single cell level using confocal microscopy,”
Photochem. Photobiol. Sci., 1
(8), 547
–552
(2002). http://dx.doi.org/10.1039/b110213n PPSHCB 1474-905X Google Scholar
S. Passarellaet al.,
“Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser,”
FEBS Lett., 175
(1), 95
–99
(1984). http://dx.doi.org/10.1016/0014-5793(84)80577-3 FEBLAL 0014-5793 Google Scholar
R. M. Castilhoet al.,
“Rac1 is required for epithelial stem cell function during dermal and oral mucosal wound healing but not for tissue homeostasis in mice,”
PloS One, 5
(5), e10503
(2010). http://dx.doi.org/10.1371/journal.pone.0010503 1932-6203 Google Scholar
L. Almeida-Lopeset al.,
“Comparison of the low level laser therapy effects on cultured human gingival fibroblasts proliferation using different irradiance and same fluence,”
Lasers Surg. Med., 29
(2), 179
–184
(2001). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
K. Ehrenreiteret al.,
“Raf-1 regulates Rho signaling and cell migration,”
J. Cell Biol., 168
(6), 955
–964
(2005). http://dx.doi.org/10.1083/jcb.200409162 JCLBA3 0021-9525 Google Scholar
C. Jimenezet al.,
“Role of the PI3K regulatory subunit in the control of actin organization and cell migration,”
J. Cell Biol., 151
(2), 249
–262
(2000). http://dx.doi.org/10.1083/jcb.151.2.249 JCLBA3 0021-9525 Google Scholar
A. J. SingerR. A. Clark,
“Cutaneous wound healing,”
N. Engl. J. Med., 341
(10), 738
–746
(1999). http://dx.doi.org/10.1056/NEJM199909023411006 NEJMAG 0028-4793 Google Scholar
M. EhrenreichZ. Ruszczak,
“Update on tissue-engineered biological dressings,”
Tissue Eng., 12
(9), 2407
–2424
(2006). http://dx.doi.org/10.1089/ten.2006.12.2407 1937-3341 Google Scholar
G. S. AshcroftS. J. MillsJ. J. Ashworth,
“Ageing and wound healing,”
Biogerontology, 3
(6), 337
–345
(2002). http://dx.doi.org/10.1023/A:1021399228395 BIOGCN 1389-5729 Google Scholar
M. M. SantoroG. Gaudino,
“Cellular and molecular facets of keratinocyte reepithelization during wound healing,”
Exp. Cell Res., 304
(1), 274
–286
(2005). http://dx.doi.org/10.1016/j.yexcr.2004.10.033 ECREAL 0014-4827 Google Scholar
V. P. Wagneret al.,
“Influence of different energy densities of laser phototherapy on oral wound healing,”
J. Biomed. Opt., 18
(12), 128002
(2013). http://dx.doi.org/10.1117/1.JBO.18.12.128002 JBOPFO 1083-3668 Google Scholar
M. A. Ribeiroet al.,
“Immunohistochemical assessment of myofibroblasts and lymphoid cells during wound healing in rats subjected to laser photobiomodulation at 660 nm,”
Photomed. Laser Surg., 27
(1), 49
–55
(2009). http://dx.doi.org/10.1089/pho.2007.2215 PLDHA8 1549- 5418 Google Scholar
G. K. Reddy,
“Photobiological basis and clinical role of low-intensity lasers in biology and medicine,”
J. Clin. Laser Med. Surg., 22
(2), 141
–150
(2004). http://dx.doi.org/10.1089/PLT.2004.22.issue-2 JCLSEO 1044-5471 Google Scholar
H. H. van BreugelP. R. Bar,
“Power density and exposure time of He-Ne laser irradiation are more important than total energy dose in photo-biomodulation of human fibroblasts in vitro,”
Lasers Surg. Med., 12
(5), 528
–537
(1992). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
A. N. Pereiraet al.,
“Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts,”
Lasers Surg. Med., 31
(4), 263
–267
(2002). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
A. V. Corazzaet al.,
“Photobiomodulation on the angiogenesis of skin wounds in rats using different light sources,”
Photomed. Laser Surg., 25
(2), 102
–106
(2007). http://dx.doi.org/10.1089/pho.2006.2011 PLDHA8 1549- 5418 Google Scholar
A. R. Medradoet al.,
“Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts,”
Lasers Surg. Med., 32
(3), 239
–244
(2003). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
L. S. Puglieseet al.,
“The influence of low-level laser therapy on biomodulation of collagen and elastic fibers,”
Braz. Oral Res., 17
(4), 307
–313
(2003). Google Scholar
L. J. Walsh,
“The current status of low level laser therapy in dentistry. Part 1. Soft tissue applications,”
Aust. Dent. J., 42
(4), 247
–254
(1997). http://dx.doi.org/10.1111/j.1834-7819.1997.tb00129.x ADEJA2 0045-0421 Google Scholar
R. Lubartet al.,
“Photochemistry and photobiology of light absorption by living cells,”
Photomed. Laser Surg., 24
(2), 179
–185
(2006). http://dx.doi.org/10.1089/pho.2006.24.179 PLDHA8 1549- 5418 Google Scholar
P. M. do Nascimentoet al.,
“A preliminary report on the effect of laser therapy on the healing of cutaneous surgical wounds as a consequence of an inversely proportional relationship between wavelength and intensity: histological study in rats,”
Photomed. Laser Surg., 22
(6), 513
–518
(2004). http://dx.doi.org/10.1089/pho.2004.22.513 PLDHA8 1549- 5418 Google Scholar
T. I. Karu,
“Mitochondrial signaling in mammalian cells activated by red and near-IR radiation,”
Photochem. Photobiol., 84
(5), 1091
–1099
(2008). http://dx.doi.org/10.1111/php.2008.84.issue-5 PHCBAP 0031-8655 Google Scholar
T. SchmelzleM. N. Hall,
“TOR, a central controller of cell growth,”
Cell, 103
(2), 253
–262
(2000). http://dx.doi.org/10.1016/S0092-8674(00)00117-3 CELLB5 0092-8674 Google Scholar
T. E. HarrisJ. C. Lawrence Jr.,
“TOR signaling,”
Sci. STKE, 2003
(212), re15
(2003). http://dx.doi.org/10.1126/stke.2122003re15 1945-0877 Google Scholar
Y. Liet al.,
“TSC2: filling the GAP in the mTOR signaling pathway,”
Trends Biochem. Sci., 29
(1), 32
–38
(2004). http://dx.doi.org/10.1016/j.tibs.2003.11.007 TBSCDB 0167-7640 Google Scholar
T. P. Neufeld,
“Body building: regulation of shape and size by PI3K/TOR signaling during development,”
Mech. Dev., 120
(11), 1283
–1296
(2003). http://dx.doi.org/10.1016/j.mod.2003.07.003 MEDVE6 0925-4773 Google Scholar
L. A. BervenF. S. WillardM. F. Crouch,
“Role of the p70(S6K) pathway in regulating the actin cytoskeleton and cell migration,”
Exp. Cell Res., 296
(2), 183
–195
(2004). http://dx.doi.org/10.1016/j.yexcr.2003.12.032 ECREAL 0014-4827 Google Scholar
E. Jacintoet al.,
“Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive,”
Nat. Cell Biol., 6
(11), 1122
–1128
(2004). http://dx.doi.org/10.1038/ncb1183 NCBIFN 1465-7392 Google Scholar
C. De VirgilioR. Loewith,
“The TOR signalling network from yeast to man,”
Int. J. Biochem. Cell Biol., 38
(9), 1476
–1481
(2006). http://dx.doi.org/10.1016/j.biocel.2006.02.013 IJBBFU 1357-2725 Google Scholar
S. M. Schiekeet al.,
“The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity,”
J. Biol. Chem., 281
(37), 27643
–27652
(2006). http://dx.doi.org/10.1074/jbc.M603536200 JBCHA3 0021-9258 Google Scholar
BiographyAna Carolina Amorim Pellicioli DDS, MsC, PhD student of the Graduation Program of Estomatopathology, School of Dentistry, State University of Campinas. She had a master’s degree experience in oral pathology at Universidade Federal do Rio Grande do Sul. Dentist by the School of Dentistry, Universidade Federal do Rio Grande do Sul. Manoela Domingues Martins DDS, PhD full professor of Oral Pathology Department, School of Dentistry, Universidade Federal do Rio Grande do Sul in Brazil. Her personal research interests are the clinical and basic aspects of laser phototherapy in oral lesions and tissue regeneration. Caroline Siviero Dillenburg DDS master’s degree student of the Department of Oral Pathology, School of Dentistry, Federal University of Rio Grande do Sul in Brazil. Her personal research interests are the clinical effects of laser phototherapy in wound repair. Marcia Martins Marques DDS, PhD full professor and chairman of the Department of Restorative Dentistry, School of Dentistry, University of São Paulo in Brazil. Her postdoc experience at the National Institutes of Health (NIH). She was an invited lecturer of the Master of Science in “Lasers in Dentistry” of the RWTH Aachen University. Her personal research interests are the basic aspects of laser phototherapy in tissue regeneration. She is an editorial board member of the Laser International Magazine of Laser Dentistry. |

